Abstract
A review of publications on the structure, properties, fabrication methods, and application fields of materials based on the Cr2AlC MAX phase is given. It is noted that the most promising method of formation of such materials is self-propagating high-temperature synthesis (SHS), one of the directions of which is SHS metallurgy. A powder mixture of chromium III and chromium VI oxides of the analytical grade, aluminum of ASD-1 grade, and carbon is used as the base charge in investigations. The adiabatic combustion temperature and composition of final products is calculated using the THERMO special program. Experiments were performed in an SHS reactor with volume V = 3 dm3 under the initial pressure of inert gas (Ar) P0 = 5 MPa. The influence of the ratio of initial reagents on SHS parameters (the combustion rate, pressure increment, and yield of the target product), composition, and microstructure of target products is investigated experimentally. A scientific approach of the formation of cast materials in the Cr–Al–C system consisting of the Cr2AlC MAX phase and phases Cr3C2 and Cr5Al8 by the SHS metallurgy method is developed. The structural-phase states of target products are studied. It is established experimentally that, varying the content of initial reagents (aluminum and carbon) in the charge, it is possible to substantially affect the synthesis regularities, composition, and microstructure of final products. An increase in the content of the Cr2AlC MAX phase in the final product and a decrease in the Cr5Al8 content occur with an increase in the carbon content (above stoichiometric) in the initial mixture. An increase in the aluminum content (above stoichiometric) in the initial mixture leads to an increase in the content of the Cr2AlC MAX phase in the final product and a decrease in the content of the Cr3C2 phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Recently, great scientific and practical interest in MAX phases of carbide and nitride compounds have appeared in Russia and abroad. MAX phases of carbide compounds are described by the general formula Mn + 1AXn, where n = 1, 2, or 3; M is the transition metal (chromium or titanium, as a rule); A is preferentially the element of IIIA and IVA subgroups of the periodic table, and X is carbon. These compounds have a layered structure in which Mn + 1Xn layers are alternated with A. Carbon atoms occupy octahedral voids formed by atoms M.
MAX phases do not have a very high hardness (2–8 GPa), but they possess increased heat and electrical conductivity, resistance to aggressive media, thermal shocks, and have a low thermal expansion coefficient [1–4]. The Cr2AlC MAX phases formed by hot pressing methods have high mechanical properties: the density of 5.17 g/cm3, Vickers hardness of 4.9 GPa, bending strength of 469 ± 27 MPa, Young modulus of 282 GPa, ultimate compression strength of 949 ± 22 MPa, and are stable to 1500°C in argon [5, 6].
The crystalline structure of the Cr2AlC MAX phase consists of six closely packed layers, of which four consist of chromium atoms and two consist of aluminum atoms. The lattice constants of the hexagonal phase are a = 2.866 Å and c = 12.82 Å.
The MAX phases are most widespread in practice as protective coatings. To fabricate thin coatings, physical (PVD) and chemical (CVD) deposition methods are developed [7]. These methods have an increased complexity of fabrication of single-phase coatings and necessity of substrate heating. To fabricate thick coatings on large-scale parts, gas-thermal methods are applied [8, 9]. However, coatings do not possess the necessary phase purity in either case. One promising deposition technology of protective coatings is electric-spark deposition (ESD), which is based on the transfer of the electrode (anode) material on a part (cathode) under pulsed discharges in a gas medium [10–12].
Among the methods of fabrication of Cr2AlC MAX phases, hot pressing and plasma sintering are most often presented in publications [5, 6, 13–16]. Herewith, the processes are usually performed at elevated temperatures (1400°C), high pressures (up to 20 MPa), and using complex equipment. They are low-productive and energy consuming.
Self-propagating high-temperature synthesis (SHS) is the most promising fabrication method of such materials; one branch of this method is SHS metallurgy. Initial mixtures consisting of metal oxides, reducer metal (aluminum), and nonmetal (carbon, boron, and silicon) are used in this method. Combustion temperatures of such mixtures usually exceed the melting points of initial reagents and final products formed in the combustion wave in the liquid-phase (cast) state [17–20].
The goal of our investigation was to develop a scientific approach for the synthesis of cast materials in the Cr–Al–C system (MAX phases Cr2AlC, Cr3C2, and Cr5Al8) with various specified compositions by the method of SHS metallurgy and to investigate their structural-phase states under various process conditions.
EXPERIMENTAL
We used mixtures of powders of chromium III and chromium VI oxides of the analytical grade, aluminum of ASD-1 grade, and carbon in our experiments.
We experimentally determined the combustion rate (Uc), pressure increment in the reactor (ΔP), and completeness of the yield of the metallic phase into the ingot (η1):
where h is the height of the mixture layer in a quartz glass, t is the combustion time, P0 and Pf are the initial and final pressures in the reactor, mm is the weight of the initial mixture, and min is the weight of the metallic phase (ingot).
Component ratios of the initial mixture were calculated using the following chemical reaction:
Herewith, the content of each component was determined according to the formula
where mi is the molecular weight of one component and M is the molecular weight of all components of the mixture.
Before the experiments, all reagents were dried in a SNOL drying oven for 3 h at 80°C. Initial mixtures of 40 and 100 g in weight were prepared manually in a porcelain mortar. When studying the combustion regularities of mixtures and phase separation of initial products, charge samples were combusted in small quartz glasses 16–25 mm in diameter and 50–60 mm in height. When studying the formation of the composition and microstructure of target products, the initial mixtures (mm = 40 and 100 g) were combusted in graphite vessels 30–40 mm in diameter and 50–100 mm in height.
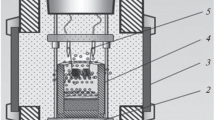
The reaction mold with the charge was placed in experiments into the SHS reactor with volume V = 3 dm3, after which, it was sealed, the initial pressure of 5 MPa of inert gas (Ar) was supplied, and the initial mixture was ignited with the help of metallic (Mo, Ni, Cr) coil by supplying voltage U = 30 V to it. The combustion process was studied visually as well as with the help of video camera.
The phase composition of synthesis products was investigated by X-ray phase analysis (XPA) with the help of a DRON-3M diffractometer using CuKα radiation with a monochromator on a secondary beam. Recording was performed in the step-by-step scanning mode in angle range 2θ = 10°–100° with a recording step of 0.02° and exposure of 2 s. The quantitative analysis was performed by the Rietveld method. The microstructure and elemental analysis of the samples were investigated using an ULTRA plus (Zeiss) ultrahigh-resolution field-emission scanning electron microscope (SEM) with an INCA 350 microanalysis system (Oxford Instruments).
The adiabatic combustion temperature and assumed composition of final products were calculated using a personal computer according to the THERMO special program.
EXPERIMENTAL RESULTS AND DISCUSSION
The thermodynamic calculation of the combustion of the mixture, the reagent ratio of which is calculated according to reaction (1), showed that Tc = 3180 K, while final products are Cr2AlCc, Al2\({\text{O}}_{3}^{{\text{c}}},\) Cr7\({\text{C}}_{3}^{{\text{c}}},\) Cr3\({\text{C}}_{2}^{{\text{c}}},\) Cr5\({\text{Al}}_{8}^{{\text{c}}},\) COg, C\({\text{O}}_{2}^{{\text{g}}},\) Alg, AlOg, and Al2Og (superscripts “c” and “g” denote condensed and gaseous products, respectively). When combusting initial mixtures with the stoichiometric ratio of reagents calculated according to reaction (1), it was found that the mixtures combust in the steady-state mode with a smooth front. Combustion rate Ug = 7.2 mm/s, and the mass spread from the reaction mold η2 ~ 3%.
Final products in the combustion wave are formed in the liquid-phase state and are separated into two layers under the effect of gravity because of the different specific weight. The bottom layer is metallic (Cr–Al–C) and the top layer is oxide (Al2O3). The yield of the target product into the ingot η1 = 44%.
X-ray phase (Fig. 1) and local microstructural (Fig. 2) analyses show that the target product mainly consists of the Cr2AlC MAX phase and a small amount of phases Cr7C3 and Cr5Al8. Diffraction lines of the Cr2AlC MAX phase are narrow (Fig. 1), which evidences the high quality of its crystalline structure. Unit-cell parameters of the experimentally formed Cr2AlC MAX phase (a = 0.286, c = 1.283) almost coincide with the theoretical values (a = 0.286, c = 1.282) of unit-cell parameters of the corresponding phase of the PDF2 crystallographic database. A quantitative analysis performed using the Rietveld method showed that the content of the Cr2AlC phase is 86%, while that of the Cr7C3 phase is 14%.

Fig. 2.
Microstructure, elemental composition, and phase composition of the metallic ingot formed from the stoichiometric mixture according to reaction (1).
Spect. | C | Al | Cr | Phase* |
|---|---|---|---|---|
1 | 8.6 | 18.7 | 72.7 | Cr2AlC |
2 | 8.4 | 18.4 | 73.2 | Cr2AlC |
3 | 0.3 | 44.2 | 55.5 | Cr5Al8 |
4 | 0.1 | 44.5 | 55.4 | Cr5Al8 |
5 | 15.5 | 0.4 | 84.1 | Cr3C2 |
6 | 14.9 | 0.3 | 84.8 | Cr3C2 |
Figures 3 and 4 show the microstructures of the surface of the ingot cleavage. It is seen that the material has a layered structure. Herewith, the nanolaminate structure with the layer thickness from 3 to 20 nm is observed (Fig. 3d). A corrugated nanolaminate structure is formed in the sample pore space (Fig. 4).
The presence of phases of chromium carbides and aluminides in the final target product is explained by the fact that SHS metallurgy is a complex and multistage process [18–20]. Carbon and aluminum form gaseous products (CO, CO2, Al, AlO, and Al2O) during combustion of initial mixture (1), which are removed from the melt. Due to this, their deficit is formed in the system when compared with the stoichiometric content, which leads to the formation of phases Cr5Al8, Cr7C3, and Cr3C2.
In subsequent experiments, we investigated the influence of the overstoichiometric carbon content (ΔC) on the synthesis regularities and formation of the composition and structure of final products. Herewith, the carbon content was calculated according to the formula
where MC is the carbon weight in the charge and \(M_{{\text{C}}}^{{{\text{stoi}}}}\) is the carbon weight in stoichiometric mixture (1).
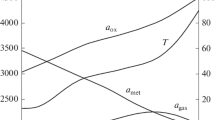
According to the data of the thermodynamic calculation, the combustion temperature (Tc) drops from 3180 to 2920 K with an increase in ΔC from 0 to 50%, and the fraction of gaseous products lowers from 13 to 9%.
It follows from the experimental results (Fig. 5) that an increase in the carbon content in the initial mixture causes a decrease in the combustion rate, the spread of the reaction mass, and the yield of the target product into the ingot, which is explained by the drop in the combustion temperature. The content of the Cr2AlC MAX phase in final products increases with an increase in the fraction of ΔC, while the Cr7C3 phase transforms into Cr3C2 (Fig. 6).
At the next stage of investigations, we studied the influence of the overstoichiometric aluminum content (ΔAl) on the synthesis regularities and the formation of the composition and structure of final products. Herewith, the aluminum content in the charge was calculated according to the formula
where MAl is the aluminum weight in the charge, while \(M_{{{\text{Al}}}}^{{{\text{stoi}}}}\) is the aluminum weight in the stoichiometric mixture formed according to reaction (1).
A thermodynamic calculation showed that the value of Tc drops from 3180 to 3000 K with an increase in the aluminum excess (ΔAl) from 0 to 50%, which in turn determines a decrease in the fraction of gaseous products.
Thus, it follows from the results of experiments (Fig. 7) that the combustion rate and spread of the reaction mass drop with an increase in ΔAl in the initial mixture with an increase in the yield of the target product into the ingot. Final products herewith consist of the Cr2AlC MAX phase and Cr5Al8 phase (Fig. 8).
CONCLUSIONS
Thus, it is shown that cast materials consisting of the Cr2AlC MAX phase, as well as Cr3C2 and Cr5Al8 phases, are formed by SHS metallurgy under the excess gas pressure from the mixture with the stoichiometric ratio of components according to (1). The presence of chromium carbide and aluminide in the final product is highly likely associated with the deficit of carbon and aluminum because of their participation in the redox reaction with the formation of gaseous oxides and suboxides (COg, \({\text{CO}}_{2}^{{\text{g}}},\) Alg, AlOg, and Al2Og) volatilizing from the melt.
An analysis of the results of this investigation and those presented in previous works on SHS metallurgy [17–20] evidences the stage character of the chemical transformations proceeding in the combustion wave. A continuous medium is formed in the heating zone after the initial oxide melts; aluminum drops and carbon particles are distributed in this medium. They interact with the initial oxide at the beginning of the chemical transformation zone independently from each other:
Thus, after the Al2O3 “bridge” formed between them, exchange reactions proceed and the carbonization of metal reduced according to reaction (2) occurs:
Thus, two condensed products Me–Al–C and Al2O3, as well as CO gas, are formed in the scope of the considered scheme. In reality, according to the thermodynamic data, other gases are also formed, such as metal vapors and suboxides (COg, \({\text{CO}}_{2}^{{\text{g}}},\) Alg, AlOg, and Al2Og).
It is established experimentally that, when varying the amount of initial reagents (aluminum and carbon) in the charge, we can substantially affect the synthesis regularities, composition, and microstructure of final target products. The concentration of the Cr2AlC MAX phase increases and the fraction of the Cr5Al8 phase decreases with an increase in the carbon content (above stoichiometric) in the initial mixture. An increase in the content (above stoichiometric) of aluminum in the initial mixture leads to an increase in the amount of the Cr2AlC MAX phase in the final product and a decrease in the Cr3C2 content.
It follows from the analysis of the investigations that cast material consisting of the Cr2AlC MAX phase and Cr3C2 and Cr5Al8 phases with their various contents can be synthesized by the SHS metallurgy method.
REFERENCES
Barsoum, M.W. and Raghy, T., The MAX phases: Unique new carbide and nitride materials, Amer. Sci., 2001, vol. 89, no. 4, pp. 336–345.
Tzenov, N.V. and Barsoum, M.W., Synthesis and characterization of Ti3AlC1.8, J. Amer. Ceram. Soc., 2000, vol. 83, no. 4, pp. 825–832.
Barsoum, M.W. and Radovic, M., Elastic and mechanical properties of the MAX phases, Annu. Rev. Mater. Res., 2011, vol. 41, pp. 195–227.
Li, H., Li, S., and Zhou, Y., Cyclic thermal shock behavior of a Cr2AlC ceramic, Mater. Sci. Eng. A, 2014, vol. 607, pp. 525–529.
Ying, G., He, X., Li, M, Du, S., Han, W., and He, F., Synthesis and mechanical properties of high-purity Cr2AlC ceramic, Mater. Sci. Eng. A, 2011, vol. 528, pp. 2635–2640.
Xiao, Li.O., Li, S.B., Song, G., and Sloof, W.G., Synthesis and thermal stability of Cr2AlC, J. Eur. Ceram. Soc., 2011, vol. 31, pp. 1497–1502.
Eklund, P., Beckers, M., Jansson, U., Högberg, H., and Hultman, L., The Mn+1AXn phases: Materials science and thin-film processing, Thin Solid Films, 2010, vol. 518, no. 8, pp. 1851–1878.
Frodelius, J., Sonestedt, M., Bjorklund, S., Palmquist, J., Stiller, K., Högberg, H., and Hultman, L., Ti2AlC coatings deposited by high velocity oxy-fuel spraying, Surf. Coat. Technol., 2008, vol. 202, pp. 5976–5981.
Pasumarthi, V., Chen, Y., Bakchi, S.R., and Agarwal, A., Reaction synthesis of Ti3SiC2 phase in plasma sprayed coating, J. Alloy Compd., 2009, vol. 484, pp. 113–117.
Verhoturov, A.D., Podchernyaeva, I.A., Pryadko, L.F., and Egorov, F.F., Elektrodnye materialy dlya elektroiskrovogo legirovaniya (Electrode Materials for Spark Alloying), Moscow: Nauka, 1988.
Zamulaeva, E.I., Levashov, E.A., Sviridova, T.A., Shvyndina, N.V., and Petrzhik, M.I., Electrospark deposition of protective coatings based on MAX-phases, Izv. Vyssh. Uchebn. Zaved. Poroshk. Metall. Funkts. Pokryt., 2013, no. 3, pp. 73–81.
Gitlevich, A.E., Mikhailov, V.V., Parkanskii, N.Ya., and Revutskii, V.M., Elektroiskrovoe legirovanie metallicheskikh pokrytii (Electric Spark Alloying of Metal Surfaces), Kishinev: Shtinitsa, 1985.
Li, S.B., Yu, W.B., Zhai, H.X., Song, G.M., Sloof, W.G., and Zwaag, S., Mechanical properties of low temperature synthesized dense and fine-grained Cr2AlC ceramics, J. Eur. Ceram. Soc., 2011, no. 31, pp. 217–224.
Zhou, W.B., Mei, B.C., and Zhu, J.Q., On the synthesis and properties of bulk ternary Cr2AlC ceramics, Mater. Sci.-Pol., 2009, vol. 27, no. 4/1, pp. 973–980.
Zhu, J., Jiang, H., Wang, F., Yang, C., and Xiao, D., Synthesis, microstructure and mechanical properties of Cr2AlC, J. Eur. Ceram. Soc., 2014, vol. 34, pp. 4137–4144.
Duan, X., Shen, L., Jia, D., Zhou, Y., Zwaag, S., and Sloof, W.G., Synthesis of high-purity, isotropic or textured Cr2AlC bulk ceramics by spark plasma sintering of pressure-less sintered powders, J. Eur. Ceram. Soc., 2015, vol. 35, pp. 1393–1400.
Merzhanov, A.G., Samorasprostranyayuschiisya vysokotemperaturnyi sintez. Fizicheskaya khimiya. Sovremennye problemy (Self-Propagating High-Temperature Synthesis. Physical Chemistry. Modern Problems), Moscow: Khimiya, 1983.
Levashov, E.A., Rogachev, A.S., Kurbatkina, V.V., Maksimov, Yu.M., and Yukhvid, V.I., Perspektivnye materialy i tekhnologii samorasprostranyayuschegosya vysokotemperaturnogo sinteza (Advanced Materials and Technologies of Self-Propagating High-Temperature Synthesis), Moscow: MISiS, 2011.
Gorshkov, V.A., Kachin, A.R., and Yukhvid, V.I., SHS metallurgy of cast Cr3C2–NiAl composite material and protective coatings based on it, Persp. Mater., 2014, no. 10, pp. 60–67.
Miloserdov, P.A., Gorshkov, V.A., and Yukhvid, V.I., High-temperature synthesis of cast Cr2AlC at an inert gas overpressure, Inorg. Mater., 2013, vol. 49, no. 8, pp. 781–785.
ACKNOWLEDGMENTS
We thank A.E. Sychev, acting head of Laboratory no. 8 at the Institute of Structural Macrokinetics of the Russian Academy of Sciences, for participating in the preparation of the material for the article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by N. Korovin
About this article
Cite this article
Gorshkov, V.A., Miloserdov, P.A., Sachkova, N.V. et al. SHS Metallurgy of Cr2AlC MAX Phase-Based Cast Materials. Russ. J. Non-ferrous Metals 59, 570–575 (2018). https://doi.org/10.3103/S106782121805005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S106782121805005X