Abstract
The present study was carried out with Kolmé clay in Liptako. X-rays diffraction results suggest that kaolinite was the predominant phase in this clay. The zero charge point of the pHpzc clay is about 7.1. The adsorption value and the percentage of fluoride ion removal by the clay increase up to a contact time of 1 h. Beyond this value, however, the fluctuation varies very little. This adsorption value increases with the fluoride ion concentration of the solution. However, it decreases considerably as the pH increases. The process of elimination of fluoride ions is done in three steps, including the diffusion of fluoride ions on the surface of the clay, the migration of fluoride ions from the surface of the clay to the active intra-particle sites and finally the chemisorption of fluoride ions on the active sites. The study of the sorption equilibrium of fluoride ions for the different concentrations indicates that the adsorption process appears to be both monolayer and multilayer and corresponds well to the Langmuir and Freundlich models. The fluoride ion adsorption kinetics can be fitted to the first and pseudo second order Lagergren models. The different velocity constants reflect a slow diffusion of fluoride ions, so this clay can be used in water defluorination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Fluoride ions in drinking water can have advantages and disadvantages for health depending on their concentration. Drinking water with high fluoride ion content can lead to conditions ranging from simple stains on the teeth to dental or bone fluorosis in severe cases [1]. However, these different diseases can be avoided by keeping the fluoride ion concentration in the water at a certain threshold [2]. Techniques to reduce the concentration of fluoride ions in water exist. Among these, techniques that rely on the contribution of calcium salts to precipitate fluoride ions into fluorine can be distinguished [2], the phenomenon of adsorption with activated alumina, activated carbon, bauxite, red soil, lanthanum-bentonite, silty clay and kaolinite as adsorbents [3] and finally the ion exchange mechanism with resins such as Ceralite IRA 400, Indion FR 10 and Amberlite IRA 400 [4]. Recent studies using membrane systems have been used to reduce the fluoride concentration in water. These include reverse osmosis, nanofiltration, electrodialysis and Donnan’s dialysis [5]. There has also been much scientific interest in water treatment using adsorbents such as white earth, red mud, montmorillonites, zeolites [6], etc. Very few studies have used kaolinite as an adsorbent in the removal of fluoride ions from water; as the adsorption capacity of this clay is lower than that of most clay minerals [2]. However, a modification of its surface by physical or chemical activation could increase its number of active sites and thus improve its adsorption capacity. The present work is centered on defluorination of water by a kaolinitic Kolmé clay. Specifically, the study will address the determination of the adsorption capacity and its mechanism.
EXPERIMENTAL
Materials
The clay sample studied comes from Kolmé, a locality located in the region of the Niger River basin called Liptako located in the Tillabery region in Niger. In Kolmé, the selected sampling point is at the following coordinates: 13°52′27.1′′ N, 01°09′7.6′′ E [7]. The clay fraction (<2 μm) of the sample is then extracted, dried and then finely ground, before being subjected to physicochemical analyzes.
All other reagents used in the present study were of analytical grade. A stock solution of a fluoride 1000 mg/L was prepared by dissolving appropriate quantity of sodium fluoride (Sigma Aldrich, United States) in distilled water.
Characterization of Kolmé Clay
XRD pattern were collected on a Siemens D5000 diffractometer, Serial A10-067, equipped with a copper anticathode (monochromatic CuKα radiation (λ = 1.54056 Ǻ), operating at a voltage of 40 kV and an emission current of 30 mA. The scanning range of 5°–50° with the rotational speed of 0.05° s–1 with a time of 5 s per phase to compensate for the loss in quality that would result from choosing a higher speed.
The Fourier transform infrared spectroscopy (FTIR) was performed before adsorption process on Bruker-IR spectrometer, IRTF 66. Spectra were recorded under 4 cm–1 resolution within the range of a 400–4000 cm–1 wavenumber.
The surface morphology of clay before adsorption was characterized by using Hitachi S-350 type microscope at 20 kV, coupled with an EDAX probe to perform a microanalysis of the samples (Link ISIS), allowing the chemical composition of the particles studied to be linked in situ.
Batch Adsorption Experiments
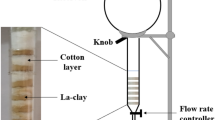
In order to study the effect of different controlling parameters like pH, initial fluoride concentration and contact time, several adsorption experiments were carried out by adding an accurate amount of Kolmé clay in 50 mL of fluoride solution to reach equilibrium with known initial concentrations ranging between 1.5 and 6 mg/L at pH 2. Hydrochloric acid and 1 M sodium hydroxide solutions were prepared to adjust the pH of the different solutions to 2 and 7 respectively. After equilibrium in reaction and centrifugation, the supernatants were filtered and analysed with the DR 5000 spectrophotometer by the colorimetric method described above.
The amount of the adsorbent (mg/g) was calculated using the Eq. (1):
where Ci and Ce are the initial and final concentrations (mg/L) of F− respectively, m is the mass of the adsorbent (g) and V is the solution volume.
RESULTS AND DISCUSSIONS
Characteristics of Adsorbent
FTIR analysis. The FTIR measurements (Fig. 1) showed two absorption bands in Kolmé clay, 3500 cm–1 band due to inner hydroxyl groups lying between the tetrahedral and octahedral sheets, and 3610 cm–1 band assigned to in-phase symmetric OH stretching vibration [8]. OH bending vibration was observed at 1630 cm–1 and Al–Al–OH bending frequency was represented by the band at 912 cm–1. The bands at 1006, 1020 and 1100 cm–1 could be assigned to O–Si–O stretching and deformation modes and Si–O–Al vibrations at 795 and 695 cm–1 are also observed. Si–O bending vibrations were shown by the bands at 470 and 530 cm–1. Bands close to 470 and 530 cm–1 have been recently assigned to Si–O–Si (429 cm–1), Si–O–Mg (456 cm–1) and Si–O–Al (524 cm–1) respectively by Hassanien et al. [9]. These results indicate the presence of kaolinite [10].
XRD analysis. The XRD pattern of Kolmé clay (Fig. 2) exhibits two prominent reflections at 12.5° and 25° 2θ, and other reflections at 21°, 31° and 35° 2θ. These reflections were matched with the kaolinite (Al2SiO2(OH)4), ICDD-PDF card no. 1-527 (unit-cell parameter a = 5.14 Å, b = 8.93 Å and c = 7.37 Å) and buttressed by reports elsewhere [11]. The results indicated that kaolinite was the predominant phase in the powdery bulk. In addition quartz (SiO2), ICDD-PDF card no. 85-865 (unit-cell parameters: a = 4.90 Å and c = 5.40 Å) was identified as minor mineral phase present in the sample. The composition of this clay is comparable to that of African clays which is dominated by kaolinite [12].
SEM analysis. SEM image of Kolmé clay before adsorption is presented in Fig. 3. The morphology of this clay possessed a well-defined sheet structure often observed in kaolinite [13].
Elemental analysis. The Kolmé clay was mainly composed of silica, alumina and iron consisting of 52.26, 25.02 and 13.99 wt % respectively, making its silica-alumina ratio to be 2.08. The clay contained little impurities with mass percent below 6.0 wt % (K2O and CaO).
Determination of the Zero Load Point
The determination of the zero charge point of any material will allow determining the pH at which its electronic charge is neutral. This pH is called pHpzc. It has been determined by the pH drift method [14]. Thus when the pH of the solution is lower than pHpzc then the material becomes positively charged. And as the pH decreases, the number of positive sites increases. But the closer it approaches pHpzc the fewer positive sites there are. It should be noted, however, that when the pH is higher than pHpzc then the material becomes negatively charged. It can thus be seen that in order to achieve a reduction in the concentration of fluoride ions in the water, the water in question must have a pH strictly below pHpzc and as low as possible.
Figure 4 gives us a pHpzc around 7.1. This result is similar to that found by Maiti et al. [15]. They used chemically treated laterite to achieve a pHpzc 7.5. In the continuation of our work, we will work at a pH 2 in order to be in the optimal conditions to obtain the best results in reducing the fluoride ion content of water. Meenakshi et al. [4] achieved a maximum adsorption value of 0.134 mg/g at pH 3 for the modified kaolinite. The fluoride ion extraction test will also be performed at pH 7 in order to evaluate the adsorption value of fluoride ions with a change in pH.
Batch Adsorption Studies
Effect of contact time on fluoride adsorption. The amount of adsorptions, qe, versus related times at pH 2 was depicted for different fluoride concentrations and present in Fig. 5. Figure 5 shows the variation of adsorption value with contact time and the percentage of fluoride ion removal by the clay increase up to a contact time of 60 min. But beyond this fluctuation varies very little for Kolmé clay. It was observed that maximum concentration of fluoride removal was attained within 60 min and there after it almost remained static for all samples. So 60 min was fixed as the period of contact for further studies. The results in Fig. 5 show that the adsorption value of the clay increases from 0.011 mg of F–/g of clay to 0.05 mg of F–/g of clay when the fluoride ion concentration of the solution varies from 1.5 to 6 mg/L. This increase in sorption capacity can be related to the specific surface area of the clay on the one hand and its Al2O3 content on the other hand. This increase in the adsorption value of the clay as a function of the variation in the initial concentration of fluoride ions in the solution can be explained by the fact that when the concentration of the solution increases, there is not only an increase in the number of collisions between the molecules of the solution but also in the functional groups of the adsorbent [16].
Influence of pH on fluoride adsorption. The removal of fluoride ions from aqueous solution was highly dependent on the solution pH in many cases as it altered the surface charge on the sorbents [4, 16]. Any solid surface can develop charge by adsorption of ions, where the solid acts as an electrode (e.g., H+ and OH– on the surface of clays). In clay aqueous systems the potential of surface is determined by the activity of ions (H+ or pH) which react with the mineral surface. So pH plays an important role and it controls the adsorption of the fluoride at the clay-solution interface. Such interface on acid-base dissociation develops positive and negative charges of the surface [17]. Defluoridation studies were carried out with Kolmé clay at pH 2 and pH 7 for 60 min.
The results show us that the adsorption value of fluoride ions by the clay decreases considerably when the pH varies from 2 to 7. This drop can go from half (1/2) to 9/10 of the capacity obtained at pH 2 depending on the initial concentration of fluoride ions in the solution. The results of Kim et al. [18] agree with the observations outlined above as they observe a decrease in adsorption value as pH increases. They link this phenomenon to a competition between F– and OH– ions for active sites. However, it should be noted that when the pH is low, in adsorption it is the chemisorption process that dominates, whereas it is the physisorption process that intervenes when the pH is higher.
Influence of diffusion. In order to better understand the process involved in the removal of fluoride ions by clay, the interparticle diffusion mass transfer model developed by Weber and Morris [19] was used. This model describes the sorption rate from the following relationship given by Eq. (2):
with \({{q}_{t}}\) (mg/g) the amount of fluoride ions adsorbed per g of adsorbent at contact time t.
The results from the above representation allowed to determine the different processes involved in the adsorption of fluoride ions by the clay.
In general, the removal of fluoride ions is essentially done by their insertion in the intercalary regions of the clay in order to balance the positive charges developed during the grinding of the adsorbent and its contact with the acid solution rich in fluoride ions [20]. According to Weber and Morris [19] during the process of adsorption of fluoride ions by the clay if particle diffusion is involved, then the relationship that binds the amount of fluoride ions adsorbed per unit of adsorbent (clay) to the square root of time would be linear. From Fig. 7, three linear segments can be distinguished during the adsorption of fluoride ions by the clay.
Thus the interpretation of these results from the intraparticle diffusion model allows concluding that the first segment corresponds to the diffusion of the fluoride ions on the surface of the clay. The second segment corresponds to the migration of fluoride ions from the clay surface to the active intraparticle sites. And the third segment corresponds to the chemisorption of fluoride ions at the active sites [21]. Thus the F- ions manage to balance the positive charge of the layers of the clay structure and sometimes they even compete with the OH– ions. We note that the interpretation of the first linear segment confirms that particle diffusion was involved in the sorption process. However, other limiting mechanisms were also involved.
Influence of adsorption isotherms. In order to study the reaction mechanisms governing the adsorption of fluoride ions by clay, the adsorption isotherms were determined from Langmuir and Freundlich models. The technique of these two models made it possible to adjust the data at equilibrium to determine the adsorption value of fluoride ions.
Freundlich’s equation is frequently used to explain the empirical way of adsorption of pollutants in aqueous media [8]. This empirical model is synonymous with heterogeneous sorption on the surface. Langmuir’s model corresponds to a monolayer sorption.
In this work, the sorption equilibrium of fluoride ions for concentrations ranging from 1.5 to 6 mg/L was adjusted from the linear equations of Langmuir and Freundlich.
Freundlich model. Freundlich’s equation is given by Eq. (3):
The linearization of this equation is done by passing to the Neperian logarithm, which leads us to Eq. (4):
where \({{K}_{{\text{F}}}}\) is the Freundlich constant characterizing the adsorbent power (or adsorption capacity) of the support, \(1{\text{/}}n~\) the affinity of the solute for the adsorbent with n the intensity of adsorption.
To determine the adsorption isotherms, the mixture consisting of the solution with an initial fluoride ion concentration of 6 mg/L and clay was chosen. The latter provided the highest correlation coefficient. The plot of ln(q) versus ln(Cr) is given in Fig. 8.
The equation thus obtained is that of a straight line of slope and whose ordinate at the origin is what allows to deduce the two characteristic parameters, namely and. Table 2 presents the different parameters obtained from the straight line shown in Fig. 8 at a temperature of 323 K.
To determine the adsorption isotherms, the mixture consisting of the solution with an initial fluoride ion concentration of 6 mg/L and the clay was chosen.
The applicability of Langmuir isotherm was also tested on fluoride adsorption on Kolmé clay. The Langmuir equation [22] was applied in the form given by Eq. (5):
where \({{q}_{{\text{m}}}}\) and \({{K}_{{\text{L}}}}\) (in L/mg) are Langmuir constants indicating the adsorption capacity and energy of adsorption, respectively. Cr is the residual concentration of fluoride ions (in mg/L), q is the amount of fluorides adsorbed per unit mass of clay (in mg/g),
The linear plot of \(\frac{{{{C}_{{\text{r}}}}}}{q}\) vs. Cr (Fig. 9) with high R2 value indicates the monolayer adsorption on Kolmé clay.
The values of \({{q}_{{\text{m}}}}\) and \({{K}_{{\text{L}}}}\) were calculated from the slopes and intercepts of the plots, respectively, and are presented in Table 3.
The KL value is in the same order as that reported by Dovonon with calcined beef bones. The value is closer to that obtained by Meenakshi et al. [4] with clay composed of 95% kaolinite.
In order to evaluate the applicability of the Langmuir model, most of the characteristics of the Langmuir isotherm can be expressed by the separation factor or by the equilibrium constant RL [19]. This factor is defined by Eq. (6):
To predict whether an adsorption process is thermodynamically favorable or not, one can use a nearly constant parameter called the separation parameter. This depends on Langmuir’s adsorption constant and is calculated from Eq. (6) above.
With RL the separation factor, KL is the Langmuir adsorption constant in L/mg and C0 the initial concentration of the solution containing fluoride ions in mg/L.
When 0 < RL < 1 then the adsorption isotherm is considered favorable. If RL is >1 then this isotherm is considered unfavorable. For RL = 1 the isotherm is linear and finally if RL = 0 then the isotherm is irreversible [23].
The separation factor RL being equal to 0.0474 then the process of adsorption of fluoride ions by the clay is thermodynamically favorable.
From Figs. 8 and 9, it can be seen that the clay sorption adjustment data from the Langmuir and Freundlich models, provide correlation coefficients of 0.9986 and 0.9897 respectively, indicating that both models appear to be suitable for the experimental data. The sorption process appears to be both monolayer and multilayer. The values of the separation factor RL and the affinity of the solute for the adsorbent 1/n prove respectively that the process of adsorption of fluoride ions by the clay is favorable in both cases. Meenakshi et al. [4] reached the same conclusion when adsorbing fluoride ions by a clay composed of 95% kaolinite in its raw and modified forms.
Thermodynamic Parameter
In order to evaluate the standard variation of free energy ΔG° during the process of adsorption of fluoride ions by clay Eq. (7) dependent on the sorption equilibrium constant is used:
With the free energy of sorption in kJ/mol, T is the temperature in Kelvin and R is the universal gas constant which is 8.314 J/mol K. The sorption equilibrium constant of the fluoride ion adsorption reaction by clay is derived from the equation of the straight line curve versus by extrapolation to zero according to the method proposed by Khan and Singh [24] given by Fig. 10.
By deduction we obtain a value of K0 = 2.2, i.e. ΔG° = –2.081 KJ/mol. The results obtained by Gourouza [16] with clay having a kaolinite component confirm these observations.
Adsorption Kinetics
In order to determine the adsorption kinetics, an experiment was carried out with the clay for 3 h. During this experiment, fluoride ion solutions of known initial concentration were mixed with two (2) grams of clay. The mixture was placed under agitation and the concentrations of the supernatant were monitored every thirty (30) min for the duration of the experiment. Adsorption kinetics was analyzed using two kinetic models: first order and Lagergren’s pseudo second order [25].
The Lagergren first-order model [21] was developed for irreversible sorption in solid/liquid systems, and is described as a function of velocity using Eq. (8):
Moving to the Naperian logarithm, we obtain Eq. (9):
With \(~{{q}_{{\text{e}}}}\) the amount of fluoride ions adsorbed per g of adsorbent at equilibrium in mg/g and the sorption constant of the clay. The curve given by the relation as a function of t is plotted in the hope of obtaining a linear relation. Figure 11 gives us this curve:
With a linear relationship having a correlation coefficient of 0.76296, the data on the reaction speed of the clay sorption can be fitted to the Lagergren first-order model. According to the model, the clay sorption constant was estimated to be 0.0089. The pseudo second order model [25] is based on the assumption that chemical sorption or chemisorption is one of the factors controlling sorption kinetics. This model is expressed through Eq. (10):
By linearizing, we have Eq. (11):
With the pseudo second order velocity constant the plot of the curve of as a function of t leads to Fig. 12.
With a correlation coefficient greater than 0.92826 and a sorption constant \({{K}_{2}}\) of 1.5017 × 10–7 (Fig. 12), the pseudo second order kinetic equation better matches the sorption data [25]. Thus, the chemisorption process can be considered. However, the different velocity constants reflect a slow diffusion of fluoride ions.
CONCLUSIONS
Experimental results reveal that Kolmé clay has a zero load point of 7.1. However, the various tests for the extraction of fluoride ions were carried out at a pH 2 to be in the optimal conditions for the adsorption of ions from these solutions. In general, the adsorption value and the percentage of elimination of fluoride ions by the clay increase up to a contact time of 1 h. This adsorption value of the clay increases from 0.011 to 0.05 when the fluoride ion concentration of the solution varies from 1.5 to 6 mg/L. However, this adsorption value of fluoride ions by the clay decreases considerably when the pH of the solution varies from 2 to 7. The process involved in the removal of fluoride ions by the clay consists of the diffusion of fluoride ions on the surface of the clay, the migration of fluoride ions from the surface of the clay to the intraparticle active sites and the chemisorption of fluoride ions at the active sites. The determination of the affinity of the solute for the adsorbent and the separation factor respectively for the Freundlich and Langmuir models confirms that the conditions are favorable for adsorption. Thus the sorption process is both monolayer and multilayer. The value of the free energy of sorption indicates that the sorption reaction of fluoride ions on clay is spontaneous. The speed of the sorption reaction of the clay fits the first- and pseudo-second order models of Lagergren and the different rate constants reflect a slow diffusion of the fluoride ions. Thus, this clay can be used in the defluorination of water at low cost. For this aspect, we suggest to evaluate the behavior of old matter clay after saturation with F− as future work.
REFERENCES
Dovonon, L.F.C., Qualité chimique des eaux souterraines dans la commune de Dassa-Zoumé (Bénin): impacts sanitaires des teneurs hors normes en fluorures et essais de traitement à l’os calciné de bovins, PhD Thesis, Bénin: Univ. d’Abomey-Calavi, 2011.
Vinati, A., Mahanty, B., and Behera, S.K., Clay and clay minerals for fluoride removal from water: A state-of-the-art review, Appl. Clay Sci., 2015, vol. 114, pp. 340–348. https://doi.org/10.1016/j.clay.2015.06.013
Ramdani, A., Taleb, S., Benghalem, A., and Ghaffour, N., Removal of excess fluoride ions from Saharan brackish water by adsorption on natural materials, Desalination, 2010, vol. 250, pp. 408–413. https://doi.org/10.1016/j.desal.2009.09.066
Meenakshi, S., Sundaram, C.S., and Sukumar, R., Enhanced fluoride sorption by mechanochemically activated kaolinites, J. Hazard. Mater., 2008, vol. 153, pp. 164–172. https://doi.org/10.1016/j.jhazmat.2007.08.031
Pontié, M., Diawara, C., Gonidec, A., Stricot, L., Lhassani, A., Dach, H., Bourseau, P., and Jaouen, P., Défluoruration des eaux par nanofiltration à grande échelle: Thiadiaye (Sénégal), une première mondiale, J. Inf. Eaux, 2010.
Peng, S., Zeng, Q., Guo, Y., Niu, B., Zhang, X., and Hong, S., Defluoridation from aqueous solution by chitosan modified natural zeolite, J. Chem. Technol. Biotechnol., 2013, vol. 88, pp.1707–1714. https://doi.org/10.1002/jctb.4022
Adamou, R., Etude de la rémanence des insecticides pyréthrinoïdes utilisés dans la lutte contre le paludisme en Afrique Subsaharienne. Caractérisation de quelques matériaux argileux du Niger et leur application dans la décontamination des eaux naturelles polluées, PhD Thesis, Niger: Univ. Abdou Moumouni de Niamey, 2012.
Madejová, J., FTIR techniques in clay mineral studies. Vib. Spectrosc., 2003, vol. 31, pp. 1–10. https://doi.org/10.1016/S0924-2031(02)00065-6
Hassanien, M.M., Abou-El-Sherbini, K.S., and Al-Muaikel, N.S., Immobilization of methylene blue onto bentonite and its application in the extraction of mercury(II), J. Hazard. Mater., 2010, vol. 178, pp. 94–100. https://doi.org/10.1016/j.jhazmat.2010.01.048
Frost, R.L., Makó, E., Kristóf, J., Horváth, E., and Kloprogge, J.T., Mechanochemical treatment of kaolinite, J. Colloid Interface Sci., 2001, vol. 239, pp. 458–466. https://doi.org/10.1006/jcis.2001.7591
Obada, D.O., Osseni, S.A., Sina, H., Salami, K.A., Oyedeji, A.N., Dodoo-Arhin, D., Bansod, N.D., Csaki, S., Atta, A.Y., Fasanya, O.O., Sowunmi, A.R., Kuburi, L.S., Dauda, M., Abifarin, J.K., and Dauda, E.T., Fabrication of novel kaolin-reinforced hydroxyapatite scaffolds with robust compressive strengths for bone regeneration, Appl. Clays Sci., 2021, vol. 215, p. 106298. https://doi.org/10.1016/j.clay.2021.106298
Fadil-Djenabou, S., Ndjigui, P.-D., and Mbey, J.A., Mineralogical and physicochemical characterization of Ngaye alluvial clays (Northern Cameroon) and assessment of its suitability in ceramic production, J. Asian Ceram. Soc., 2015, vol. 3, pp. 50–58. https://doi.org/10.1016/j.jascer.2014.10.008
Duarte-Silva, R., Villa-García, M.A., Rendueles, M., and Díaz, M., Structural, textural and protein adsorption properties of kaolinite and surface modified kaolinite adsorbents, Appl. Clay Sci., 2014, vol. 90, pp. 73–80. https://doi.org/10.1016/j.clay.2013.12.027
Jia, Y.F., Xiao, B., and Thomas, K.M., Adsorption of metal ions on nitrogen surface functional groups in activated carbons, Langmuir, 2002, vol. 18, pp. 470–478. https://doi.org/10.1021/la011161z
Maiti, A., Basu, J.K., and De, S., Chemical treated laterite as promising fluoride adsorbent for aqueous system and kinetic modeling. Desalination, 2011, vol. 265, pp. 28–36. https://doi.org/10.1016/j.desal.2010.07.026
Gourouza, M., Caractérisation de Matériaux (Argiles, Gypse et Os calcinés); études quantitative, cinétique et thermodynamique de l’adsorption des ions fluorures par l’argile de Sabon-Karré et les Os calcinés, PhD Thesis, Niger: Univ. Abdou Moumouni de Niamey, 2013.
Worrl, W.E., Textbook of Clays: Their Nature, Origin and General Properties, London: Maclaren, 1968. https://www.abebooks.com/first-edition/Clays-Nature-Origin-General-Properties-1968/885835062/bd.
Kim, J.H., Lee, C.G., Park, J.A., Kang, J.K., Choi, N.C., and Kim, S.B., Use of pyrophyllite clay for fluoride removal from aqueous solution. Desalin. Water Treat., 2013, vol. 51, pp. 3408–3416. https://doi.org/10.1080/19443994.2012.749198
Weber, W.J. and Morris, G.C., Removal of biologically-resistant pollutants from waste waters by adsorption, Advances in Water Pollution Research, Proc. 1st Int. Conf. on Water Pollution Research, New York: Pergamon, 1962, pp. 231–266.
Lv, L., He, J., Wei, M., and Duan, X., Kinetic studies on fluoride removal by calcined layered double hydroxides, Ind. Eng. Chem. Res., 2006, vol. 45, pp. 8623–8628. https://doi.org/10.1021/ie050363d
Ho, Y.S. and McKay, G., Pseudo-second order model for sorption processes, Process Biochem., 1999, vol. 34, pp. 451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Langmuir, I., The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc., 1916, vol. 38, pp. 2221–2295. https://doi.org/10.1021/ja02268a002
Ho, Y.S., Second order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and nonlinear methods, Water Res., 2006, vol. 40, pp. 119–125. https://doi.org/10.1016/j.watres.2005.10.040
Khan, A.A and Singh, R.P., Adsorption thermodynamics of carbofuran on Sn(IV) arsenosilicate in H+, Na+ and Ca2+ forms, Colloids Surf., 1987, vol. 24, pp. 33–42. https://doi.org/10.1016/0166-6622(87)80259-7
Lagergren, S., About the theory of so-called adsorption of soluble substances, K. Sven. Vetenskapsakad. Handl., 1898, vol. 24, pp. 1–39.
Funding
We thank the Islamic Development Bank for funding this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Salifou Issa, Osseni, S.A., Obada, D.O. et al. Removal of Fluoride Ions from Water by Kolmé Clay. J. Water Chem. Technol. 45, 455–466 (2023). https://doi.org/10.3103/S1063455X23050090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X23050090