Abstract
An experimental study is performed of the plasma dynamic synthesis of ultra-dispersed titanium dioxide powders upon a change in the energy supplied to the accelerator. It is shown that the energy of synthesis affects the anatase/rutile mass percentage ratio in the composition of the final product. The maximum content of anatase at a level of 80.0% is obtained at a supplied energy of ~33 kJ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Titanium dioxide TiO2 is a polymorphic semiconductor material that exists in three modifications: anatase, brookite, and rutile [1–3]. Due to its low cost, nontoxicity, and unique physical properties that include a broad band gap and high permittivity, this chemical compound is widely used as a photocatalyst, polymer filler, and luminescent gas-sensitive material [4–7]. It is also used in producing pigments and ceramics [8, 9]. Titanium dioxide can be obtained from minerals, titanium salts, or alkoxides via different types of synthesis, particularly sol–gel, hydrothermal, sulfate, or chloride [5, 10–12]. In recent decades, special attention has been given to physical means of synthesizing titanium dioxide [13–15].
The rutile phase is stabler than anatase and brookite in the photocatalytic use of TiO2, since the latter two are metastable forms of TiO2 [16]. The anatase modification is preferred because of its higher surface energy and Fermi level [17, 18]. However, many recent works have shown the efficiency of using combinations of the modifications: anatase/rutile, brookite/rutile, and anatase/brookite [2, 19, 20].
In [21], we demonstrated the fundamental possibility of synthesizing dispersed titanium dioxide via direct plasma dynamic synthesis. It was shown that the final product contained two crystalline modifications of titanium dioxide: anatase and rutile. A key factor affecting the characteristics of the product of plasma dynamic synthesis, particularly the phase composition, was in this case energy W supplied and released in an accelerator channel. In this work, we consider the effect the energy supplied to an accelerator has on the operation of the latter and the phase composition of the product of synthesis.
EXPERIMENTAL
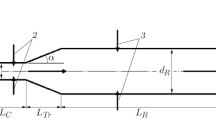
The plasma dynamic synthesis of titanium dioxide is based on the operation of a pulsed coaxial magneto-plasma accelerator (CMPA) with titanium electrodes. Its operating principle was described in [21, 22]. The energy supplied to the accelerator was increased by raising charging voltage Uch of capacitive energy storage (CES) from 2.0 to 3.0 kV at a constant capacitance of C = 14.4 mF. The ratio between oxygen and argon partial pressures in the reactor chamber was р(О2)/р(Ar) = 1 : 1. Current and voltage oscillograms were recorded with Tektronix TDS2012 digital oscilloscopes. Dynamic characteristics of the plasma flow in the free space of the reactor chamber were obtained through high-speed photodetection on the VFU-1 and Photron FastCAM SA1.1 setups. A qualitative X‑ray phase analysis was performed using the Search-Match software and the PDF 4+ structural database. A quantitative analysis was performed using the Rietveld PowderCell 2.4 software. Scanning electron microscopy was used to study the synthesized material on a JSM-6700F microscope.
RESULTS AND DISCUSSION
The comparison of energy characteristics in Fig. 1 shows that a substantial regular increase in the discharge current amplitude, power supply Im (126.0 and 149.4 kA), maximum charge power Рm (124.3 and 162.9 MW), and total supplied energy W (26.5 and 35.1 kJ) was observed upon raising Uch from 2.2 to 2.5 kV and initial energy Wc from 34.85 to 45.0 kJ under similar CMPA parameters, particularly the comparable initial resistances of the carbon jumper between the electrodes (680 and 700 Ω, respectively). We can also see an increase of 76.0 to 78.0% in the efficiency of energy transfer from the CES to the CMPA load.
Typical oscillograms for current I(t) and voltage U(t), curves of power P(t) and supplied voltage W(t), photograms of a hypersonic single-pulse plasma jet, curves of law of motion L(t) and rate of decay υ(t) for the shock front of a supersonic plasma jet after leaving the accelerating channel in experiments with W = (а) 26.5 and (b) 35.1 kJ.
The dynamics of the shock wave structure was analyzed using photograms. Figure 1 shows typical L(t) curves for the law of motion of the jet fronts with highest energy W = 35.1 kJ and lowest energy W = 26.5 kJ. The laws of motion were approximated by polynomial functions. When differentiated, they yielded estimated curves for the laws of attenuation of bow shock wave velocity υ(t) over time, presented in the same plot.
The mutual arrangement of the curves the higher bow shock wave front velocities throughout the range of observation after leaving the accelerating channel at higher supplied energies W. Average initial velocities υav estimated in the range of ∆t ≈ 5.0 µm are ~2.8 and ~2.4 km/s for the higher and lower energies, respectively. However, according to the gas-dynamic laws of supersonic flows, the area of the bow shock wave boundary’s surface rapidly broadens and increases behind the accelerating channel cut, resulting in fast damping of the wave’s velocity.
All other things being equal, known patterns [23] indicate an increase in W raises mass m of titanium eroded and removed from the accelerating channel, the main precursor of synthesis (see Fig. 2). The dependence is clearly quadratic and can be approximated with the equation
However, an increase in the mp value is only observed when energy W is raised to ≈27.0 kJ and a value of mp ≈ 2.3 g is attained with subsequent stabilization or some reduction. The utilization of metallic titanium for synthesizing the oxide product, defined as the ratio of the mass of Ti in the dioxide to m (2),
is no higher than 30%, due to the limited free recession of the synthesized material at the walls of the reactor chamber. The oxide particles that manage to crystallize are subsequently deposited in the form of a dispersed product. The microdroplets with no time to crystallize reach the walls of the reactor chamber, precipitate, and solidify. The content of the product’s coarse particles [22] is independent of W and ~13% on average.
Statistical analysis of the granulometric composition of the fine fraction of the products synthesized at energies of 27.5 to 37.7 kJ showed no appreciable differences in the laws of size distribution. In both cases, the size of most particles was ~80 nm, which is very close to the data in [21]. All other things being equal, these results show indirectly that mp can be raised by increasing the overall dimensions (diameter and length) of the reactor chamber and its volume.
The material of the product was studied via X-ray diffractometry and identified as two titanium dioxide polymorphs: anatase aTiO2 (tetragonal syngony, sp. gr. I41/amd {141}) and rutile rTiO2 (tetragonal syngony, sp. gr. P42/mnm {136}). It was found that W strongly affects the phase composition of the product [22]. An increase in the intensity of aTiO2 reflections and a reduction in rutile rTiO2 are observed upon raising W, with a minor drop in the crystallinity of the product in the range of 97.0–98.0%. The corresponding change in the mass percentage calculated for anatase ω(aTiO2) and rutile ω(rTiO2) upon a change in W is illustrated in Fig. 3.
The maximum value ω(aTiO2) ≈ 80% and the minimum value ω(rTiO2) ≈ 20% are reached when W ≈ 33 kJ. The established effect of W is due to an increase in the velocity of the plasma flow and the surface area of the bow shock wave front, from which more micro- and nanodroplets of the synthesized material are washed away.
In free recession, the droplets crystallize in the anatase structure. The termination of ω(aTiO2) growth upon a further increase in W is caused by the liquid droplets being deposited onto the wall of the reactor chamber and their crystallization in the rutile structure, as was confirmed by XRD data. According to the calculated average sizes of coherent scattering regions (CSRs; see Figs. 3b), in the range of W = 20–40 kJ, there is a weak tendency of CSR growth for both phases with some excess rTiO2. The average size of a CSR for the anatase phase is ~72 nm. It is 83 nm for rutile and 80 nm for both phases in general. It should be noted that the size of the CSR is often identical to the average size of the crystallite in a nanopowder. The size of a CSR is in this case conventionally 10–15% smaller than the fine particle (grain) size determined via electron microscopy, since the CSR corresponds to the internal (ordered) portion of the grain and does not include strongly distorted boundaries [24, 25].
X-ray diffractometry thus shows that titanium dioxide powders obtained via plasma dynamic synthesis at W ≈ 30–35 kJ have a predominantly anatase crystal structure.
CONCLUSIONS
We established the optimum energy parameters (С = 14.4 mF and Uch = 2.5 kV) for a supply of power at which the maximum discharge current does not exceed Im = 150 kA, the supplied energy is W ≈ 30 kJ at a power of Pm ≈ 160 MW and a pulse length of tpl ≈ 450 µs, and high thermal and electrodynamic stability is ensured for all elements of the system. Under optimum conditions, the growth in mass mp of the powdered product and utilization KU of metallic titanium as W rises are, according to CMPA parameters, limited by the dimensions and volume of the reactor chamber. The content of ω(aTiO2) shoots to ~80% when W > 20–25 kJ. The content ω(rTiO2) plummets to ~20% and stabilizes at W ≈ 33.0 kJ. At the same time, the average size of the CSRs grows somewhat to ~72 nm for anatase and ~83 nm for rutile.
REFERENCES
Borisov, S.V., Magarill, S.A., and Pervukhina, N.V., J. Struct. Chem., 2019, vol. 60, no. 11, p. 1191.
Kozhevnikova, N.S., Ul’yanova, E.S., Shalaeva, E.V., et al., Kinet. Catal., 2019, vol. 60, no. 3, p. 325.
Lopes, J.N.L., Filho, J.C.S., Messias, D.N., et al., J. Lumin., 2021, vol. 240, 118461.
Wang, D., Wu, X., and Gao, Q., Ceram. Int., 2021, vol. 47, no. 20, p. 28557.
Ahmad, R.A.R., Harun, Z., Azhar, F.H., et al., Mater. Today. Proc., 2021, vol. 46, p. 2122.
Solanki, K., Parmar, D., Savaliya, C., et al., Mater. Today. Proc., 2021, vol. 50, no. 6, p. 2576.
Komaraiah, D., Radha, E., Sivakumar, J., et al., Opt. Mater., 2020, vol. 108, 110401.
Yang Yu, Yu Zhao, Tian-Dong Zhang et al., Ceram. Int. 2018, vol. 44, no. 6, p. 6866.
Guan, R., He, Zh., Liu, Sh., et al., Powder Technol., 2021, vol. 380, p. 334.
Azimi-Fouladi, A., Hassanzadeh-Tabrizi, S.A., and Saffar-Teluri, A., Ceram. Int., 2018, vol. 44, no. 4, p. 4292.
Siwińska-Stefańska, K., Kubiak, A., Piasecki, A., et al., Appl. Surf. Sci., 2019, vol. 463, p. 791.
Sharma, R., Sarkar, A., Jha, R., et al., Int. J. Appl. Ceram. Technol., 2020, vol. 17, no. 3, p. 1400.
Kim, K., ACS Catal., 2019, vol. 9, no. 10, p. 9206.
Shanenkov, I., Nikitin, D., Ivashutenko, A., et al., Ceram. Int., 2020, vol. 47, no. 5, p. 6884.
Shanenkov, I., Nikitin, D., Ivashutenko, A., et al., Surf. Coat. Technol., 2020, vol. 389, 125639.
Allen, N.S., Mahdjoub, N., Vishnyakov, V., et al., Polym. Degrad. Stab., 2018, vol. 150, p. 31.
Du, P., Niu, P., Yang, Y., et al., J. Phys. Chem. Lett., 2022, vol. 13, p. 4244.
Ding, Y., Yang, I.S., Li, Z., et al., Prog. Mater. Sci., 2020, vol. 109, 100620.
Gorbunova, V.A. and Slepneva, L.M., Nauka Tekh., 2018, vol. 17, no. 6, p. 521.
Suwannaruang, T., Hildebrand, J.P., Taffa, D.H., et al., J. Photochem. Photobiol., A, 2020, vol. 391, 112371.
Sivkov, A.A., Nasyrbaev, A., Nikitin, D.S., et al., Inorg. Mater., 2021, vol. 57, no. 4, p. 337.
Sivkov, A., Vympina, Yu., Ivashutenko, A., et al., Ceram. Int., 2022, vol. 48, no. 8, p. 10862.
Shanenkov, I., Sivkov, A., Ivashutenko, A., et al., J. Alloys Compd., 2019, vol. 774, p. 637.
Gusev, A.I., Nanomaterialy, nanostruktury, nanotekhnologii (Nanomaterials, Nanostructures, Nanotechnologies), Moscow: Fizmatlit, 2007.
Gusev, A.I. and Kurlov, A.S., Metallofiz. Noveishie Tekhnol., 2008, vol. 30, no. 5, p. 679.
Funding
The reported study was funded by RFBR, project number 20-33-90060.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Bondareva
About this article
Cite this article
Sivkov, A.A., Vympina, Y.N., Rakhmatullin, I.A. et al. Studying the Effect of Supplied Energy on the Phase Composition of the Product of Plasma Dynamic Synthesis in a Ti–O System. Bull. Russ. Acad. Sci. Phys. 86, 1242–1245 (2022). https://doi.org/10.3103/S1062873822100203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1062873822100203