Abstract
It has been shown that the ultrafiltration treatment of Dnieper water with the use of PA-20, PAN-20, and UPM-20 membranes does not provide the necessary level of total organic carbon for centralized water supply (≤8 (mg C)/dm3) in all the cases. However, the best result is obtained on the UPM-20 membrane. The application of an OPMN-P nanofiltration membrane results in that the total organic carbon decreases even below the established normative value, i.e., to 1.1–1.7 (mg C)/dm3, and the specific capacity of the selected membrane further sustains no changes up to the permeate takeoff degree of 90% after decrease for the first 10 min of operation in contrast to the ultrafiltration membranes. When processing the experimental data in compliance with the convective filtration theory, it has been established that the ultrafiltration UMP-20 membrane first (~40 min) results in the clogging of its pores with the further transition through an intermediate mechanism to filtration with the formation of a precipitate on its surface. The nanofiltration membrane is characterized by the immediate formation of a precipitate on its surface, being the most advantageous variant from the economic viewpoint. The remineralization of the formed nanofiltration permeate by means of dolomite grains provides the production of water, which is full in calcium and magnesium ions from the physiological viewpoint. The results from the biotests of water samples at the level of a fish organism has shown the absence of any acute and chronic toxicity and, however, the input Dnieper water was revealed to have a genotoxic effect on the blood samples of selected test objects. Chronic water toxicity has been revealed at the level of a hydra and daphnia organisms. The biological testing of the remineralized nanofiltration permeate has shown that it meets the biological parameters of potable water supply.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Dnieper river water is the main source of water supply in Ukraine. In particular, 30% of potable water in Kyiv is treated at the Dnieper Water Supply Station (DWSS) [1]. In the existing water treatment systems on the territory of Ukraine, the water of surface sources is subjected to primary chlorination, coagulation, clarification on a filtering load, and post chlorination for conservation before supply to consumers in most cases [2].
A specific feature inherent in the composition of Dnieper water is an increased content of natural organic compounds (NOCs), which attain >50 (mg C)/dm3 of total organic carbon (TOC). In this case, 77.5% of TOC are compounds of humic nature [3].
It has been established that humic compounds as such are not hazardous for human health and, on the contrary, they are promising as potential medicine remedies of new generation: they have an immunomodulatory, anti-inflammatory, antifungal, and antiviral effect [4]. However, the presence of humic compounds in water worsens its organoleptic properties. A much more serious problem is the formation of humic acid derivatives, which are toxic, mutagenic, and cancerogenic compounds as pointed out in [5], during the treatment of natural water with chlorine-containing compounds.
The traditional and least expensive method for the removal of humic compounds from the water or surface sources is coagulation [6]. However, the content of fulvic acids in Dnieper water is ~20–40 times higher than the content of humic compounds to attain 95–98% of natural organic compounds. It contains only 11% of fulvic acids with molecular mass (m.m.) of more than 1000 Da, whereas the molecular mass of the major fraction of fulvic acids must be ranged within 200–1000 Da. The degree of removal by coagulation is relatively low for such humic compounds and ranged within 23.0–41.1% [3].
This problem is especially urgent for Dnieper water due to the specifics of its composition, as it requires the deep purification from humic compounds at the stage of postchlorination.
In recent years, the traditional methods for the purification of surface water are successfully replaced by baromembrane techniques. Thus, there are more than 100 water treatment stations based on ultrafiltration in Norway, nanofiltration is used in France (Meru-Sur-Oise), Great Britain, the Netherlands [7], the United States (Florida) [8], and Japan [9], and an ultrafiltration station is also used in Russia [5].
Ultrafiltration membranes retain colloid particles of clay and iron oxide, algae, coloring natural organic compounds, and pathogenic microorganisms. Nanofiltration membranes provide a higher efficiency of water purification from dissolved organic compounds and ionic contaminants. Depending on the composition, ultra- or nanofiltration may be selected for its purification.
The objective of this study was to investigate the possibilities of ultra- and nanofiltration in the removal of fulvic acids from Dnieper basin water.
EXPERIMENTAL
The used object of study was Dnieper water taken at the Dnieper Water Supply Station. In this study, ultrafiltration membranes MIFIL PAN-20 and PA-20 (Institute of Physicoorganic Chemistry, National Academy of Sciences of Belarus) and VLADIPOR UPM-20 and a nanofiltration membrane OPMN-P (ZAO NTTs Polimersintez, Vladimir, Russia) were used.
The efficiency of fulvic acid removal from Dnieper water was studied by means of frontal filtration in a baromembrane cell with a magnetic stirrer, whose rotation speed was 300 rpm. The cell volume was 420 cm3, the working surface area of membranes was 12.57 × 10–4 m2, and the experiments were performed at 22–25°C. To avoid the effect of shrinkage in the porous structure of the membranes on experimental results, they were preliminary pressurized before experiments by forcing distilled water through them under pressure until constant specific capacity values were attained. Ultrafiltration and nanofiltration were performed at a pressure of 0.5 and 1.5 MPa, respectively. The regenerative cleaning of membranes after experiment was performed with the use of a phosphate–Trilon solution [10].
The nanofiltration permeate was remineralized in a column loaded with the 5–10-mm fraction of dolomite chips (Stone Plus Company) with a diameter of 3.3 cm and a height of 70 cm at a filtration rate of 5.9 m3/h.
The concentration of natural organic compounds in the inlet Dnieper and purified water (permeate) was judged from the total organic carbon content determined by catalytic combustion at 800°C on a Shimadzu TOC-V CSN analyzer (Japan). The Ca2+ and Mg2+ concentration was determined by trilonometric titration.
The toxicity of inlet water samples and water after nanofiltration with further remineralization was estimated by biotesting methods: hydra test (with the use of Hydra attenuate) described in [11, 12], Daphnia test (with the use of Daphnia magna) [12], and micronuclear test (on the gills, blood cells and caudal fin of fishes) [13]. The used reference sample was synthetic fresh water prepared as described in [12].
RESULTS AND DISCUSSION
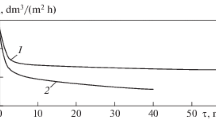
The study on the treatment of Dnieper water with the use of ultrafiltration membranes of different trademarks has shown that it has not been ever managed to attain the TOC content admitted for centralized water supply (≤8 (mg C)/dm3 [14]): the PA-20 and PAN-20 membranes decreased the TOC content in the filtrate to ~13 (mg C)/dm3, and a slightly better result of 11.2–11.7 (mg C)/dm3 was demonstrated by the UPM-20 membrane (Fig. 1a, curves 1–3). A decrease in the specific capacity Jw as a result of clogging in the UPM-20 membrane was the lowest (see Fig. 1b, curves 1'–3'). The nanofiltration membrane has provided the permeate with a TOC content of 1.1–1.7 (mg C)/dm3 (see Fig. 1a, curve 4). The value of Jw for the OPMN-P membrane was not further subjected to any changes up to k = 90% after decrease for the first 10 min in contrast to the ultrafiltration membranes (see Fig. 1b, curve 4').
(a) Degree of purification from total organic carbon and (b) specific capacity of membranes (1), (1') PA-20, (2), (2') PAN-20, (3), (3') UPM-20, and (4), (4') OPMN-P and decrease in the content of (4") Ca2+ and (4'") Mg2+ ions versus permeate takeoff during the ultra- and nanofiltration of Dnieper water.
As shown by the processing of data on the change in Jw of membranes in the course of ultra- and nanofiltration in compliance with the convective filtration theory [15], membrane pores were clogged for ~40 min, when theUPM-20 membrane was used (Fig. 2a, curve 1). This was followed by the transition through an intermediate mechanism (see Fig. 2a, curve 2) to filtration with the formation of a precipitate on the surface of a membrane (Fig. 2b, curve 3).
In contrast to the results measured in the process of filtration though the UPM-20 membrane, the nanofiltration treatment of Dnieper water is accompanied only by the formation of a precipitate on its surface (see Fig. 2b, curve 4). Here, it should be taken into account that the rate of growth in the total resistance upon the clogging of pores with an increase in the withdrawn filtrate amount is 3/2 of the resistance, proportional to the resistance for the intermediate mechanism, and remains constant for the formation of a precipitate on the surface of a membrane [15]. In addition, the comparison of filtration constants in the case of ultra- and nanofiltration (0.91 × 107 and 0.43 × 107 s/m2, respectively) shows that the formation of a precipitate on the surface of the OPMN-P membrane is almost twice slower.
Hence, nanofiltration has not only provided a decrease in the TOC concentration to 1.1–1.7 (mg C)/dm3, increasing this parameter to 91.1–93.9% (see Fig. 1a, curve 4) in comparison with coagulation treatment (23.0–41.1%) [3], but also has proven to be more cost-effective in comparison with ultrafiltration. Here, let us point out that surface water basins can be purified with the use of a polyamide membrane, which is modified with zirconium dioxide or zirconium hydrophosphate and resistant to the deposition of organic precipitates on its surface [16].
However, the permeate obtained after nanofiltration at a 90-% takeoff contained 23.0 mg/dm3 of calcium ions and 3.0 mg/dm3 of magnesium ions at a physiologically substantiate normative of 25–75 and 10–50 mg/dm3, respectively. Remineralization by passing the purified nanofiltration water through CaMg(CO3)2 dolomite grains has completed the content of Ca2+ ions to 47.8 mg/dm3 and the content of Mg2+ ions to 12.3 mg/dm3.
The results of biotesting the water samples have shown the absence of acute and chronic toxicity at the level of fish organisms (Table 1).
At the same time, the appearance of double nuclei inside red blood cells was observed in the fish blood samples taken from the test objects residing in the inlet Dnieper water (Fig. 3a) to evidence morphological transformations inside the cells [19]. The amount of revealed pathologies exceeded the normative values of genotoxicity [14]. Hence, Dnieper water does not correspond to the quality of potable water by its biological parameters, i.e., is toxic and, therefore, can not be recommended or use in potable water supply [19]. At the same time, the test objects of purified and remineralized water did not observed to have any quantitative changes in the cell characteristics corresponding to the normative requirements (see Fig. 3b).
Biotesting at the level of a hydra organism has shown that the inlet water sample exhibits chronic toxicity (14.29%) with respect to the selected test object as evidenced by its morphological transformations (see Fig. 3c), whereas the purified and remineralized water sample does not manifest any acute and chronic toxicity (see Fig. 3d) [14].
The Daphnia test of the inlet Dnieper water has shown a 40-% mortality rate (see Table 1), which is four times higher than the normatively admissible level, and indicates the manifestation of chronic toxicity, whereas the purified water does not exhibit any chronic toxicity for the selected organism [19, 20].
CONCLUSIONS
The results of studying the possibility to use ultra- and nanofiltration for the purification of Dnieper water from natural organic compounds have shown that ultrafiltration does not provide the TOC content admissible for centralized water supply, whereas the application of a nanofiltration membrane has resulted in a permeate with a TOC content of 1.1—1.7 (mg C)/dm3 up to the permeate takeoff of 90%. The use of remineralization for this permeate by passing through dolomite grains has completed the content of Ca2+ and Mg2+ ions to the physiologically substantiated normative level, and biotesting results have shown that the biological characteristics of Dnieper water after nanofiltration purification and dolomite remineralization satisfy the normative requirements to potable water in contrast to the inlet water. The studied potable water preparation method can be used at local stations.
REFERENCES
What kind of water do people of Kiev drink from taps and pump rooms, Glavkom, 2013. https://glavcom.ua/kyiv/news/287156-kakuju-vodu-pjut-kievljane-iz-kranov-i-bjuvetov.html. Accessed July 31, 2020.
Modern technologies and equipment for water treatment. https://knute.edu.ua/. Accessed August 14, 2020.
Klimenko, N.A., Samsoni-Todorova, E.A., Savchina, L.A., Lavrenchuk, I.N., and Zasyad’ko, T.N., Seasonal variations of characteristics of organic matter in the Dnieper River water, J. Water Chem. Technol., 2012, vol. 34, pp. 154–161.
Savchenko, I.A., Korneeva, I.N., Luksha, E.A., and Pasechnik, K.K., Biological activity of humic substances: Prospects and problems of their application in medicine, MediAl, 2019, no. 1, pp. 54–60.
Goncharuk, V.V., Klimenko, N.A., Savchina, L.A., et al., Minimizing the genetic risk through development of modern drinking water technologies, J. Water Chem. Technol., 2000, vol. 22, no. 5, pp. 487–503.
Matilainen, A., Vepsäläinen, M., and Sillanpää, M., Natural organic matter removal by coagulation during drinking water treatment, Adv. Colloid Interface Sci., 2010, vol. 159, no. 2, pp. 189–197.
Thorsen, T. and Fløgstad, H., Nanofiltration in Drinking Water Treatment: Literature Review, Nieuwegein: Techneau, 2006.
Bartels, C., Wilf, M., Casey, W., and Campbell, J., New generation of low fouling nanofiltration membranes, Desalination, 2008, vol. 221, nos. 1–3, pp. 158–167.
Costa, A.R. and de Pinho, M.N., Performance and cost estimation of nanofiltration for surface water treatment in drinking water production, Desalination, 2006, vol. 196, nos. 1–3, pp. 55–65.
Goncharuk, V.V., Balakina, M.M., Kucheruk, D.D., and Skubchenko, V.F., UA Patent 76544, 2006.
Nanieva, A.V., Pelishenko, A.V., Kovalenko, V.F., and Goncharuk, V.V., J. Water Chem. Technol., 2019, vol. 41, pp. 329–333.
DSTU (State Standard of Ukraine) 4173:2003: Water Quality. Determination of Extreme Lethal Toxicity on Daphnia magna Straus and Cerio daphnia affinis Lilljeborg (Cladosera, Crustacea), Kyiv: Ukr. Nauk.-Doslid. Inst. Ekol. Probl., 2003.
DSTU (State Standard of Ukraine) 7387:2013: Water Quality. Method for Determination of Cyto- and Genotoxicity of Water and Aqueous Solutions on Blood Cells of Freshwater Zebrafish (Brachydanio rerio Hamilton-Buchanan), Kyiv: Inst. Koloidn. Khim. Khim. Vodi im. A.V. Dumans’kogo, Nats. Akad. Navuk Ukr., 2013.
DSTU (State Standard of Ukraine) 7525:2014: Drinking Water. Requirements and Methods of Quality Control), Kyiv: Minist. Ekon. Razvit. Ukr., 2014.
Zhuzhikov, V.A., Fil’trovanie: teoriya i praktika razdeleniya suspenzii (Filtration: Theory and Practice of Suspension Separation), Moscow, 1980.
Zmievskii, Y., Rozhdestvenska, L., Dzyazko, Y., Kornienko, L., Myronchuk, V., Bildukevich, A., and Ukrainetz, A., Organic-inorganic materials for baromembrane separation, in Nanophysics, Nanomaterials, Interface Studies, and Applications, Springer Proc. Phys. Ser., vol. 195, Cham: Springer, 2017, pp. 675–686. https://doi.org/10.1007/978-3-319-56422-7_51
DSTU (State Standard of Ukraine) 4075-2001: Water Quality. Determination of Extreme Lethal Toxicity of Chemicals and Water on Freshwater Fish (Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae), Part 2: Semi-Static Method (ISO 7346-2:1996, MOD), Kyiv, 2014.
Arkhipchuk, V.V., Garan’ko, N.M., and Goncharuk, V.V., UA Patent 67315, 2006.
Goncharuk, V.V., Nauka o vode (The Science about Water), Kyiv, 2010.
DSTU (State Standard of Ukraine) 4174:2003: Water Quality. Determination of Chronic Toxicity of Chemicals and Water on Daphnia magna Straus and Cerio daphnia affinis Lilljeborg (Cladosera, Crustacea) (ISO 10706:2000, MOD), Kyiv: Ukr. Nauk.-Doslid. Inst. Ekol. Probl., 2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
About this article
Cite this article
Balakina, M.M., Seminska, O.O., Osmalena, O.V. et al. Capabilities of Ultra- and Nanofiltration in the Purification of Dnieper Water from Natural Organic Compounds. J. Water Chem. Technol. 43, 342–347 (2021). https://doi.org/10.3103/S1063455X21040032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X21040032