Abstract
Electron probe microanalysis (EPMA) has been used to obtain concentration curves and calculate the bulk diffusion coefficients of Sn in solid solutions of the copper–tin system in a tin concentration range of less than 13.9% weight (7.96 at %) and temperature range from 500 to 650°C. Diffusion couples are made from pure Cu (99.995%) and a two-component alloy of Cu with chemically pure Sn by direct alloying metallic Cu with Sn in an Ar–H2 atmosphere at 1100°C for 2 h. Diffusion coefficients were calculated using the Matano–Boltzmann and Grube methods from the upper part of the concentration curve (from 6 to 8 at % (D1)) and the lower part (from 2 at % to zero (D2)). It is shown that Sn diffusion coefficients in a concentrated solution were several times greater than Sn diffusion coefficients in dilute solution. Both values of diffusion activation energy, especially the second, coincide with isotope data on Sn diffusion in a pure copper (187 kJ/mol). A qualitative interpretation is proposed of the effect of accelerating the diffusion of tin in a concentrated solid solution of the copper–tin system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The Cu–Sn system is widely used in electrical engineering because it has high thermal conductivity, satisfactory mechanical (antifriction) properties, good corrosion resistance, and is economically profitable [1]. In addition, tin is one of the main elements of solders, including for copper alloys [2]; therefore, the features of the diffusion behavior of tin in significant concentrations in copper are of certain interest.
It should be noted that there are a number of works in the literature related to the study of diffusion processes in the Cu–Sn system. To a greater extent, these studies were devoted to the formation of intermetallic phases of Cu3Sn and Cu5Sn between the copper substrate and solder, as well as the study of the Kirkendall effect, which leads to the appearance of excess vacancies and their coagulation into pores [3–6]. In a significant part of the work, direct measurements were made (isotopic and by the method of X-ray microspectral analysis (EPMA)) of diffusion coefficients (D) [7–14]; however, the main data were obtained at high (over 700°С) temperatures, which is apparently connected with the difficulty of measuring D at relatively low temperatures.
According to the phase diagram (Fig. 1 [15]), the Cu-based α-solid solution exists in a rather wide concentration range (up to 9.1 at % Sn at t = 586°C), and the solubility of tin is almost constant in the range t = 350–798°C. At low temperatures (about 350°C and below), the solubility begins to decrease sharply. The solid solution is in equilibrium with the liquid phase at t > 798°С, with the β phase with А2 structure (equilibrium concentrations 13.1–14.9 at % Sn) in the range t = 586–798°С, with the γ phase with DO structure3 (equilibrium concentrations 15.5–16.5 at % Sn) at t = 520–586°C, and with δ and ε phases at lower temperatures.
Phase diagram of the Cu–Sn system [15].
The thermodynamic data for the Cu–Sn system allow one to fairly accurately describe these equilibria so that the chemical potentials of the components and their activity can be considered known [16, 17].
The aim of this work was to measure the diffusion parameters in a wide concentration range at moderately high temperatures (500–650°С), for which the method of X-ray microanalysis was chosen.
RESEARCH METHOD
Diffusion pairs were made of pure copper and a two-component Cu–Sn alloy with a Sn concentration corresponding to the solubility of tin in copper at a peritectic reaction temperature of 798°С. A two-component alloy was obtained by the direct fusion of metallic copper with tin in an argon atmosphere with hydrogen at a temperature of 1100°C in a quartz reactor for 2 h. To prepare the alloy, copper with a purity of 99.995% and chemically pure tin were used. The composition of the alloy was controlled by an inductively coupled plasma atomic emission method (ICP-AES). The analysis showed that the tin content in various areas of the ingot ranged from 8 to 8.3 at %.
Plates about 1-mm-thick were cut from alloy and pure copper; their surface was ground and polished to a mirror shine using a diamond slurry. After that, the alloy plate was clamped between two plates of pure copper in a graphite clamp. The resulting sample was preliminarily annealed in an inert atmosphere at a temperature of 800°C for 1 h and the reliability of the compound was checked.
Diffusion annealing of the samples was carried out in vacuum quartz ampoules at a pressure of 3 × 10–3 Pa in the temperature range 500–650°C. Then, the samples were cut along the direction of the diffusion flow, after which the previously polished and polished samples were etched in a 40% aqueous ammonia solution and a 1–2% aqueous solution of 10% hydrogen peroxide [12].
Tin concentration profiles were obtained using a JEOL JSM-6480LV low vacuum scanning electron microscope (SEM) with an INCA ENERGY DryCool attachment for energy dispersive spectrometry, as well as a FEGFEINavaNanoSEM microscope with a Bruker attachment. The operating voltage for elemental analysis was 20 kV, the vacuum in the chamber was 3 × 10–3 Pa, and the electron start diameter is about 100 nm. Accordingly, the diameter of the analysis region was approximately 1–3 μm. The instrument error did not exceed a few tenths of a percent.
To determine the diffusion coefficients, the elemental composition was studied at points located perpendicular to the initial copper/alloy interface, far from the grain boundaries. A typical micrograph of the sample surface is shown in Fig. 2.
RESULTS
Tin concentration dependencies were obtained (C) from the depth of penetration (x) after annealing at 500 (100 h), 580 (200 h), and 650°C (50 h). A short annealing time at 500°C was chosen in connection with the prospect of measuring grain boundary diffusion.
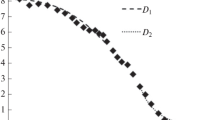
The asymmetric form of the concentration dependence (Fig. 3) is noteworthy. It is clearly seen that the curvature of the upper part of the curve is less than the lower one. Such asymmetry, as a rule, is associated with the dependence of the diffusion coefficient on the concentration D(C).
In this case, to determine value D, use the Matano–Boltzmann method [18] or its analogues (for example, [19]). For the concentration profile obtained after annealing at 650°C for 50 h, the position of the Matano plane is shown in Fig. 4 (xM = 82 μm and CM = 4.3 at %).
The diffusion coefficient was calculated at C1 = 7 at % and C2 = 1 at %, i.e., at the points most differing in the concentration of tin in solid solutions, according to the formulas:
where τ is the annealing time.
Note that, in the Matano method, the coefficient of mutual diffusion is determined. However, according to Darken [20], its value is described as follows:
where Di is the partial diffusion coefficients of the components. Because DSn > DCu and the mole fraction of tin XSn\( \ll \)XCu, D ~ DSn; i.e., the mutual diffusion coefficient is approximately equal to the partial diffusion coefficient of Sn in the solid solution.
The calculation results according to the formula (7) give values D1 = 3.8 × 10–15 and D2 = 1.6 × 10–15 m2/s, respectively.
Thus, the diffusion coefficient of tin in a more concentrated solution, as could be assumed by the type of concentration profile, is greater than in a diluted one, but the difference is small (only 2.4 times). Therefore, dependence DSn(C) is weak, especially considering (and concentration profiles were measured on 4 samples) that the statistical error was about 20% in a more concentrated solution and about 10% in a more diluted solution.
It is known [21] that the determination of the coefficient of mutual diffusion D by the Matano–Boltzmann method at the ends of the concentration profile leads to gross errors. Therefore, we used the more accurate and easy-to-use Grube method [22], which is applicable in the case of a weak dependence D(C) and, in fact, is an implementation of the finite difference method based on the replacement of derivatives by difference schemes. In this case, the concentration profile is divided into small segments for which D = const and we use the solution of the second Fick equation for two semi-infinite plates [23]:
where erfc is the addition to the error function, with the following initial and boundary conditions:
In the calculations, took C0 = 8.2 at % and x0 = 4.3 μm, and the diffusion coefficient was determined by the upper part of the concentration profile, in the range of tin concentrations from 6 to 8 at % (D1) and at the bottom from 2 at % to zero (D2).
At t = 500°C, diffusion coefficients were calculated for three concentration profiles, at 580°C for nine and at 650°C for four. It should be noted that measurements at 500°C were the least accurate due to the small diffusion path. The results are shown in Table 1 in comparison with published data on the diffusion of tin in pure copper. The statistical error of the calculations by the Grube method ranged from 7 to 17%.
In all cases, the values D2 are close to literary data and the ratio D1/D2 makes up several units.
Figure 5 presents the Arrhenius dependence of the obtained data.
The temperature dependence of the diffusion coefficients can be represented in the form of the following equations:
Both the first and, especially, the second values of activation energy (–194 and –188 kJ/mol) almost completely coincide with the results of isotopic measurements of tin diffusion in pure copper (187 kJ/mol). Note that coefficient D1 corresponds to diffusion in a solid solution with a liquidus temperature much lower than the melting temperature of pure copper; therefore, a decrease in the activation energy should be expected, although our results showed that this does not happen.
DISCUSSION
Among the results, we note a slight increase DSn in the Cu–Sn α-solid solution when compared to the diffusion of Sn in pure Cu. It is advisable to consider two possible causes of this effect. The first may be related to the thermodynamic factor. According to Darken [20], the diffusion coefficients in a concentrated (nonideal) solution (D) and dilute solution (D*) are related by
where γ is the activity coefficient.
This explanation correlates with thermodynamic data because, according to [16], in the Cu–Sn α-solid solution, the activity coefficient Sn increases with increasing concentration (Table. 2).
Thus, D > D *. The estimate shows that the second term in Eq. (7) varies from 1 to 0.9 in the concentration range of interest to us, which gives a doubling of the diffusion coefficient.
Another reason for increasing D in the alloy can be associated with the formation of excess vacancies due to the greater mobility of tin atoms in the alloy compared to pure copper. This idea was first put forward by the authors of [24, 25] when analyzing the relationship between the Kirkendall effect and the processes of vacancy formation/disappearance. According to this model, the difference in atomic fluxes is compensated by the flux of vacancies. Excess vacancies are formed in the area of the material where diffusion is faster.
As is known [26], with the vacancy diffusion mechanism,
where Dv is the diffusion coefficient of vacancies, Xv is the molar fraction of vacancies, and \(E_{{\text{v}}}^{{\text{f}}}\) and \(E_{{\text{v}}}^{{\text{m}}}\) are the energy of the formation and movement of vacancies.
In accordance with the result, we assume that the energies of the formation and movement of vacancies are constant. Then, the excessive concentration of vacancies (\(X_{{\text{v}}}^{{{\text{ex}}}}\)) is associated only with the excess entropy of their formation (\(S_{{\text{v}}}^{{{\text{ex}}}}\)):
where \(X_{{\text{v}}}^{{{\text{eq}}}}\) is the equilibrium concentration of vacancies and R is the gas constant.
In our case, the concentration of vacancies increases with respect to the equilibrium as D1/D2, i.e., 2–3 times. This corresponds to an excess entropy of vacancy formation of approximately R; therefore, the concentration of vacancies doubles.
CONCLUSIONS
(1) Using EPMA, concentration profiles were obtained and the diffusion coefficients of Sn in the solid solution of the Cu–Sn system with a concentration of Sn of up to 8 at % in the temperature range 500–650°C were calculated.
(2) It is shown that the diffusion coefficients of tin in a concentrated solution are several times greater than in a diluted solution.
(3) The temperature dependences of the diffusion coefficients in concentration ranges from 6 to 8 at % and from 2 at % To zero can be represented in the form of Eqs. (5) and (6). The activation energy values (–194 and –188 kJ/mol) included in them almost completely coincide with the results of isotopic measurements of tin diffusion in pure copper (187 kJ/mol).
(4) A qualitative interpretation of the effect of accelerating the diffusion of tin in a concentrated solid solution of the Cu–Sn system is proposed based on the fact that the coefficient of thermodynamic activity of tin increases with an increasing concentration of tin in the α-solid solution of Cu–Sn, as well as under the assumption of the occurrence of excess vacancies in the alloy due to the greater mobility of tin atoms in the alloy when compared with pure copper.
REFERENCES
Kostrzhitskii, A.I. and Gusareva, O.F., Patterns of formation of the structure of iron-chromium coatings during ion deposition, Izv. Akad. Nauk SSSR. Met., 1987, no. 1, pp. 169–172.
Wang, J., Leinbach, C., Liu, H.S., Roth, M., and Jin, Z.P., Re-assessment of diffusion mobilities in the face-centered cubic Cu–Sn alloys, CALPHAD, 2009, no. 33, pp. 704–710.
Minho, O., Vakanas, G., Moelans, N., Kajihara, M., and Zhang, W., Formation of compounds and Kirkendall vacancy in the Cu–Sn system, Microelectron. Eng., 2014, vol. 120, pp. 133–137.
Baheti, V.A., Kashuap, S., Kumar, P., Chattopadhya, K., and Paul, A.J., Solid-state diffusion-controlled growth of the intermediate phases from room temperature to an elevated temperature in the Cu–Sn and the Ni–Sn systems, J. Alloys Compd., 2017, vol. 727, pp. 832–840.
Labie, R., Ruythooren, W., and Van Humbeeck, J., Solid state diffusion in Cu–Sn and Ni–Sn diffusion couples with flip-chip scale dimensions, Intermetallics, 2007, no. 15, pp. 396–403.
Chao, B., Chae, S.-H., Zhang, X., Lu, K.-H., Im, J., and Ho, P.S., Investigation of diffusion and electromigration parameters for Cu–Sn intermetallic compounds in Pb-free solders using simulated annealing, Acta Mater., 2007, no. 55, pp. 2805–2814.
Gorbachev, V.A., Klotsman, S.M., Rabovskii, Y.A., Talinskii, V.K., and Timofeev, A.N., Diffusion of impurities in copper. IV. Diffusion of tin and antimony in single crystals of copper, Fiz. Met. Metalloved., 1973, no. 35, pp. 889–893.
Fogelson, R.L., Ugay, Y.A., and Akimova, I.A., Diffusion of tin in copper, Phys. Met. Metallogr., 1974, no. 37, pp. 1107–1108.
Oikawa, H. and Hosoi, A., Interdiffusion in Cu–Sn solid solutions confirmation of anomalously large Kirkendall effect, Scr. Metall., 1975, no. 9, pp. 823–828.
Hoshino, K., Iijima, A.Y., and Hirano, K.I., Inter-diffusion and Kirkendall effect in Cu–Sn alloys, Trans. Jpn. Inst. Met., 1980, vol. 21, no. 10, pp. 674–682.
Santra, S. and Paul, A., Vacancy wind effect on interdiffusion in a dilute Cu (Sn) solid solution, Philos. Mag. Lett., 2012, vol. 92, no. 8, pp. 1–11.
Onishi, M. and Fujibuchi, M., Reaction-diffusion in the Cu–Sn system, Trans. Jpn. Inst. Met., 1975, no. 16, pp. 539–547.
Paul, A., Ghoshi, C., and Boetinger, W.J., Diffusion parameters and growth mechanism of phases in the Cu‒Sn system, Metall. Mater. Trans. A, 2011, vol. 42, no. 4, pp. 952–963.
Kumar, S., Handwerker, C., Dayamanda, M., and Phase, J., Intrinsic and interdiffusion in Cu–Sn system, J. Phase Equilib. Diffus., 2011, vol. 32, no. 4, pp. 309–319.
Massalski, B., Binary Alloy Phase Diagrams, Metals Park, OH: American Society for Metals, 1986.
Predel, B. and Schallner, U., Thermodynamic investigation of the Cu–Ga, Cu–In, Cu–Ge, and Cu–Sn systems, Mater. Sci. Eng., 1972, no. 10, pp. 249–258.
Wallbrecht, R.C., Blachnik, R., and Mills, R.C., The heat capacity and enthalpy of some hume-rothery phases formed by copper, silver and gold. Part II. Cu + Ge, Cu + Sn, Ag + Sn, Au + Sn, Au + Pb systems, Thermochim. Acta, 1981, no. 46, pp. 167–174.
Matano, C., On the relation between the diffusion coefficient and concentration of solid metals, Jpn. J. Phys., 1933, no. 8, pp. 109–113.
den Broeder, E.J.A., A general simplification and improvement of the Matano–Boltzmann method in the determination of the interdiffusion coefficients in binary systems, Scr. Metall., 1969, vol. 3, no. 5, pp. 321–325.
Darken, L., Diffusion, mobility and their interrelation through free energy in binary metallic systems, Trans. AIME, 1948, no. 175, pp. 184–201.
Adda, Y. and Philibert, J., La Diffusion dans les solides, Paris: Press Universitaires de France, 1966.
Grube, G. and Jedele, A., Die Diffusion der Metalle im esten Zustand, Z. Elektrochem., 1932, no. 38, pp. 799–807.
Crank, J., Mathematics of Diffusion, Oxford: Clarendon Press, 1956.
Bardeen, J. and Herring, C., Atom Movements, Cleveland, OH: A.S.M., 1951, pp. 87–288.
Bardeen, J. and Herring, C., Imperfections in Nearly Perfect Crystals, New York: John Wiley and Sons, 1952.
Bokstein, B.S., Mendelev, M.I., and Srolovitz, D.J., Thermodynamics and Kinetics in Materials Science, Oxford: Oxford Univ. Press, 2005.
ACKNOWLEDGMENTS
We thank V. Yesin (Center des materiaux, CNRS UMR 7633, Paris, France) for help in conducting the experiments.
Funding
This study was financed by the Russian Science Foundation (project no. 16-12-10478).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
About this article
Cite this article
Nikulkina, V.P., Rodin, A.O. & Bokshtein, B.S. Diffusion of Tin in a Solid Solution of the Copper–Tin System. Russ. J. Non-ferrous Metals 61, 291–296 (2020). https://doi.org/10.3103/S1067821220030116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821220030116