Abstract
A simple co-precipitation method was employed in the synthesis of carbon-magnetic cobalt aluminum layered double hydroxide (Fe3O4/Co–Al LDH) and the obtained nano-structured material was further applied as an innovative adsorbent for the removal of two anionic dyes, namely Tartrazine (TA) and Indigo Carmine (IC). The synthesized nanosorbent was identified by scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD). In addition, Fe3O4/C/Co–Al LDH was magnetic and easily recovered by a small bar magnet after the sorption operation. The batch adsorption experiments were carried out to optimize different essential parameters such as contact time, initial dye concentration, solution pH, and adsorbent mass. Response surface methodology (RSM) involving central composite design (CCD) was also employed to obtain a suitable regression model for adsorption system and subsequently investigate the most influential parameters. Based on ANOVA results, all of the main parameters were significant. In particular, the initial dye concentrations and sorbent mass let to the largest variances on response values. The results showed the removal percentage of the dye increased by increase in the amount of the nanosorbent. In the contrary, the adsorption value of the nanosorbent for dye decreased by increasing the sorbent amounts. Moreover, the removal percentage and the adsorption value of the adsorbent decreased for each dye in the presence of another dye due to the competition between dyes for the available adsorbent surface area. Furthermore, the pseudo second-order model was used to describe the adsorption kinetics. The Langmuir isotherm model was the most suitable to fit the experimental data. The sorption capacities for TA and IC were obtained to be 52.3 and 61.7 mg/g, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
With booming industrial development, environmental concerns has raised toward wastewater discharges from various industries, which release tons of toxic organic chemicals such as different synthetic dyes [1–3]. Amongst are the anthraquinone, azo, sulfur, indigoid, triphenylmethyl, xanthene and phthalocyanine derivates, which are more frequently used [1–6]. Therefore, it is of utmost importance to solve this environmental issue by developing cost-effective technologies; thus leading to effective removal of toxic dyes from contaminated soil and water.
To remove these pollutants from wastewaters, several techniques have been introduced so far, including chemical oxidation, coagulation/flocculation, biological treatment, membrane separation and ion exchange; each of which are known to suffer from different restrictions [1–3]. Among the aforementioned approaches, adsorption has been considered to be superior to other techniques for water treatment in terms of initial cost, flexibility, ease and simplicity of design and operation. To date, various sorbents have been developed with the aim of lowering the cost and enhancing their efficiencies, namely zeolites, clay materials, industrial waste products, agricultural wastes, biosorbents, nano-sized materials, polymers, etc.(starch, cyclodextrin, cotton) [2, 3, 7–9]. Among mentioned adsorbents, layered double hydroxides (LDHs) can be considered as environmentally benign, appropriate adsorbents for the removal of dyes both in surface adsorption and anion exchange techniques due mainly to their high charge density of the sheets and the exchangeability of the interlayer anions [10–13]. The LDH materials are composed by the general formula [\({\text{X}}_{{1 - n}}^{{2 + }}\)\({\text{X}}_{n}^{{3 + }}\)(OH)2]n+(Ym–)n/m⋅zH2O, where X2+ and X3+ are divalent metal cations (Ca2+, Mg2+, Zn2+, Cu2+, Ni2+, Co2+, etc.) and trivalent metal cations (Al3+, Fe3+, Ga3+, Cr3+, etc.), respectively. The cations occupy the octahedral sites within the hydroxide layers, n is the molar ratio between divalent and trivalent cations X3+/(X2+ + X3+) while Ym– (Cl–, Br–, I–, OH–, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{{2 - }}\), etc.) is the interlayer anion with m indicating the charge of the anion [14, 15]. The LDHs composition can be easily changed by replacing the interlayer anions with other anions or replacing the metal ions in the hydroxide sheets. As a result, LDHs are considered as efficient anion exchangers. Furthermore, a combination of Fe3O4 nanoparticles (NPs) with sorbents such as LDHs can improve the separation and re-dispersion of magnetic sorbent from aqueous solution [16–22]. The magnetic LDH strongly interacts with external magnetic fields making the separation process much easier under a moderate magnetic field. However, LDH-based materials have limitations on the removal of organic pollutants due to their lower surface area than carbon-based adsorbents. It should be noted that carbon-based nanosorbents have been widely used to remove water contaminants due to their high surface area and the presence of numerous surface functional groups. Therefore, the fabrication of adsorbents as a composite, i.e. porous carbon, and a LDH in the form of nanocomposites can increase their adsorption capacity to remove various inorganic and organic pollutants simultaneously [16, 17]. Since the efficiency of adsorption process is mainly affected by parameters such as pH, time, the initial dye concentrations and adsorbent mass [1, 8], it is necessary to make an appropriate design to maximize the removal efficiency (R %) and uptake capacity (q) of dye using this new nanosorbent.
The multivariate optimization has greatly drawn attraction of researchers with respect to conventional “one-at-a-time” method to optimize the adsorption process [23–25]. In fact, more information with fewer experiments can be obtained using a response surface methodology (RSM) approach which leads to a predictive model considering the responses of adsorption process with effective parameters and their interactions [1, 8]. Derringer’s desirability functions can be used for simultaneous optimization of dye removal efficiencies (% RTA and % RIC) [1, 8].
In this work, we were aimed to synthesize Fe3O4/C/Co–AlLDH via a simple route, and study its capability of removing some anionic dyes, TA and IC from aqueous solution. Subsequently, the central composite design (CCD) was employed as an RSM approach to give us further insight into the most effective adsorption factors and obtain optimum conditions for maximum dye removal efficiency by magnetic Fe3O4/C/Co–Al LDH. In addition, the equilibrium and kinetics data were also studied so that we can obtain the appropriate descriptive models. To the best ofour knowledge, magnetic Co–Al LDH has not been hitherto employed in the removal of the studied dyes from aqueous samples.
EXPERIMENTAL
Reagents and Materials
All chemicals such as Co(NO3)2, Al(NO3)3⋅9H2O, FeCl3⋅6H2O, FeCl2⋅4H2O, ammonium hydroxide (25%), Tartrazine and Indigo Carmine were purchased from Merck (Darmstadt, Germany). The prepared solutions were diluted with ultra-pure water to obtain mixed standards.
Apparatus
The chemical composition of the synthesized magnetic C/Co–Al LDH was analyzed using EDX by applying VEGA\\TESCAN SEM instrument. The particle size and morphology of magnetic nanosorbent was imaged using SEM (MIRA3-TESCAN). In addition, an XRD pattern of the magnetic C/Co–Al LDH was recorded, using a Philips X-ray diffractometer (model PW 3040/60 X’pert pro). The XRD measurements were made using Phillips powder diffractometer model PW3040 with CuKα radiation. The pH of the solutions was adjusted by a digital pH meter Metrohm (model 744). The analysis of the studied dyes was performed by a UV-Vis spectrophotometer (Analytikjena SPECORD250).
Preparation of Magnetic C/Co–Al Layered Double Hydroxide
A chemical co-precipitation method was used to prepare Fe3O4 NPs. Briefly, FeCl3⋅6H2O (8.48 g) and FeCl2⋅4H2O (2.25 g) were dissolved in 400 mL deionized water under N2 atmosphere under vigorous stirring (1000 rpm) at 80°C for 20 min [20]. Magnetic C/Co–Al LDH was accordingly synthesized by a modified coprecipitation method. 2.1 g Co(NO3)2 and 0.83 g Al(NO3)3 were dissolved in 50 mL deionized water to prepare a mixed solution. Then 0.35 g Fe3O4 NPs was introduced in this mixed solution and followed by ultrasonic for 15 min. 0.30 g Activated carbon was added into solution under stirring for 24 h. The solution pH was adjusted to 10.5 by dropwise addition of 2 mol/L NaOH solution. The solution was stirred vigorously throughout the process. Subsequently, the mixed solution was kept stirring at 60°C for 2 h at a constant pH of 10.5. The magnetic C/Co–Al LDH was separated by a magnet and washed several times with deionized water, and dried after wards in a vacuum oven at 60°C for 2 h.
Batch Procedure
The stock solutions of TA and IC were made at the same concentration of 1000 mg/L. Accordingly, the test solutions were prepared by diluting and/or mixing the certain amounts of TA and IC stock solutions. In a typical experiment, 10 mL of the dye solution was used, and a certain amount of dried nanosorbent was added to each solution. Prior to the adsorption process, the initial pH value of each test solution was adjusted by adding a small amount of either 0.1 M HNO3 or 0.1 M NaOH solutions. The mixture was then shaken at 300 rpm (IKA-OS2 Basic Orbital Shaker, Germany) at a known temperature for 60 min. After wards, the synthesized nanosorbent was separated from the solution with a magnet (10 × 5 × 4 cm with a 1.4 Tesla magnetic field). Thenceforth, the concentrations of the studied dyes were determined by UV-Vis spectrophotometer.
The adsorption capacity q and removal ratio (R %) were given as follow [1]:
where m is the sorbent mass (g), Cf is the equilibrium dye concentration, Ci is the dye initial concentration (mg/L) and V is the volume of solution (L).
Central Composite Design as Optimization Approach
CCD, a rotatable five-level design, is composed of several superimposed designs: a full factorial design (2k), a star design (axial points 2k) and replicates at center points C0, where k denotes the number of factors and C0 replicates at the center points. The total number of experiments (N) in CCD equals N = 2k + 2k + C0. Moreover, each of the parameters was coded at five levels: –α, –1, 0, +1 and + α [24, 25]. Therefore, for four variables and seven center points, 31 experiments have been carried out. The effective variables and their levels used in the CCD are given in Table 1.
The relationship of response, main parameters, and interactions can be formulated as a quadratic model:
here A is response (removal percent), α0 is a constant, αi is coefficient of linear effect, αij is the coefficient of squared effect, αij is the coefficient of interaction effect and ε is random error. The regression analysis of the obtained data and evaluation of the coefficient of regression model were obtained using the Minitab 17 statistical software.
RESULTS AND DISCUSSION
Characterization of Magnetic C/Co–Al LDH
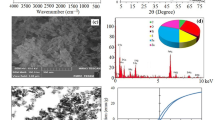
SEM images were obtained to further investigate the synthesized nanosorbents with regard to their morphology and surface features. Fe3O4/C/Co–Al LDH was shown to be in nanosize scale and formed aggregates (Fig. 1). The atomic composition of the Fe3O4/C/Co–Al LDH was evaluated by EDX analysis (Fig. 2), where peaks associated with Co, Al, Fe, and O can be observed well. The peaks at (220), (311), (400), (511), and (440) were similar to the XRD pattern of Fe3O4 NPs. The XRD pattern of the Fe3O4/C/Co–Al LDH is given in Fig. 3. The (003) and (006) reflected of a typical Fe3O4/C/Co–Al LDH material are also clearly observed.
According to results (Fig. 4), 0.04 g of Fe3O4/LDH can remove 80% of IC (200 mg/L) and 75% of TA (200 mg/L) from the aqueous solution; while Fe3O4/C/LDH can remarkably remove 99% IC and 97% TA, respectively. It is obvious that the adsorption of IC and TA from aqueous solution increased using Fe3O4/C/LDH.
In addition, the point of zero charge for Fe3O4/C-LDH was obtained by the mass titration process developed by Noh and Schwarz [26]. The value of pH (PZC) was found to be 7.5 for Fe3O4/C-LDH. At lower pH, the surface of the Fe3O4/C/LDH is positively charged and shows higher adsorption due to electrostatic interactions between the positively charged Fe3O4/C/LDH and the negatively charged pollutants. The adsorption capacity of TA and IC on the surface of Fe3O4/C/LDH was found to be higher at lower pH, which is due to electrostatic attraction between TA and IC and positively charged Fe3O4/C/LDH. As the pH of the solution increases, the adsorption capacity decreases due to the electrostatic repulsion between the anionic, TA and IC dyes and the negative charge of the adsorbent surface.
The synthesized magnetic C-LDH showed significant adsorption capacity for removal of both organic dyes from aqueous solutions due to the synergistic effect of the carbon and LDH content. In the other word, the combination of porous carbon and LDH in nanoadsorbent structure enhances the surface area, chemical stability and oxygen containing functional groups. As a result, the effective active adsorption sites are increased, resulting in improved adsorption. In general, adsorption occurred via electrostatic interaction, ion exchange and complex formation mechanisms. IC and TA are adsorbed on the Fe3O4/C/LDH by electrostatic attraction between the dye molecules and the LDH. In addition, the carbon in the LDH facilitates the formation of H-bonding between anionic dye and carbon. Therefore, the cooperative contribution of electrostatic attraction and hydrogen bonding between the dye molecules (IC and TA) and Fe3O4/C/LDH is useful for the enhancement of the adsorption capacity.
Response Surface as Optimization Approach
In the recent study, the CCD was used to identify the effects of the main significant parameters on dye removal percent and to find the optimum conditions resulting in maximum removal efficiency [1, 8]. Four important variables, such as CTA and CIC), pH and m were applied as independent parameters. According to the derived matrix design for running CCD 31 experiments were performed (Table S1 (ESM_4.pdf)). The multiple regression analysis of the experimental data led to the empirical relationship between RIC and RTA and effective parameters as coded values:
where RIC and RTA represent the predicted dye removal percentages.
To evaluate the model coefficients, the analysis of variance (ANOVA) was considered (Table 2).
In this respect, values of Prob > F (less than 0.050) were considered as the significant parameters for RTA and RIC in obtained model, while the non-significant lack of fit (more than 0.05) (0.72 and 0.74) indicated that the quadratic model is valid for the present data (Table S2 (ESM_5.pdf). The adjusted R2 (Adj-R2) which indicates the good fitness of predictive model with the experimental data was calculated to be 0.999 and 0.998 for TA and IC, respectively.
Also, the response surface plots (Figs. S1 and S2 (ESM_1.pdf and ESM_2.pdf)) can clearly represent the effects of the main parameters and their mutual interactions on RTA and RIC. Based on ANOVA results, all of the main parameters were significant (P < 0.05). In particular, CTA and CIC and m let to the largest variances on RTA and RIC. As it was predicted, RTA and RIC increased by accretion in the amount of the nanosorbent, most probably owning to the enhanced sorbent surface and accessibility of more active sites with the increase in the nanosorbent amount. In the contrary, q decreased by increasing m, attributing to overlapping or aggregation of active sorption sites resulting in a decrease in total sorbent surface area and an increase in diffusion path length [1, 8]. In addition, it is well known that increase in Ci causes an increase in 2q due to further contact between nanosorbent particles and dye molecules while leading to the decrease in removal efficiency of the dye [1, 8, 23]. Moreover, the removal percentage and the sorption value of the adsorbent decreased for each dye in the presence of another dye due to the competition between dyes for the available adsorbent surface area.
Adsorption Isotherm Models
The dye adsorption behavior with magnetic C/Co–Al LDH was evaluated by Langmuir and Freundlich isotherm models [1, 8]:
where qe is the adsorption capacity at equilibrium (mg/g), qmax is the maximum amount of adsorption (mg/g), b is the adsorption equilibrium constant (L/mg), Ce is the equilibrium concentration of sorbate in the solution (mg/L) and KF is the constant (mg/g) representing the adsorption capacity. Nonlinear least-squares regression analysis using Microsoft Excel solver was employed to estimate the models’ parameters (Table 3).
In non-linear regression analysis, it is important to determine the bestfitting isotherm model, thus three error functions, namely coefficient of determination (R2), Chi-square (χ2) test and normalized standard deviation (q) were applied [1, 8]:
where n is the number of data points, qe,exp and qe,cal are the experimental and model calculated adsorption capacities (mg/g). These criteria provide a numerical estimate to measure the goodness of fit for a given mathematical model to the data. The smaller values of χ2 and Δq indicate that data calculated by the model are closer to the experimental values.
According to error functions in Table 3, the adequacy of fit for Langmuir model suggests that the dye adsorption is limited to monolayer coverage and the surface is relatively homogenous in terms of functional groups with significant interaction with TA and IC molecules. Maximum adsorption capacity (qmax) of Fe3O4/C/Co–Al LDH was obtained to be 52.3 and 61.7 mg/g, for TA and IC, respectively.
Adsorption Kinetics
The kinetic parameters are favorable for predicting the adsorption rate and giving important information for designing and modeling the adsorption processes. Therefore, the kinetics of understudied dyes adsorption on Fe3O4/C/Co–Al LDH was studied by pseudo-first-order, pseudo-second-order and intraparticle diffusion models [1, 8, 23]. To study the sorption kinetics of TA and IC on Fe3O4/C/Co–Al LDH, the initial concentrations were set to 100 and 87 mg/L for each dye, respectively while changing the contact time in the range of 2 to 90 min in both single and binary systems (Fig. S3 (ESM_3.pdf)). Moreover, the similarity between the experimental and model calculated data was revealed by the determination coefficients (R2 ~ 1). The closer the R2 values to 1, the more adequacy of fit for the model to describe the kinetics of TA and IC adsorption on Fe3O4/C/Co-Al LDH. The pseudo-second-order kinetic model (Table 4) was shown determination coefficient of 0.998 that can strongly predict experimental data.
The relative difference between the q(exp) and q(cal) is a great criterion to evaluate the suitability of them. As given in Table 4, the qe,exp and the qe,cal values obtained from the pseudo-second-order model are very similar in values, obviously indicating the suitability and applicability of the pseudo-second-order model.
CONCLUSIONS
In this study, the magnetic C/Co–Al LDH was successfully prepared and used for the simultaneous removal of TA and IC from aqueous solution. The magnetic C/Co–Al LDH was characterized by SEM, EDX and XRD. CCD was then applied to determine a suitable model that can describe the effect of significant variables. The kinetic experimental data showed the sorption process could reach equilibrium quickly in about 60 min and the pseudo-second-order model was accurately described the adsorption kinetics. The Langmuir isotherm model was the most suitable to fit the experimental data. The sorption capacities for TA and IC were obtained to be 52.3 and 61.7 mg/g, respectively.
The adsorption isotherm data were in good agreement with the Langmuir equation; and the sorption capacities for TA and IC were obtained to be 52.3 and 61.7, respectively. Furthermore, the magnetic C/Co–Al LDH can be easily separated using a small magnet bar after the adsorption of contaminant on magnetic sorbent.
REFERENCES
Bagtash, M. and Zolgharnein, J., Crossed mixture-process design for optimization of simultaneous adsorption of Tartrazine and Indigo Carmine dyes by cobalt hydroxide nanosorbent, J. Chemom., 2018, vol. 32, no. 9, e3039. https://doi.org/10.1002/cem.3039
Crini, G., Non-conventional low-cost adsorbents for dye removal: A review, Bioresour. Technol., 2006, vol. 97, pp. 1061–1085.
Gupta, V.K. and Suhas, Application of low-cost adsorbents for dye removal—A review, J. Environ. Manage., 2009, vol. 90, pp. 2313–2342.
Mobinikhaledi, A., Moghanian, H., and Deinavizadeh, M., PTSA-catalyzed condensation of xylenols and aldehydes under solvent-free conditions: One-pot synthesis of 9H-xanthene or bisphenol derivatives, C. R. Chim., 2013, vol. 16, no. 11, p. 1035–1041.
Moghanian, H., Mobinikhaledi, A., and Baharangiz, Z., Synthesis, characterization and magnetic properties of novel heat resistant polyimide nanocomposites derived from 14H-dibenzo[a,j]xanthene, J. Polym. Res., 2014, vol. 21, no. 7, 513.
Moghanian, H., Mobinikhaledi, A., and Deinavizadeh, M., Efficient, one-pot synthesis of xanthene derivatives using boron sulphonic acid as a solid heterogeneous catalyst under solvent-free conditions, Res. Chem. Intermed., 2015, vol. 41, no. 7, pp. 4387–4394.
Hua, M., Zhang, S., Pan, B., Zhang, W., Lv, L., and Zhang, Q., Heavy metal removal from water/wastewater by nanosized metal oxides: A review, J. Hazard. Mater., 2012, vols. 211–212, pp. 317–331.
Bagtash, M. and Zolgharnein, J., Removal of brilliant green and malachite green from aqueous solution by a viable magnetic polymeric nanocomposite: Simultaneous spectrophotometric determination of 2 dyes by PLS using original and first derivative spectra, J. Chemom., 2018, vol. 32, no. 7, e3014.
Baileya, S.E., Olinb, T.J., Brickab, R.M., and Adriana, D.D., A review of potentially low-cost sorbents for heavy metals, Water Res., 1999, vol. 33, no. 11, pp. 2469–2479.
Zhu, M.-X., Li, Y.-P., Xie, M., and Xin, H.-Z., Sorption of an anionic dye by uncalcined and calcined layered double hydroxides: A case study, J. Hazard. Mater., 2005, vol. 120, pp. 163–171.
Seftel, E.M., Popovici, E., Mertens, M., De Witte, K., Van Tendeloo, G., Cool, P., and Vansant, E.F., Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application, Microporous Mesoporous Mater., 2008, vol. 113, pp. 296–304.
El Gaini, L., Lakraimi, M., Sebbar, E., Meghea, M., and Bakasse, A., Removal of Indigo Carmine dye from water to Mg–Al–CO3-calcined layered double hydroxides, J. Hazard. Mater., 2009, vol. 161, pp. 627–632.
Shabanian, M., Ardeshir, H., Haji-Ali, S., Moghanian, H., Hajibeygi, M., Faghihi, K., Khonakdar, H.A., and Salimi, H., Efficient poly(methyl-ether-imide)/LDH nanocomposite derived from a methyl rich bisphenol: From synthesis to properties, Appl. Clay Sci., 2016, vol. 123, pp. 285–291.
Huang, S., Song, S., Zhang, R., Wen, T., Wang, X., Yu, S., Song, W., Hayat, T., Alsaedi, A., and Wang, X., Construction of layered double hydroxides/hollow carbon microsphere composites and its applications for mutual removal of Pb(II) and humic acid from aqueous solutions, ACS Sustainable Chem. Eng., 2017, vol. 5, pp. 11268–11279.
Zhang, M., Yao, Q., Lu, C., Li, Z., and Wang, W., Layered double hydroxide–carbon dot composite: High-performance adsorbent for removal of anionic organic dye, ACS Appl. Mater. Interfaces, 2014, vol. 6, pp. 20225–20233.
Kundu, S. and Naskar, M.K., Carbon-layered double hydroxide nanocomposite for efficient removal of inorganic and organic based water contaminants—Unravelling the adsorption mechanism, Mater. Adv., 2021, vol. 2, pp. 3600–3612.
Zhang, X., Wang, J., Li, R., Dai, Q., Gao, R., Liu, Q., and Zhang, M., Preparation of Fe3O4@C@layered double hydroxide composite for magnetic separation of uranium, Ind. Eng. Chem. Res., 2013, vol. 52, pp. 10152–10159.
Afradi, M., Foroughifar, N., Pasdar, H., and Moghanian, H., L-Proline N-sulfonic acid-functionalized magnetic nanoparticles: A novel and magnetically reusable catalyst for one-pot synthesis of 3,4-dihydropyrimidine-2-(1H)-thiones under solvent-free conditions, RSC Adv., 2016, vol. 6, no. 64, pp. 59343–59351.
Mobinikhaledi, A., Moghanian, H., and Souri, Z., Efficient synthesis of 2-amino-4H-chromene derivatives in the presence of piperazine-functionalized Fe3O4/SiO2 magnetic nanoparticles as a new heterogeneous reusable catalyst under solvent-free conditions, Lett. Org. Chem., 2014, vol. 11, no. 6, pp. 432–439.
Mobinikhaledi, A., Moghanian, H., and Ghanbari, M., Synthesis and characterization of sodium polyaspartate-functionalized silica-coated magnetite nanoparticles: A heterogeneous, reusable and magnetically separable catalyst for the solvent-free synthesis of 2-amino-4H-chromene derivatives, Appl. Organomet. Chem., 2018, vol. 32, e4108.
Moghanian, H., Fard, M.A.B., Mobinikhaledi, A., and Ahadi, N., Bis(p-sulfoanilino)triazine-functionalized silica-coated magnetite nanoparticles as an efficient and magnetically reusable nano-catalyst for Biginelli-type, Res. Chem. Intermed., 2018, vol. 44, no. 7, pp. 4083–4101.
Shabanian, M., Moghanian, H., Khaleghi, M., Khaleghi, M., Hajibeygi, M., Khonakdar, H.A., and Vahabi, H., Chitosan and imide-functional Fe3O4 nanoparticles to prepare new xanthene based poly(ether-imide) nanocomposites, RSC Adv., 2016, vol. 6, pp. 112568–112575.
Zolgharnein, J., Bagtash, M., Feshki, S., Zolgharnein, P., and Hammond, D., Crossed mixture process design optimization and adsorption characterization of multi-metal (Cu(II), Zn(II) and Ni(II)) removal by modified Buxus sempervirens tree leaves, J. Taiwan Inst. Chem. Eng., 2017, vol. 78, pp. 104–117.
Massart, D.L., Vandeginste, B.G.M., Buydens, L.M.C., De Jong, S., Lewi, P.J., and Smeyers-Verbeke, J., Handbook of Chemometrics and Qualimetrics, Part A, Data Handling in Science and Technology, Amsterdam: Elsevier, 1997, vol. 20A.
Bruns, R.E., Scarmino, I.S., and de Barros Neto, B., Statistical Design—Chemometrics, Data Handling in Science and Technology, Amsterdam: Elsevier, 2006.
Noh, J.S. and Schwarz, J.A., Estimation of the point of zero charge of simple oxides by mass titration, J. Colloid Interface Sci., 1989, vol. 130, pp. 157–164.
ACKNOWLEDGMENTS
Authors appreciate the deputy of Iran national Science foundation for its financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Maryam Bagtash, Javad Zolgharnein Carbon-Magnetic Layered Double Hydroxide as a New Nanosorbent for Efficient Removal of Tartrazine and Indigo Carmine Dyes from Water Solutions; Multivariate Optimization and Adsorption Characterization. J. Water Chem. Technol. 44, 259–268 (2022). https://doi.org/10.3103/S1063455X2204004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X2204004X