Abstract
The ion–molecular model of water, which assumes a high concentration of short-lived ions of Н3О+ and ОН– and their proton exchange with neutral Н2О molecules, indicates that water cohesion is achieved in the form of electrostatic interaction between oscillating ions and the molecular dipoles that surround them. It is shown that these oscillators with a frequency of 5 THz are responsible for the specific heat capacity of water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The atomic–molecular structure of liquid water has yet to be established with certainty, and is still actively discussed in the literature. It is generally accepted that water is an ensemble of indivisible Н2О molecules bound by a network of hydrogen bonds [1, 2]. All modern studies follow this concept [3]. The question of how translation of Н2О molecules occurs in a network of hydrogen bonds remains unresolved. This movement is responsible for the incredible variety of water’s properties (mechanical, thermal, and electrical) [4].
A system of hydrogen bonds is considered to be a specific feature of liquid water, and the reservoir of its internal energy. The energy of a single hydrogen bond is calculated by dividing the enthalpy of water’s evaporation (2240 kJ kg−1 at the boiling point) by the concentration of molecules (55.5 mol L−1) and four bonds per molecule. The result is 10 kJ (or 0.1 eV) per bond. It is believed the bonds are broken and restored in picosecond times, allowing molecules to rotate and translationally move at the moment of freedom [3]. Modern models of such dynamics are complex and specific. The search for a clear physical model remains relevant, as “Water’s structure and dynamics continue to defy a simple, consistent description” [4].
In [5], we described the dielectric spectra of liquid water using a model in which the concept of hydrogen bonding was not employed. We presented water as a dense gas of particles (molecules and ions) undergoing thermal collisions and exchanging protons (in other words, transforming each other). The model successfully described the electrodynamics of water with an unexpected condition: a high concentration of ions in water that is 7 orders of magnitude higher than the one commonly accepted (1.5 × 1027 m−3 instead of 6.6 × 1019 ions of both signs for pH 7).
A strong concentration of separated charges would mean liquid water has an electric field with high energy density that could serve as a cohesive factor (and actually be a material embodiment of a network of hydrogen bonds). In this work, we explore the possibility of describing the thermal properties of water (heat of vaporization and heat capacity) using a model based on the concepts of electrostatics and hydrodynamics.
MODEL
A model medium with the structure of liquid water is shown in Fig. 1. The molecules of Н2О with diameter d = 2.8 × 10−10 m, separated by one quarter of diameter δ = 0.7 × 10−10 m, are indicated by the circles [1, 3]. Such sets of molecules are usually shown entangled in a network of hydrogen bonds, with the circles connected by lines.
In our case, the molecules are free. The medium is a dense gas whose molecules undergo thermal collisional motion. We assume the expansion of the molecules is restrained by the Coulomb field of the Н3О+ and ОН− ions present in the medium (marked with positive and negative signs). The ions are H2O molecules that either lack a proton or have an excess one. A charged ion is the center of attraction and polarization for the surrounding neutral dipole H2O molecules. The medium appears to consist of ion-centered molecular clumps of n molecules.
Excess protons and holes exchange hosts in collisions. Charges pass from molecule to molecule, so that molecules and ions transform one another; i.e., each molecule periodically becomes a charged ion, and each ion becomes a neutral molecule. Charged centers wander randomly. The polarization cells surrounding the charges move with them (an ion-centered polarization configuration). Ions in the hydration shell move like large particles of variable composition.
We believe the motion of particles in water obeys the Brownian law of diffusion. This means the continuous renewal of the structure of molecules with step 𝓁 and ions with step L proceeds in the process of diffusion, where 𝓁 and L are the distances between the centers of molecules and ions, respectively. We assume the tight packing of the spheres is satisfied, so the concentration of molecules N0 of the medium is
The concentration is in turn considered as the ratio of the density ρ of water to its molecular mass \({{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\) = 3 × 10–26 kg: N0 = \({{\rho } \mathord{\left/ {\vphantom {{\rho } {{{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}}}} \right. \kern-0em} {{{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}}},\) where we consider the reference density ρ of liquid water [6] to be the basic characteristic of the model medium shown in Fig. 1, and quantities N0 and ℓ to be derived from it. At room temperature, ρ = 103 kg m−3, N0 = 3.3 × 1028 m−3, and 𝓁 = 3.5 × 10−10 m. The gap between the molecules in the first approximation is δ ~ 𝓁 − d ~ 7 Å, where d = 2.8 × 10−10 m is the diameter of an H2O molecule [1].
Local rearrangements occur in picoseconds. The time scale is determined by the thermal velocity of the molecule \({\upsilon } = \sqrt {{{3kT} \mathord{\left/ {\vphantom {{3kT} {{{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}}}} \right. \kern-0em} {{{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}}}} \) and, accordingly, by the time it spans the distance δ (t = δ/υ).
In dynamic behavior, the medium has the solid state property described by Frenkel [7]: in the process of diffuse thermal motion, an ion (a circle with plus or minus in Fig. 1) interrupts the translational movement for a while and oscillates inside the cell from polarized molecules (dipoles). Like any system of oscillating charges, such an oscillator emits/absorbs electromagnetic radiation. According to our assumption, this oscillatory motion manifests itself in the infrared absorption spectra in the form of a peak at a frequency of 5.3 THz [5].
In works that mention the 5 THz peak, it is noted as special: the peak weakly depends on temperature, disappears in a supercritical state, and cannot be described by molecular dynamics methods: “even polarizable models fail in reproducing this feature” [8]. It is believed that the peak reflects the dynamics of the stretching of hydrogen bonds, H-bond stretching [9].
The weak sensitivity of the peak to the isotope effect, i.e., the replacement of protons by heavier deuterons, is fundamentally important for us [10]. This fact suggests that heavy particles oscillate with a response of 5 THz, namely, oxygen atoms with an odd number of protons, i.e., ions.
COHESION AND HEAT CAPACITY
Figure 1 illustrates a characteristic molecular motif in the form of an ion surrounded by neutral H2O molecules (four such formations are located at the corners of the figure). The formations diffusely quickly move in the volume of the medium due to thermal motion. This fact suggests that, on average, dipole H2O molecules are constantly located in the field of attraction of ions. We express the energy of this attraction by the formula of the electrostatic ion-dipole interaction Eqp [11]
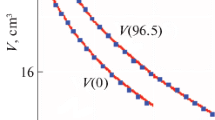
where q = 1.6 × 10–19 C is an elementary charge; p = 6.14 × 10–30 C m is a dipole moment of Н2О molecule; ε0 = 8.85 × 10–12 F m–1 is the dielectric constant of vacuum; ℓ is the distance between oxygen atoms (3.5 × 10–10 m at room temperature). The energy calculated using formula (2) is compared to the experimental enthalpy of water evaporation from the database in [12]. We find that the calculated curve in a wide range of temperatures lies in the experimental dependence (the lower curve in Fig. 2). We may therefore conclude that the ion–dipole interaction of mutually transforming ions and molecules is responsible for the retention of H2O molecules.
Enthalpy of evaporation H and specific heat capacities CP and CV of liquid water as a function of temperature. The dots represent experimental data from [12]; the grey lines, calculations using electrostatic formulas (4) and (10); the black lines, fitting with formulas 11a and 11b.
We consider obtained energy Н = N0Eqp to be convertable into the kinetic energy of microparticles constituting the medium as a result of thermal fluctuations. The oscillation of an ion with a frequency of 5 THz in a cell formed by surrounding centrally polarized H2O molecules is assumed to be the mechanism of conversion. The vibrational energy is expressed by the classical formula
where κ = \(m{\omega }_{0}^{2}\) is the elastic constant of the oscillator; m = 3 × 10−26 kg is the mass of an oxygen atom (in a molecule or an ion); ω0 = 2πν0, where ν0 = 5.3 is the frequency of the oscillator in THz; and δ is the amplitude of oscillations.
It is difficult to consider the operation of the oscillator, since the center of oscillations moves diffusely as a result of proton exchange between the ions and Н2О molecules (as a result of the transfer of protons from oxygen to oxygen) [5]. The polarization configuration of neutral H2O molecules follows the delayed movement of the center. The configuration is shifted by the redistribution of protons on the H2O molecules surrounding an ion. The described mechanism is obviously complex. We use two simplifications to continue our discussion: (a) We increase the number of degrees of freedom of the oscillator in formula (3) from the standard three to n; i.e., n is the number of particles involved in the operation of the oscillator. (b) The mutual transformation of molecules and ions is allowed for; i.e., energy H is distributed between oscillating ions and the dipole H2O molecules located in their fields, according to the principle of equal distribution of energy over degrees of freedom.
Based on the above, we consider energy H to be the sum of the energies of the oscillators and write it in two terms:
where Ni is a concentration of ions such that nNi = N0.
Assumption (b) means that each of the terms, is equal to total energy H when doubled. Formula (2) then forms a system of three equations, the solution to which provides the expressions for δ, n and Ni:
The magnitudes of the listed values for room temperature are shown in Table 1. Figure 3 is a graphic illustration of formula (4).
Bonding energy of an ensemble of mutually transforming charged and dipole particles, depending on the distance between the centers of the particles calculated using formula (4). The position of minimum and the value of energy in the minimum correspond to the parameters of liquid water with an accuracy of several percent.
According to the logic of the model, the second term in formula (4),
represents the energy of an oscillator responsible for the accumulation of heat. Q then has equal potential QP and kinetic QK components. Allowing for elastic constant κ = m(2πν0)2, vibration frequency ν0 = υ/δ, and thermal velocity υ = (3kBT/m)1/2 of a particle, the latter are reduced to
where α is a dimensionless fitting coefficient that we define below. In the chosen approach, the derivative of QK with respect to temperature is specific heat capacity

where the derivation with respect to T is indicated by the prime mark.
The two upper curves in Fig. 2 show the result from comparing calculations using formula (10) and the experimental findings from the database in [12]. The heat capacities of liquid water at constant pressure CP and constant volume СV are the same in the range of room temperature, and they are accurately conveyed by formula (10) with coefficient α close to unity (0.935). At elevated temperatures, experimental CP and СV diverge. This effect in formula (10) is contained in the specific temperature behavior of factor (αN0T)'. Formal fitting of α to experimental CP and СV yields
We do not consider the origin of the difference between СP and CV; instead, we focus on the coincidence between the calculations using (10) and the findings from experiments at room temperature.
RESULTS AND DISCUSSION
Figure 2 shows that the time-averaged energy of ion–molecular interactions in water is expressed by simple electrostatic formula (4). As a result of the mutual transformation of charged and dipole particles, this energy has a pronounced minimum that depends on the distance between molecules. The minimum parameters correspond exactly to the parameters of liquid water (enthalpy of evaporation H = 2.4 × 103 kJ kg−1 and distance 𝓁 = 3.5 × 10–10 m between the centers of molecules). In dynamics, this means molecules with dipole moment p that are trying to expand are held back by charge q, which pulls them together at stationary distance 𝓁.
Electrostatic model (4) yields a high concentration of separated charges (ions) at the greatest density: Ni ≈ 1.5 × 1027 m–3, exactly the one we obtained earlier in our model description of the dielectric characteristics of water [5]. At this concentration, the ions are separated by distances of L = 1 ± 0.05 nm. This would seem to be a fundamental parameter of the model that determines the behavior of water under the conditions of its spatial structuring (in porous media).
The mutual transformation of ions and molecules proceeds as a result of proton exchange during thermal ion–molecular collisions. The characteristic period of exchange is determined by thermal velocity υ = \(\sqrt {{{3kT} \mathord{\left/ {\vphantom {{3kT} {{{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}}}} \right. \kern-0em} {{{m}_{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}}}} \) of a particle and thus the mean free path: t = λυ = 0.7 × 10−10/(6.4 × 102) s ≈ 0.1 ps. If this is so, the collisional redistribution of protons on n = 24 molecules around an ion takes ~2.5 ps (the lifetime of the charge in the hydration cell). A similar time is found in modern models, but it has a different meaning; i.e., it is considered the characteristic time of the breaking and reforming of hydrogen bonds (the lifetime of a hydrogen bond) [3].
According to our model, heat Q is accumulated in liquid water via the energy pumping of an oscillator. The 24 molecules of H2O, each of which becomes an Н3О+ ion or an ОН– ion for 2.5 ps as a result of collisions and proton exchange, participate in the process. The efficiency of this mechanism is απ2 ≈ 3 times higher than that of the classical harmonic oscillator EOSC = 3kT.
There is another approach to describing the properties of liquids from the viewpoint of solid state physics: interpreting the heat capacity of liquids in terms of phonons [13]. The wandering oscillators considered in this work correspond to this approach, as they give macroelasticity to water. This problem would seem promising for theoretical analysis.
CONCLUSIONS
It was shown that the Coulomb field of charges (protons and proton holes) which moves in Brownian fashion over H2O molecules is a factor in the connecting of liquid water molecules within the ion–molecular model we proposed earlier. The model explains the stability of water as a system of oxygen and hydrogen atoms through the competition between two processes: the thermal scattering of Н2О molecules and their gathering through the Coulomb attraction of ions.
In this model, liquid water accumulates heat through ion oscillators with a frequency of 5 THz; these are groups of ~24 molecules of H2O gathered by the charge pulling them together with a localization time of 2.5 ps.
REFERENCES
Eisenberg, D. and Kauzman, W., The Structure and Properties of Water, Oxford: Clarendon, 1969.
Malenkov, G.G., J. Struct. Chem., 2006, vol. 47, p. S1.
Brini, E., Fennell, C.J., Fernandez-Serra, M., et al., Chem. Rev., 2017, vol. 117, p. 12385.
Henchman, R.H., J. Phys.: Condens. Matter, 2016, vol. 28, p. 384001.
Volkov, A.A., Artemov, V.G., Volkov, A.A., Jr., and Sysoev, N.N., J. Mol. Liq., 2017, vol. 248, p. 564.
Kell, G.S., J. Chem. Eng. Data, 1975, vol. 20, no. 1, p. 97.
Frenkel, J., Kinetic Theory of Liquids, Oxford Univ. Press, 1946.
Sega, M. and Schroder, C., J. Phys. Chem. A, 2015, vol. 119, no. 9, p. 1539.
Shi, L., Ni, Y., Drews, S.E.P., and Skinner, J.L., J. Chem. Phys., 2014, vol. 141, p. 084508.
Zelsmann, H.R., J. Mol. Struct., 1995, vol. 350, p. 95.
Landau, L.D. and Lifshitz, E.M., Elektrodinamika sploshnykh sred (Electrodynamics of Continuous Media), Moscow: Nauka, 1982, 2nd ed.
Rivkin, S.L. and Aleksandrov, A.A., Teplofizicheskie svoistva vody i vodyanogo para (Thermophysical Properties of Water and Water Vapor), Moscow: Energiya, 1980.
Bolmatov, D., Brazhkin, V.V., and Trachenko, K., Sci. Rep., 2012, vol. 2, p. 421.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Obrezanova
About this article
Cite this article
Volkov, A.A., Vasin, A.A. & Volkov, A.A. Cohesion and Heat Capacity of Liquid Water from the Viewpoint of an Electrostatic Model. Bull. Russ. Acad. Sci. Phys. 84, 48–52 (2020). https://doi.org/10.3103/S1062873820010281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1062873820010281