Abstract
Predicting the misrun formation in thin-walled castings of magnesium alloys is a critical task for foundry. The computer simulation of casting processes can be used to solve this problem. Adequate results of simulation can be attained in the presence of the correct thermal properties of the alloy and a mold in a wide temperature range, interface heat-transfer coefficient between the casting and a mold, and the critical solid fraction (at which the melt flow in a mold is stopped). In this work, the interface heat-transfer coefficient between the ML5 (AZ91) magnesium alloy and a no-bake sand mold is found by comparing simulation spiral test lengths with experimental spiral test lengths under the same pouring conditions. Its values above the liquidus temperature are hL = 1500 W/(m2 K) at pouring temperatures of 670 and 740°C and hL = 1800 W/(m2 K) at 810°C. Below the solidus temperature, hS = 600 W/(m2 K). The critical solid fraction for the ML5 (AZ91) magnesium alloy was also determined for no-bake mold casting (with a cooling rate of ~2 K/s)—its value was 0.1–0.15. The critical solid fraction is refined by comparing the position of misruns by the results of simulation and in an actual “Protective cap” ML5 (AZ91) alloy casting poured into the no-bake mold. Castings are poured at temperatures of 630 and 670°C, and the critical solid fraction is 0.1 in both cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
To predict the misrun formation in castings, the computer simulation of foundry processes is widely used [1, 2]. Simulating filling of thin-wall castings of magnesium alloys is an especially important problem, because magnesium alloys do not possess high fluidity due to their wide solidification range.
Melt fluidity depends on many factors such as the melt composition, melt superheating, alloy grain size, the presence of grain-refining additions, thermal properties of the mold material and its temperature, melt purity and viscosity, and the presence of coatings on the mold surface [3]. Fluidity is measured in industrial practice as the known-section channel length, which is filled by melt in the standard fluidity test [4]. Spiral, strip, and other tests are used [5]. There are few works in which the fluidity of magnesium alloys was studied [6–8], which is associated with complexity of their melting and casting.
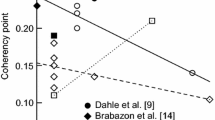
Melt flow in the mold also continues at the moment when the melt is in the mushy state. The fraction of the solid phase, at which the melt flow in the mold is stopped, is called critical [9]. There are certain difficulties in its experimental determination. Another parameter, which is called the coherency point [12], is similar and determinable experimentally [10, 11]. The coherency point is the solid fraction; upon reaching it, the melt starts to acquire strength [10].
Values of coherency point for the AZ91 (ML5) are presented in Table 1 and in Fig. 1. According to [13], an increase in the cooling rate (Vcool) promotes an increase in the AZ91 alloy coherency point. In other publications [14–18], higher values are presented for the AZ91 alloy at Vcool = 0.4–1.0 K/s.
One important parameter, which should be known to simulate the fluidity and misrun formation, is the interface heat-transfer coefficient [3]. It is usually calculated based on experimentally determined temperatures in the casting and in the mold [19]. The temperature dependence of the interface heat-transfer coefficient is best suitable for simulation [20]; however, its dependence on time, which cannot be used for simulation, is most often presented in publications [19]. The interface heat-transfer coefficient is not constant and depends on many parameters [21–27].
The authors of [20] have found the following values of the interface heat-transfer coefficient for the AZ91 magnesium alloy during casting into the sand mold hardened by furan resin: hL = 600 W/(m2 K) above the liquidus temperature and hS = 50 W/(m2 K) below the solidus temperature. We failed to find more data for the comparison.
The authors of [1, 28, 29], in order to evaluate alloy fluidity and the subsequent determination of the interface heat-transfer coefficient and critical solid fraction by comparing the results of simulation and experimental data, used the spiral test.
The goal of our work was to find the interface heat-transfer coefficient between the ML5 (AZ91) magnesium alloy and the no-bake sand mold, as well as the critical solid fraction for the ML5 (AZ91) alloy when casting into the no-bake sand mold using procedures approved for the A356 alloy [29].
EXPERIMENTAL
We used the following raw materials as the charge for the alloy preparation: magnesium (99.9%), aluminum (99.99%), zinc (99.975%), and master alloy Mg–10 wt % Mn. Melting was performed in a steel crucible in a resistance furnace under cover of the carnallite-based flux (KCl · MgCl2). Melt refining to remove non-metallic inclusions was performed at 740–760°C by mixing molten carnallite into the melt. After this, the melt was held for 15 min to remove the flux and pouring was performed. The composition of prepared alloys is presented in Table 2. The chemical composition was determined by energy dispersive X-ray spectrometry over the area of 1 × 1 mm using a Tescan Vega SBH3 scanning electron microscope (SEM) (Tescan, Czech Republic) with an Oxford energy dispersive microanalysis attachment.
To determine the melt fluidity, a spiral test was used. The mold for its pouring was made from furan resin binded sand. Pouring temperatures were tp = 670, 740, and 810°C. To compare the misrun locations in an actual casting with the results of simulation, the Protective Cup casting was poured at tp = 630 and 670°C. The mold making procedure and casting parameters of spiral test and Protective Cup is presented in [29]. So long as the molds were intended for magnesium alloy pouring, the ignition inhibitor—the potassium tetrafluoroborate powder in an amount of 0.5 wt % of the sand weight—was added during their fabrication.
Simulation was performed in the ProCast 2016 software. The simulation process, as well as the procedure for finding the interface heat-transfer coefficient and critical solid fraction, are described in [29]. We used the alloy thermal properties calculated with the help of the PanMg 2014 thermodynamic database (CompuTherm, United States) included into the ProCast 2016 program and thermal properties of furan binded sand mold taken from [30]. The thermal properties of MPG-6 graphite (graphite stopper was used when pouring the spiral test) were taken from [31], while the interface heat-transfer coefficient between the melt and graphite was taken from [32].
RESULTS AND DISCUSSION
Results of Experimental and Simulated Spiral Test Pouring of the ML5 (AZ91) Alloy
We simulated casting of spiral tests at several values of the interface heat-transfer coefficient between the casting and a mold above the liquidus temperature (606°C) hL = 600–2100 W/(m2 K) every 300 W/(m2 K) and at a constant value of the interface heat-transfer coefficient below the solidus temperature (413°C) hS = 600 W/(m2 K). The interface heat-transfer coefficient below the solidus temperature was determined previously in [33]. The interface heat-transfer coefficient between the liquidus and solidus temperatures was specified in the form of a linear dependence between mentioned two.
A thermocouple measuring the melt temperature in a sprue was mounted into the parting line of the mold before pouring. We found cooling curves for the thermocouple arranged in the mold parting line using the simulation. The best coincidence of cooling curves found with the help of simulation and experimental curves was observed at hL = 1500 W/(m2 K) at tp = 670 and 740°C an hL = 1800 W/(m2 K) at tp = 810°C. The results of a comparison of experimental and simulated cooling curves at the above values of the interface heat-transfer coefficient are presented in Fig. 2. It is seen that these curves are rather close. These values of the interface heat-transfer coefficient were subsequently used. They differ from value hL = 1100 W/(m2 K) presented in [33], but this difference is not as significant for pouring temperatures of 670 and 740°C.
Amounts of the solid phase at the coherency point presented in Fig. 1 and Table 1 are expressed as volume fractions. The critical solid fraction is expressed in the ProCast casting simulation software as weight fraction; therefore, it is necessary to determine what the difference of the volume fraction of the solid phase for the ML5 (AZ91) alloy from the weight fraction is. Volume and weight fractions of the solid phase of the ML5 (AZ91) alloy with the Mg–9Al–0.7Zn–0.3Mn (wt %) composition were calculated in the Thermo-Calc program using the TTMG3 thermodynamic database. To determine the volume fraction, the density of this alloy was additionally calculated in the ProCast program. It was determined that the volume fraction of the solid phase at t = 575°C is 0.456, while the weight fraction is 0.464; i.e., they differ no more than by 3%. This allows us to affirm that volume fractions of the solid phase presented in Table 1 almost coincide with the weight fractions.
According to the results of simulation, the average cooling rate when pouring spiral tests of the ML5 (AZ91) alloy was ≈2 K/s. The value of the coherency point for the ML5 (AZ91) alloy at a high cooling rate (2.5 K/s) presented in the literature is 0.13 [13] (Fig. 1). Therefore, we determined the lengths of spiral tests using the simulation at critical solid fractions \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.1 and 0.15, which correspond to the coherency point of the ML5 (AZ91) alloy. Values of the interface heat-transfer coefficient above the liquidus temperature were specified as hL = 1500 W/(m2 K) for pouring temperatures of 670 and 740°C and hL = 1800 W/(m2 K) for tp = 810°C.
Lengths of poured spiral tests depending on the pouring temperature are presented in Fig. 3 and denoted by solid line 1. Their average values were 332, 446, and 603 mm for tp = 670, 740, and 810°C, respectively. Figure 3 also shows spiral lengths found using the simulation at critical solid fraction \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.15 (dashed line 2) and 0.1 (dotted line 3). It is seen that the experimental and simulated spiral lengths are closest for tp = 670°C at \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.15, for tp = 740°C at \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.1, and for tp = 810°C at \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.15. Our results do not allow us to note the critical solid fraction, but in general we can consider that it is in the range of 0.1–0.15.
Experimental (1) and found at the critical solid fraction of (2) 0.15 and (3) 0.1 dependences of the length of the spiral test made of the ML5 (AZ91) alloy on the pouring temperature. The used interfacial heat-transfer coefficients were hL = 1500 W/(m2 K) at tp = 670 and 740°C and hL = 1800 W/(m2 K) at tp = 810°C.
Results of Pouring and Simulation of the Protection Cup Casting Made of the ML5 (AZ91) Alloy
Figure 4a shows the Protection Cup casting poured at 630°C. The critical solid fraction was specified as 0.1. Casting regions where the solid fraction during filling equals zero are shown gray 1. Regions where solid fraction is between zero and a critical value of 0.1, and consequently, there is a low probability of the misrun formation, are denoted darker 2. Zones with a high probability of the misrun formation (the solid fraction exceeds the critical one) are fulfilled black 3. It is seen that the results of the simulation and experiment are rather similar. We also performed simulation at \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.15. The coincidence with the experiment was much worse in this case.
The position of misruns in the Protective Cup casting at a pouring temperature of 670°C is shown in Fig. 5a. It is seen that misruns are present only in a small segment at the casting top. Figure 5b shows the solid fraction distribution in the casting during filling found using simulation at tp = 670°C. The critical solid fraction is 0.1. In general, the area of misruns by the results of simulation is much larger than in an actual casting, but their position (in the top casting part) coincides very well. Simulation at \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.15 did not match the experiment as well.
The disagreement of misruns positions between experimentally produced casting and results of simulation in ProCast observed in Fig. 5 is explained by the imperfection of simulating the melt flow when filling the mold. It is seen (Fig. 5a) that the direction of the main melt flow (a bright region in the photograph of the casting staring in the gate region and passing with elevation upward to the opposite casting wall) forms the superheated melt zone in the wall opposite to the gate, while melt is cold there according to the results of simulation (Fig. 5b) and the mold is filled along the wall adjoining the gate. This implementation feature of filling simulation (rather than the thermal calculation) is the main cause of the mismatch between misrun simulation results and experimental ones.
CONCLUSIONS
By comparing the experimental data on pouring the spiral tests and results of simulation, the interface heat-transfer coefficient between the ML5 (AZ91) alloy casting and a no-bake mold is determined. It is hL = 1500 W/(m2 K) above the liquidus temperature and hS = 600 W/(m2 K) below the solidus temperature for pouring temperatures of 670 and 740°C. For the pouring temperature of 810°C, hL = 1800 W/(m2 K) above the liquidus temperature. The critical solid fraction (at which the melt flow in the mold is stopped) for the ML5 (AZ91) alloy during pouring into a furan binded sand mold is in a range of 0.1–0.15.
Based on experimentally found values of the interface heat-transfer coefficient, it is established that the results of simulation in the ProCast program and the native experiment data for the Protection Cup casting are well-matched at \(f_{{\text{S}}}^{{{\text{cr}}}}\) = 0.1 and hL = 1500 W/(m2 K).
REFERENCES
Jakumeit, J., Subasic, E., and Bünck, M., Prediction of misruns in thin wall castings using computational simulation, in: Shape Casting: 5th Int. Symp., San Diego: Wiley, 2014, pp. 253-260.
Humphreys, N.J., McBride, D., Shevchenko, D.M., Croft, T.N., Withey, P., Green, N.R., and Cross, M., Modelling and validation: Casting of Al and TiAl alloys in gravity and centrifugal casting processes, Appl. Math. Model., 2013, vol. 37, nos. 14–15, pp. 7633–7643.
Di Sabatino, M. and Arnberg, L., A review on the fluidity of Al based alloys, Metall. Sci. Technol., 2004, vol. 22, no. 1, pp. 9–15.
Pikunov, M.V., Plavka metallov, kristallizatsiya splavov, zatverdevanie otlivok: Uchebnoe posobie dlya vuzov (Melting of Metals, Crystallization of Alloys, and Solidification of Castings: Textbook for Higher School), Moscow: MISiS, 2005.
Di Sabatino, M., Arnberg, L., Brusethaug, S., and Apelian, D., Fluidity evaluation methods for Al–Mg–Si alloys, Int. J. Cast Met. Res., 2006, vol. 19, pp. 94–97.
Li, Y., Wu, G., Chen, A., Liu, W., Wang, Y., and Zhang, L., Effects of processing parameters and addition of flame-retardant into moulding sand on the microstructure and fluidity of sand-cast magnesium alloy Mg–10Gd–3Y–0.5Zr, J. Mater. Sci. Technol., 2017, vol. 33, no. 6, pp. 558–566.
Hua, Q., Gao, D., Zhang, H., Zhang, Y., and Zhai, Q., Influence of alloy elements and pouring temperature on the fluidity of cast magnesium alloy, Mater. Sci. Eng. A, 2007, vol. 444, nos. 1–2, pp. 69–74.
Koltygin, A.V. and Plisetskaya, I.V., Influence of small calcium additives on fluidity of magnesium alloys, Liteishchik Rossii, 2011, no. 6, pp. 41–43.
Ravi, K.R., Pillai, R.M., Amaranathan, K.R., Pai, B.C., and Chakraborty, M., Fluidity of aluminum alloys and composites: A review, J. Alloys Compd., 2008, vol. 456, nos. 1–2, pp. 201–210.
Dahle, A.K. and Arnberg, L., Development of strength in solidifying aluminium alloys, Acta Mater., 1997, vol. 45, no. 2, pp. 547–559.
Veldman, N.L., Dahle, A.K., StJohn, D.H., and Arnberg, L., Dendrite coherency of Al–Si–Cu alloys, Metall. Mater. Trans. A, 2001, vol. 32, no. 1, pp. 147–155.
Dahle, A.K., Tundel, P.A., Paradies, C.J., and Arnberg, L., Effect of grain refinement on the fluidity of two commercial Al–Si foundry alloys, Metall. Mater. Trans. A, 1996, vol. 27, no. 8, pp. 2305–2313.
Kryl, M., Tacski, T., Matula, G., Snopinski, P., and Tomiczek, A.E., Analysis of crystallisation process of cast magnesium alloys based on thermal derivative analysis, Arch. Metall. Mater., 2015, vol. 60, no. 4, pp. 2993–2999.
Liang, S.M., Chen, R.S., Blandin, J.J., Suery, M., and Han, E.H., Thermal analysis and solidification pathways of Mg–Al–Ca system alloys, Mater. Sci. Eng. A, 2008, vol. 480, nos. 1–2, pp. 365–372.
Gourlay, C.M., Meylan, B., and Dahle, A.K., Shear mechanisms at 0–50% solid during equiaxed dendritic solidification of an AZ91 magnesium alloy, Acta Mater., 2008, vol. 56, no. 14, pp. 3403–3413.
Gourlay, C.M., Meylan, B., and Dahle, A.K., Rheological transitions at low solid fraction in solidifying magnesium alloy AZ91, Mater. Sci. Forum, 2007, vol. 561–565, pp. 1067–1070.
Hou, D.-H., Liang, S.-M., Chen, R.-S., Dong, C., and Han, E.-H., Effects of Sb content on solidification pathways and grain size of AZ91 magnesium alloy, Acta Metal. Sinica (Engl. Lett.), 2015, vol. 28, no. 1, pp. 115–121.
Barber, L.P., Characterization of the solidification behavior and resultant microstructures of magnesium-aluminum alloys: A Master Degree Thesis, Worchester: Worchester Polytech. Inst., 2004.
Rajaraman, R. and Velraj, R., Comparison of interfacial heat transfer coefficient estimated by two different techniques during solidification of cylindrical aluminum alloy casting, Heat Mass Transfer, 2008, vol. 44, no. 9, pp. 1025–1034.
Chen, L., Wang, Y., Peng, L., Fu, P., and Jiang, H., Study on the interfacial heat transfer coefficient between AZ91D magnesium alloy in silica sand, Exp. Thermal Fluid Sci., 2014, vol. 54, pp. 196–203.
Wang, D., Zhou, C., Xu, G., and Huaiyuan, A., Heat transfer behavior of top side-pouring twin-roll casting, J. Mater. Process. Technol., 2014, vol. 214, no. 6, pp. 1275–1284.
Griffiths, W. and Kawai, K., The effect of increased pressure on interfacial heat transfer in the aluminium gravity die casting process, J. Mater. Sci., 2010, vol. 45, no. 9, pp. 2330–2339.
Sun, Z., Hu, H., and Niu, X., Determination of heat transfer coefficients by extrapolation and numerical inverse methods in squeeze casting of magnesium alloy AM60, J. Mater. Process. Technol., 2011, vol. 211, no. 8, pp. 1432–1440.
Nishida, Y., Droste, W., and Engler, S., The air-gap formation process at the casting-mold interface and the heat transfer mechanism through the gap, Metall. Trans. B, 1986, vol. 17, no. 4, pp. 833–844.
Tikhomirov, M.D., Simulation of thermal and shrinkage processes during the solidification of castings of high-strength aluminum alloys and development of the computer analysis system of the casting technology, Extended Abstract of Cand. Sci. (Eng.) Dissertation, St. Petersburg: SPb Gos. Politekh. Univ., 2004.
Bouchard, D., Leboeuf, S., Nadeau, J.P., Guthrie, R.I.L., and Isac, M., Dynamic wetting and heat transfer at the initiation of aluminum solidification on copper substrates, J. Mater. Sci., 2009, vol. 44, no. 8, pp. 1923–1933.
Lu, S.-L., Xiao, F.-R., Zhang, S.-J., Mao, Y.-W., and Liao, B., Simulation study on the centrifugal casting wet-type cylinder liner based on ProCAST, Appl. Thermal Eng., 2014, vol. 73, no. 1, pp. 512–521.
Di Sabatino, M., Arnberg, L., and Bonollo, F., Simulation of fluidity in Al–Si alloys, Metall. Sci. Technol., 2005, vol. 23, no. 1, pp. 3–10.
Bazhenov, V.E., Petrova, A.V., and Koltygin, A.V., Simulation of fluidity and misrun prediction for the casting of 356.0 aluminum alloy into sand molds, Int. J. Metalcasting, 2018, vol. 12, no. 3, pp. 514–522.
Palumbo, G., Piglionico, V., Piccininni, A., Guglielmi, P., Sorgente, D., and Tricarico, L., Determination of interfacial heat transfer coefficients in a sand mould casting process using an optimised inverse analysis, Appl. Thermal Eng., 2015, vol. 78, pp. 682–694.
Zhmurikov, E.I., Savchenko, I.V., Stankus, S.B., and Tecchio, L., Measurements of thermal properties of graphite composites for a neutron target converter, Vestn. NGU. Ser. Fiz., 2011, vol. 6, no. 2, pp. 77–84.
Bazhenov, V.E., Koltygin, A.V., Tseloval’nik, Yu.V., and Sannikov, A.V., Determination of interface heat transfer coefficient between aluminum casting and graphite mold, Russ. J. Non-Ferrous Met., 2017, vol. 58, no. 2, pp. 114–123.
Bazhenov, V.E., Petrova, A.V., Koltygin, A.V., and Tseloval’nik, Yu.V., Determination of heat transfer coefficient between AZ91 magnesium alloy casting and no-bake mold, Tsvetn. Met., 2017, no. 8, pp. 89–96.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education and Science of the Russian Federation, contract no. 03.G25.31.0274 dated May 27, 2017, and a Grant of the President of the Russian Federation to young scientists and postgraduates who are carrying out scientific investigations and developments in the priority directions of the Russian Federation’s economy modernization (2016–2018 contest).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by N. Korovin
About this article
Cite this article
Petrova, A.V., Bazhenov, V.E. & Koltygin, A.V. Prediction of Misruns in ML5 (AZ91) Alloy Casting and Alloy Fluidity Using Numerical Simulation. Russ. J. Non-ferrous Metals 59, 617–623 (2018). https://doi.org/10.3103/S1067821218060159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821218060159