Abstract
Tetracycline (TC) is one of the most commonly applied antibiotic and due to the risk posed by antibiotic-resistant pathogens, the need for treating these wastewater is beyond question. Photocatalytic degradation of TC in aqueous solution was studied using TiO2 based on natural zeolite (clinoptilolite, CP) under UV irradiation in a batch reactor. For the first time, in order to propose an accurate model for the photocatalytic degradation of TC under UV light, central composite design based on response surface methodology was employed to assess the individual and interactive effects of several classic parameters on both TC degradation and TOC removal (mineralization). In order to analyze the process, three significant variables pH, catalyst concentration, and tetracycline concentration and tetracycline degradation as the process response were studied. The results of the experiments were fitted to quadratic polynomial models. A maximum degradation of TC of more than 77% was achieved at the optimum conditions (pH 5.9, catalyst concentration of 0.30 g L–1 and TC concentration of 8 mg L–1) within 60 of irradiation. The mineralization or oxidation profile, represented as the total organic carbon, was decreased significantly during the optimum conditions, achieving value near 54% after 4 h. The experimental values agreed with the predicted ones, indicating suitability of the model employed and the success of model in optimizing the conditions of photocatalysis. The quadratic model had high R2 coefficient (0.980) and high F-value, confirming that the model was valid to predict the experimental results. Moreover, the adjusted R2 is very close to the R2 value as another evidence for goodness of the model fit. Initial TC concentration and catalyst dosage are the major factors affecting the photocatalytic degradation of TC using TiO2/CP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Over the past few years, antibiotics and their metabolites have been detected in surface water, ground water, wastewater, and drinking water at levels ranged from ng L–1 to μg L–1 [1, 2]. The wide use of antibiotics is beneficial for preserving human health, but results in their undesirable accumulation in different environmental compartments as a secondary effect, which cannot be effectively eliminated by traditional biological methods [3–5]. Tetracycline (TC) is one of the most commonly applied antibiotic and its production and usage rank second worldwide after antimicrobial sulfonamides [6]. Because it is biologically active, stable and non-biodegradable, its release to the environment threatens aquatic life and increases the risk posed by antibiotic-resistant pathogens [7]. Thus the need for treating these wastewaters is beyond question. Recently, heterogeneous photocatalytic degradation of organic pollutants in the presence of nanostructured semiconductors attained the maximum efficiency of mineralization [2, 8–12]. Investigation of photocatalytic degradation of pollutants has usually been made by the single-variable-at-a-time (SVAT, the most common practice holding all other variables constant) method in previous studies [11–13]. Nevertheless, the SVAT approach possesses many drawbacks, such as being time-consuming, inability to account for interactions between different variables and inefficiency in predicting the true optimum conditions. Response surface methodology (RSM) is a useful statistical method which can determine the optimal condition and reduce the study time and the cost by the comprehensive analysis of mathematics [14]. For the first time, in order to propose an accurate model for the photocatalytic degradation of TC under UV light, central composite design (CCD) based on RSM was employed to assess the individual and interactive effects of several classic parameters on both TC degradation and total organic carbon (TOC) removal (mineralization). TiO2 based on natural zeolite was used as photocatalyst for the study.
EXPERIMENTAL
TiO2 based on natural zeolite (TiO2/clinoptilolite: TiO2/CP) was prepared via simple method by mixing 1 g TiO2 P25 with 5 g of natural zeolite in powder form with subsequent treatment according to our previous investigation [15]. Common TC (C20H24N2O8) pharmaceutical 250 mg capsule was purchased from Daru Company, Iran. All the water used was deionized.
Photocatalytic Experiments
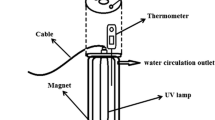
Photocatalysis experiments were carried out in the homemade cylindrical photocatalytic reactor. It was made of Pyrex with an effective volume of 300 mL and equipped with four UV-C lamps (4W from Philips Co., Japan) as irradiation source (Fig. 1). Prior to photocatalytic experiments, adsorption study was carried out. Samples of the reaction solution were obtained at regular intervals, filtered by centrifuge and analyzed by UV−Vis spectrophotometer. The degradation efficiency of TC was calculated by (C0 – C)/C0 × 100.
Experimental Design and Statistical Analysis
Based on the literature [9], three operational factors: pH (4–10), catalyst (0.3–0.8 g L–1) and initial TC concentration (8–14 mg L–1) were introduced as those with the most significant impact, which were considered as the system variables, and TC degradation efficiency was calculated as the process response. CCD has been extensively used along with RSM to optimize various physical, chemical and biological processes using Design Expert software version 8.0.4.1 [16]. Total organic carbon measurements were performed by TOC analyzer (Anatoc, Australia). UV−Vis spectra for determination of TC concentration were obtained on a Shimadzu UV-1650PC spectrophotometer.
RESULTS AND DISCUSSION
Statistical Analysis
Experimental and predicted values obtained for degradation of TC at the design points are presented in Table 1. Degradation efficiency of TC (%) is correlated to independent variables with a second-order polynomial by the following equation:
Statistical analysis of the model was performed to evaluate the analysis of variance (ANOVA), indicating F-value of 49.79 and R2 = 0.98, implying that the model was statistically significant (Table 2) [17, 18]. Moreover, the adjusted R2 is very close to the R2 value, which is another evidence for goodness of the model fit [19, 20]. The lack of fit F-value of 0.82 is not significant as the P-value is 0.5814. From these results, it can be concluded that the obtained model is adequate to describe the relationship between the degradation efficiency and three factors for degradation of TC. Initial TC concentration and catalyst dosage are the major factors affecting the photocatalytic degradation of TC using TiO2/CP. The three-dimensional response surface plots represent an infinite number of combinations of the two selected variables with the other maintained at its respective zero coded level. The results of Fig. 2, a revealed that the rate of degradation of TC decreased with increase in concentration irrespective of the dose of catalyst. By increasing the initial concentration of TC, higher amounts of reactant and reaction intermediates are adsorbed at the surface of the photocatalyst. Therefore, the demand of reactive species (\(^{•}{\text{OH}}\) and \(^{•}{{{\text{O}}}^{{2 - }}}\)) needed for the degradation of TC also increases. However, the formation of \(^{•}{\text{OH}}\) and \(^{•}{{{\text{O}}}^{{2 - }}}\) on the catalyst surface remains constant for a given light intensity, catalyst amount, and duration of irradiation. Hence, the available \(^{•}{\text{OH}}\) radicals are inadequate for the TC degradation at higher concentrations. Similar results were reported by others and usually attributed to the limited amount of active sites available for the adsorption of reactant at higher concentration [21, 22]. According to Fig. 2b, at constant pH, efficiency increased by increasing the amount of catalyst dosage. The saddle nature of the plots confirms that a significant interaction exists between these two factors (BC). It is found that when TiO2/CP loading were increased, the photodegradation was enhanced. Increased loading of the catalyst increases the generation rate of electron/hole pairs and, thus, the formation of \(^{•}{\text{OH}}\) radicals leading to the enhancement of photodegradation. Maximum degradation of tetracycline more than 94% was achieved at tetracycline concentration of 8 mg L–1, catalyst concentration of 0.8 g L–1 and pH 10 within 60 min of irradiation (see Table 1).
Optimization of Independent Variables and Validation
In the present study, efforts have been made to minimize the use of the catalyst and to maximize the degradation of TC, during optimization. The optimum conditions for the maximum possible degradation of TC under the described constraints were found out to be: dose of TiO2/CP 0.3 g L–1, concentration of TC 8 mg L–1 and pH of reaction mixture 5.9. Under these conditions the model predicted the degradation of TC to be 77.9%. The result showed the efficient use of RSM to find out the optimum conditions for the photocatalytic degradation of TC using TiO2/CP. The degradation efficiency was found out from the validation experiment to be about 75%. It was in good agreement with the predicted result, which confirms the adequacy and validity of the model.
Mineralization Assessment
TOC analysis provided valuable information about the efficiency of the photocatalytic process. The destruction of organic pollutants should be regarded as an overall degradation process that ultimately involves mineralization of both the parent substance and its intermediates. The mineralization or oxidation profile, represented as the TOC, was decreased significantly during the optimum conditions, achieving value near 54% after 4 h. It is remarkable that the TOC removal reached good value, indicating that the oxidation of intermediates promoted a continuous cleavage of the initial organic structures.
CONCLUSIONS
The photocatalytic degradation of TC in an aqueous solution under UV light using an experimental design methodology by titania based on natural zeolite was firstly studied. From the quadratic models developed for degradation and subsequent ANOVA test using Design Expert software, the initial concentration of TC and catalyst dosage were found to be the most influential factors. The quadratic model had high Required coefficient (0.98) and high F-value, confirming that the model was valid to predict the experimental results.
The process was optimized under two applied constraints of minimum use of catalyst and maximum degradation of TC under the constraints. The optimum values of the three independent variables were found out as dose of TiO2/CP 0.3 g L–1, initial concentration of TC 8 mg L–1 and pH of reaction mixture 5.9 under the applied constraints. Under the optimum condition the degradation of TC was predicted to be 77.9%. Furthermore, the TOC result (54% removal after 4 h) revealed that modified semiconductor could effectively mineralize TC.
REFERENCES
Javid, A., Nasseri, S., Mesdaghinia, A., Hossein Mahvi, A., Alimohammadi, M., and Aghdam, R.M., Performance of photocatalytic oxidation of tetracycline in aqueous solution by TiO2 nanofibers, J. Environ. Heal. Sci. Eng., 2013, vol. 11, no. 1, pp. 24–38.
El Kemary, M., El Shamy, H., and El Mehasseb, I., Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles, J. Lumin., 2010, vol. 130, pp. 2327–2331.
Zhu, X. and Wang, Y.D., TiO2 photocatalytic degradation of tetracycline as affected by a series of environmental factors, J. Soils Sediments, 2014, vol. 14, no. 8, pp. 1350–1358.
Shakir, M., Faraz, M., Asif Sherwani, M., and Al Resayes, S.I., Photocatalytic degradation of the Paracetamol drug using Lanthanum doped ZnO nanoparticles and their in-vitro cytotoxicity assay, J. Lumin., 2016, vol. 176, pp. 159–167.
Hong, Y., Ren, A., Jiang, Y., He, J., Xiao, L., and Shi, W., Sol–gel synthesis of visible-light-driven Ni(1‒x)Cux-Fe2O4 photocatalysts for degradation of tetracycline, Ceram. Int., 2015, vol. 41, no. 1, pp. 1477–1486.
Oturan, N., Wu, J., Zhang, H., Sharma, V.K., and Oturan, M.A., Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: effect of electrode materials, Appl. Catal., B, 2013, vol. 140, pp. 92–97.
Liu, S., Zhao, X.R., Sun, H.Y., Li, R.P., Fang, Y.F., and Huang, Y.P., The degradation of tetracycline in a photo-electro-Fenton system, Chem. Eng. J., 2013, vol. 231, pp. 441–448.
Cataldo, S., Ianni, A., Loddo, V., Mirenda, E., Palmisano, L., Parrino, F., and Piazzese, D., Combination of advanced oxidation processes and active carbons adsorption for the treatment of simulated saline wastewater, Sep. Purif. Technol., 2016, vol. 171, pp. 101–111.
Saadati, F., Keramati, N., and Mehdipour Ghazi, M., Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: a review, Crit. Rev. Environ. Sci. Technol., 2016, vol. 46, no. 8, pp. 757–782.
Zhu, X.D., Wang, Y.J., Sun, R.J., and Zhou, D.M., Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2, Chemosphere, 2013, vol. 92, pp. 925–932.
Zielińska-Jurek, A., Wysocka, I., Janczarek, M., Stampor, W., and Hupka, J., Preparation and characterization of Pt–N/TiO2 photocatalysts and their efficiency in degradation of recalcitrant chemicals, Sep. Purif. Technol., 2015, vol. 156, pp. 369–378.
Liu, X.J., Li, W.W., Lam, P.K.S., and Yu, H.Q., Photodegradation of perfluorooctane sulfonate in environmental matrics, Sep. Purif. Technol., 2015, vol. 151, pp. 172–176.
Ai, C., Zhou, D., Wang, Q., Shao, X., and Lei, Y., Optimization of operating parameters for photocatalytic degradation of tetracycline using In2S3 under natural solar radiation, Sol. Energy, 2015, vol. 113, pp. 34–42.
Guo, W.Q., Meng, Z.H., Ren, N.Q., Zhang, Z.P., and Cui, F.Y., Optimization of key variables for the enhanced production of hydrogen by Ethanoligenens harbinense W1 using response surface methodology, Int. J. Hydrogen Energ., 2011, vol. 36, no. 10, pp. 5843–5848.
Saadati, F., Keramati, N., and Mehdipour Ghazi, M., Synthesis of nanocomposite based on Semnan natural zeolite for photocatalytic degradation of tetracycline under visible light, Adv. Environ. Technol., 2016, vol. 2, pp. 63–70.
Sakkas, V.A., Islam, Md.A., Stalikas, C., and Albanis, T.A., Photocatalytic degradation using design of experiments: a review and example of the Congo red degradation, J. Hazard. Mater., 2010, vol. 175, pp. 33–44.
Torrades, F. and García-Montaño, J., Using central composite experimental design to optimize the degradation of real dye wastewater by Fenton and photo-Fenton reactions, Dyes Pigm., 2014, vol. 100, pp. 184–189.
Moradi, M., Ghanbari, F., Manshouri, M., and Angali, K.A., Photocatalytic degradation of azo dye using nano-ZrO2/UV/persulfate: response surface modeling and optimization, Korean J. Chem. Eng., 2016, vol. 33, pp. 539–546.
Davarnejad, R., Mohammadi, M., and Ismail, A.F., Petrochemical wastewater treatment by electro-Fenton process using aluminum and iron electrodes: statistical comparison, J. Water Process Eng., 2014, vol. 3, pp. 18–25.
Chun, S.Y., An, S.W., Lee, S.J., Kim, J.T., and Chang, S.W., Optimization of sulfamethoxazole degradation by TiO2/hydroxyapatite composite under ultraviolet irradiation using response surface methodology, Korean J. Chem. Eng., 2014, vol. 31, no. 6, pp. 994–1001.
Ahmed, S., Rasul, M.G., Martens, W.N., Brown, R., and Hashib, M.A., Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments, Desalination, 2010, vol. 261, pp. 3–18.
Royaee, S.J., Sohrabi, M., and Jabari Barjesteh, P., Performance evaluation of a continuous flow Photo-Impinging Streams Cyclone Reactor for phenol degradation, Chem. Eng. Res. Des., 2012, vol. 90, pp. 1923–1929.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Farzaneh Saadati, Keramati, N. & Ghazi, M.M. Optimization of Photocatalytic Degradation of Tetracycline Using Titania Based on Natural Zeolite by Response Surface Approach. J. Water Chem. Technol. 42, 30–35 (2020). https://doi.org/10.3103/S1063455X20010087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X20010087