Abstract
Results are presented from studying the effect ion-stimulated diffusion has on the profile of the distribution of chemical elements at a film–substrate interface during analysis with optical emission spectroscopy of a glow discharge. Sputtered films of iron garnets on a gadolinium–gallium garnet substrate before and after granulation via thermal annealing are investigated. The role of diffusion processes in shaping the distribution profile of elements at a film–substrate interface is shown. A technique is proposed for restoring the true shape of an interface profile without considering the effect the ion flux has on the film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The physics of micro- and nanomaterials is largely determined by the properties of their surface, while the surface can be either free (usually in contact with gases or vacuum), or represents an interface (usually, two condensed substances, for example, the interface between a film and a substrate). Therefore, the formation of the surface and near-surface layers with desired physical and chemical properties is one of the key tasks of micro- and nano-engineering.
To analyze the distribution profile of chemical elements near the sample surface and at the interfaces of thin films with a substrate, methods of ion sputtering of the surface with the subsequent analysis of chemical composition of either the sputtered material (laser and secondary ion mass spectrometry, emission spectroscopy of glow and arc discharge, etc.), or directly the surface after etching (Auger spectroscopy, energy dispersive X-ray microanalysis, etc.) [1–8] are often used. Thus, by etching the sample in layers and analyzing its chemical composition, it is possible to obtain a picture of the distribution profile of elements. However, experience shows that these profiles differ from the actual distribution of elements. This is especially clearly observed when analyzing sharp boundaries of thin-film interfaces and heterojunctions. It manifests in the form of blurring of the experimentally measured profile, often with notable asymmetry [9–14]. There is diffusion mixing of atoms in near-surface layers (ion-stimulated diffusion) when a surface is bombarded with a directed flow of high-energy ions, which distorts data on the true distribution profile of elements. The aim of this work was to study and analyze the effect different mechanisms of diffusion have on shaping the distribution profile of chemical elements at a film–substrate interface, and to assess the possibility of restoring the true distribution profile of elements (before exposure to an ion beam).
EXPERIMENTAL
We studied films of bismuth-substituted iron garnet before and after heat treatment. The distribution profile of chemical elements at the film–substrate interface was analyzed experimentally via optical emission spectroscopy of the glow discharge. The effect different mechanisms of diffusion had on the distribution profile of elements was assessed theoretically using the numerical solution to the equation of diffusion in an approximation of spatial–temporal discretization.
Preparing Samples
Samples of films of bismuth-substituted iron garnet (BiIG) were obtained via the ion-reactive sputtering of a target with the composition Bi1.0Lu0.5Gd1.5Fe4.2Al0.8O12 in an Ar (25 mol %) + O2 (75 mol %) atmosphere at a pressure P = 8.7 × 10–2 Pa (pressure of residual gases is not worse than Pres = 10–3 Pa). Wafers 500 μm thick and made of polished gadolinium–gallium garnet Gd3Ga5O12 (GGG) single crystal with (111) surface orientation were used as substrates. Our BiIG/GGG films were 100 ± 5 nm thick. The films were in the amorphous state immediately after deposition. They were crystallized via thermal annealing in air for 20 min at a temperature of 700°C.
Investigating the Distribution Profile of Elements
Optical emission spectroscopy (OES) of an RF glow discharge on a Horiba GD Profiler 2 spectrometer [12] was used to study the distribution profile of chemical elements at the film–substrate interface. Figure 1 shows the scheme of OES.
The surface of a sample sputters when bombarded with argon ions. Atoms of the sputtered substance are excited in the plasma of the glowing RF discharge, with the subsequent emission of optical quanta at a certain wavelength. The resulting radiation is decomposed into a spectrum on a diffraction grating with 2400 lines/mm and analyzed on a polychromator in real time. The intensity of the corresponding characteristic spectral lines determines the content of elements on surface of the test sample. The depth of etching is determined by recalculating the period of etching according to preliminary calibrations.
OES was performed at argon pressure PAr = 200 Pa. The discharge power was 20 W, and the diameter of the anode window was 4 mm. The mode of pulsed plasma generation was used by applying short packets of high-frequency (13.68 MHz) pulses with a frequency of 5 kHz in order to study the interface zones in depth with high resolution. The rate of surface sputtering was 2 nm/s.
Analyzing Diffusion Processes
To analyze thermally activated diffusion at the film–substrate interface during the thermal treatment of garnets, a numerical solution is found for the one-dimensional classical equation of diffusion
where C(x,t) is the one-dimensional dynamic function of the spatial distribution of the concentration of a chemical element, Dx is the coefficient of diffusion in direction x, and φ(x,t) is the one-dimensional dynamic field of diffusion fluxes.
This equation is solved in a spatial–temporal discretization approximation (i.e., the change in the concentration of a chemical element ∂C in interval ∂x over period of time ∂t is found from difference between fluxes ∂φ at the boundaries of this interval (the flux gradient). This is done by creating a two-dimensional matrix of elements with dimension [k; 2k]:
where the columns define the partitioning of space into discrete intervals ∂x perpendicular to the plane of the interface, and the rows define the partition into unit time intervals ∂t.
The elements of matrix (2) contain the probability of finding a chemical element in a given interval of space at a given time. Columns from 0 to k correspond to the film, while those from k + 1 to 2k correspond to the substrate. The initial conditions in row 1 for the distribution of elements in the film and substrate are set at a conditional zero moment in time. The resulting profile can be stepwise or take another form. Columns 1 and 2k contain the boundary conditions (normally the maximum probability of an element being in the film or the substrate, respectively).
The rest of the matrix elements are filled according to the formula
where \(D_{x}^{'} = {{{{D}_{x}}} \mathord{\left/ {\vphantom {{{{D}_{x}}} {\partial x}}} \right. \kern-0em} {\partial x}}\) is the coefficient of diffusion in the direction perpendicular to the plane of the interface, normalized for the interval of spatial partitioning.
The elements of the matrix in each row i thus define the spatial distribution of the chemical element in space 0–2k through conditional time interval i − 1.
Other conditions are considered to analyze the process of ion-stimulated diffusion when film elements are sputtered with Ar+ ions:
(1) The coefficient of diffusion varies exponentially between layers, starting from the film’s surface:
where \(D_{{0x}}^{{\text{'}}}\) and λ are variable constants whose values are selected individually for each chemical element and determined by the energy of the bombarding ions and the spatial partitioning.
(2) An arbitrary profile, the stepwise distribution profile of a chemical element, or data obtained by calculating a profile under thermal diffusion can be used to establish the initial conditions.
(3) After each time interval, all boundary conditions are shifted to the right by one spatial interval, and all elements to the left are reset to zero (surface sputtering is modeled).
(4) The resulting profile is the array of extreme left non-zero values (on the surface of the film) obtained at each time interval can be used.
RESULTS AND DISCUSSION
Distribution Profile of Elements in a BiIG/GGG Film
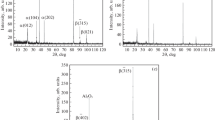
Figure 2 shows profiles of the distribution of different chemical elements over depth in the BiIG/GGG film immediately after deposition on the substrate at room temperature (Fig. 2a) and after 20 min of heat treatment at 700°C (Fig. 2b).
Several key points in Fig. 2 should be noted. A considerable change in the concentration of chemical elements is observed in two regions (highlighted in the figure by a dotted line). Region 1 is the outer surface of a garnet film and region 2 is the film–substrate interface. Analysis of the profiles in region 2 shows that the interface had some blurring and the distribution of elements over the depth had an asymmetric form (sharper on the side of the film, and flatter on the side of the substrate). Note that a symmetric distribution profile of the element formed upon thermally activated diffusion (with homogeneity of the temperature field). Asymmetry therefore cannot be a result of heat treatment; instead, it is a consequence of ion-stimulated (shock) diffusion caused by bombarding the film’s surface with high-energy Ar+ ions during sputtering, which is an essential part of using the GD Profiler 2 profilometer.
It should be noted that the contribution from thermal diffusion is minimal in Fig. 2a (deposition at room temperature without heat treatment), and the profile is mainly determined by the contribution from the mechanism of ion-stimulated diffusion. We can also see that Ga was detected in the volume of the film after heat treatment (Fig. 2b), although it was not in the composition of the target or the spectra of the film before heat treatment. A change in the content of Gd in the film relative to the substrate was also observed after annealing.
The strongest characteristic is the increased widths of the surface and interface regions after heat treatment, which is undoubtedly a consequence of thermally activated diffusion during annealing.
The change in the intensity of the oxygen line at the interface could not be due to a change in its content, since it is equal to 12 formula units in the garnet cell for both the film and the substrate. Such changes were obviously due to a change in the sputtering rate, which altered the intensity of the discharge glow. The lines of all elements were therefore first normalized to that of oxygen and then to their own maximum value when analyzing the data further.
Analyzing the Contribution from Different Diffusion Processes to the Distribution Profiles of Chemical Elements
Figure 3 shows results from analyzing the distribution profiles of individual chemical elements at the film–substrate interface before and after heat treatment. Dots correspond to experimental results, and the solid line represents results from analyzing the model. There is good agreement between the theory and experiments. The dotted line shows the initial conditions for calculating ion-stimulated diffusion (i.e., the true distribution profile of the elements). They take the form of a stepped profile immediately after deposition on a cold substrate or a blurred profile as a result of thermal diffusion during annealing. Table 1 gives the parameters used in the model analysis.
Analysis of the distribution profiles of chemical elements (indicated in the figures) at the film–substrate interface: (a), (c), (e), (g), and (i) before heat treatment; (b), (d), (f), (h), and (k) after heat treatment. The dots indicate experimental data; the solid line, analysis of the model; the dotted line, the true shape of the profile without considering ion-stimulated diffusion.
Figures 3a, 3c, 3e, 3g, and 3i show profiles before heat treatment. In our theoretical analysis, we ignored the contribution from thermal diffusion and considered the true distribution profile (initial conditions) of chemical elements to be stepwise. The boundary conditions were determined by the content of the element in the film and in the substrate, respectively. The distribution profile observed in the experiment was mainly due to ion-stimulated diffusion as a result of bombarding the surface with high-energy ions.
The distribution profile of the elements observed experimentally when studying the film before heat treatment was therefore mostly an artifact caused by the means of research, while the true shape of the profile differed greatly from the experimental results and was close to stepwise. However, analysis of the sample before annealing allowed us to estimate the parameters of ion-stimulated diffusion and consider them when analyzing the contribution from thermal diffusion after annealing.
Figures 3b, 3d, 3f, 3h, and 3k present the distribution profiles of the elements after heat treatment. The dotted line shows the shape of the profile formed by thermal diffusion without considering ion-stimulated diffusion. We can see that bombarding the surface with argon ions considerably distorted the distribution profile of elements in relation to the true profile.
We can therefore reconstruct the true distribution profile of elements without the influence of ion-stimulated diffusion by analyzing the contribution from different mechanisms of diffusion to the formation of the observed distribution profile of elements. Figure 4 compares the experimentally observed distribution profile of chemical elements (Fig. 4a) and the reconstructed true profile (Fig. 4b). The area of the interface is marked with a dotted line. We can see that the left edge of the area of the interface is the same in both figures. The right edge differs considerably, with the observed area of the interface being ~1.5 times wider than in the true profile (around 75 and 50 nm, respectively).
CONCLUSIONS
Deposited films of bismuth-substituted iron garnet were used to analyze the contribution from different mechanisms of diffusion to the distribution profile of chemical elements on a film–substrate interface while investigating it via optical emission spectroscopy of a glow discharge. We found that directed ionic-stimulated diffusion occurred when the surface was sputtered with high-energy Ar+ ions, which distorted the experimentally observed distribution profile of elements at the interface relative to the true profile. A procedure was proposed for a theoretical analysis of the profiles obtained in the experiment that allowed us to restore the true form of the profile without considering the effect of ion-stimulated diffusion as an artifact of the means of research. The experimentally observed profile for a BiIG/GGG film (100 ± 5 nm thick after 20 min of heat treatment at 700°С) showed an area of the film–substrate interface that was ~1.5 times wider than in the restored true profile.
REFERENCES
Alharbi, A., Boguslavskiy, A.E., Austin, D., et al., Sci. Rep., 2018, vol. 8, p. 3789.
Levinea, J., Savina, M.R., Stephan, T., et al., Int. J. Mass Spectrom. Ion Processes, 2009, vol. 288, p. 36.
Priamushko, T.S., Mikhaylov, A.A., Babikhina, M.N., et al., Metals, 2018, vol. 8, p. 372.
Moutarlier, V., Lakard, S., Patois, T., and Lakard, B., Thin Solid Films, 2014, vol. 550, p. 27.
Zaide, Z., Kaizhong, Z., Xiandeng, H., and Hong, L., Appl. Spectrosc. Rev., 2005, vol. 40, no. 2, p. 165.
Avsec, K., Jenko, M., Conradi, M., et al., Coatings, 2019, vol. 9, p. 868.
Ong, J.L. and Lucas, L.C., Biomaterials, 1998, vol. 19, nos. 4–5, p. 455.
Inamoto, Sh. and Otsuka, Yu., Microscopy, 2020, vol. 69, no. 1, p. 26.
Suzuki, S. and Kakita, K., J. Surf. Anal., 2005, vol. 12, p. 174.
Vontorova, J., Dobias, V., and Mohyla, P., Chem. Pap., 2017, vol. 71, p. 1507.
Smentkowski, V.S., Zorn, G., Misner, A., et al., J. Vac. Sci. Technol., A, 2013, vol. 31, no. 3, 030601.
Fedorenko, A.A., Berzhansky, V.N., Karavainikov, A.V., et al., Tech. Phys., 2021, vol. 66, no. 2, p. 343.
Levy, M., Borovkova, O.V., Sheidler, C., et al., Optica, 2019, vol. 6, no. 5, p. 642.
Suturin, S.M., Korovin, A.M., Bursian, V.E., et al., Phys. Rev. Mater., 2018, vol. 2, no. 10, 104404.
Funding
This work was supported by the Russian Science Foundation, project no. 19-72-20154.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by S. Rostovtseva
About this article
Cite this article
Tomilin, S.V., Fedorenko, A.A., Berzhansky, V.N. et al. Effect of Ion-Stimulated Diffusion on the Distribution Profile of Elements on a Film–Substrate Interface. Bull. Russ. Acad. Sci. Phys. 86, 546–551 (2022). https://doi.org/10.3103/S1062873822050276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1062873822050276