Abstract
Thermal transformations in calcium iodate were studied by differential scanning calorimetry in the temperature range of 300–973 K. The process was found to proceed by the scheme: Ca(IO3)2∙H2O → Ca(IO3)2 → Ca5(IO6)2 + I2 + 9O2. Calculated kinetic parameters adequately described experiment. X-ray diffraction patterns of the intermediate products formed at 523, 723, and 873 K well agreed with the available literature data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Oxygen compounds of iodine, iodic acids, metal iodates are strong oxidizers, which release iodine and oxygen during thermal decomposition. In view of this, these compounds find application in energetic thermite mixtures whose combustion products produce a biocidal effect on dangerous bacteria and their spores [1, 2]. To date, numerous studies have been focused on the behavior and combustion characteristics of such compounds [3–8]. Another urgent problem is the withdrawal from usage of perchlorate-containing pyrotechnic compositions because of their ecological incompatibility, in particular, it was proposed to use periodates instead of perchlorates in pyrotechnic compositions [9].
Calcium iodate Ca(IO3)2 is of especial interest since this salt is present in nature as lautarite mineral and hence is environment-friendly. Calcium iodate is fed to animals as food supplement E916. It is also added to cosmetic ointments and lotions as an antiseptic and deodorant. The advantages of Ca(IO3)2 over KClO4 as an oxidizing agent are as follows: (a) Ca(IO3)2 contains more oxygen than KClO4 and (b) the melting (decomposition) temperature of Ca(IO3)2 (813 K) is lower than that of KClO4 (855 K) only slightly.
As is known, decomposition of KClO4 in thermite reactions yields KCl that is rather stable at elevated temperatures. Products of Ca(IO3)2 decomposition contain CaI2 which must partially dissociate into iodine and CaI in the vapor phase at high temperatures. The latter ones are highly reactive and hence are capable of (a) deactivating dangerous bacteria and (b) accelerating chemical reactions due to more efficient gas-phase transfer of intermediates.
In this context, basic knowledge about the chemical transformations taking place during thermolysis of calcium iodate becomes of key importance. To date, thermal decomposition of KClO4 has been studied in detail [10, 11] while that of calcium iodate, inadequately, largely because of its complexity as is evidenced by the reactions of potassium iodate in aqueous solutions [12]. Synthesis and thermolysis of Ca(IO3)2⋅6H2O was reported in [13, 14]. It was found to involve the stages of dehydration and formation of a non-aqueous product, followed by decomposition with evolution of gaseous iodine and oxygen. Thermodynamic parameters of the process were calculated. The reaction rate was found to affect a type of product modification: α-Ca(IO3)2 or β-Ca(IO3)2; while ambient pressure, a kind of product: calcium oxide or calcium iodide.
Freshly prepared calcium iodate monohydrate was studied in [13, 14]. In this work, we explored the behavior of commercially available reagent that contained smaller (and unknown) amount of crystallization water (compared to Ca(IO3)2⋅H2O monohydrate). In addition, the commercial calcium iodate could undergo aging during its storage. To our knowledge, the thermolysis of aged calcium iodate has not been explored so far.
Here we report on (i) determining the amount of crystallization water in commercial calcium iodate and (ii) the processes occurring during thermal decomposition of the above calcium iodate and relevant kinetic parameters.
2 EXPERIMENTAL
Calcium iodate (from Almerdale Assets) was used as the object of our study. Its thermal decomposition was studied by differential scanning calorimetry (NETZSCH STA 409 PC/PG analyzer, under Ar). After holding samples at 523, 723, and 873 K for 1 h, their phase composition was determined with a RIKOR portable X-ray diffractometer (Cu-Kα radiation).
3 CALCULATING KINETIC PARAMETERS
Kinetic parameters for decomposition of calcium iodate were calculated using the algorithm described in [15]. The calculation procedure involved the following steps.
(1) Extent of conversion α is calculated for each temperature Ti using the values of initial sample mass m0, end sample mass me, and running sample mass mi at the ith point on a TG curve:
αi = (m0 – mi)/(m0 – me).
(2) Derivatives (dα/dT)I are numerically calculated for each Ti using the method of rectangles.
(3) Maximum values—Tmax, (dα/dT)max, and αmax—are obtained from the curve plotted at Step 2 or from the DTG, DTA, DSC curves. Reaction order n, used as an initial approximation, is obtained by interpolating the values presented in Table 1.
(4) The values of Yi and Xi (expressions (2) and (3), respectively) are calculated using Eq. (1) (taken from [15]):
where A is pre-exponential factor, β heating rate, E activation energy, and R universal gas constant.
For each temperature Ti within the temperature range of a given step, coefficients a and b in the equation Yi = aXi + b are obtained using the linear LS method to calculate parameters E = –aR and A = βexp b.
Correlation coefficient r for Yi and Xi is calculated as follows: (a) when r ≠ 1, n is changed and Step 4 is repeated until the absolute value of r approaches to unity as close as possible and (b) the values of n, E, and A obtained at the last iteration are taken as final.
All calculations were performed using MS Office Excel 365, Windows 10.
4 RESULTS AND DISCUSSION
The thermograms of calcium iodate decomposition are shown in Fig. 1. The process is seen to have three endothermic stages. The first one (413–493 K) is a decrease in sample mass; the second one peaked at 683 K proceeds without mass change; and the third one is a two-step process accompanied by significant mass loss.
As follows from Fig. 2a, starting calcium iodate represented a mixture of two phases, Ca(IO3)2⋅H2O and Ca(IO3)2. Our calculations based on the TG curve have shown (see Table 2) that these are present in the amounts of 44 and 56 wt %, respectively. After holding at 523 K for 1 h, the sample contained only the Ca(IO3)2 phase (see Fig. 2b), which is indicative of complete water removal.
Figure 3 shows processed TG data for the first stage of calcium iodate decomposition. Figure 4 presents these data linearized according to Eq. (2) while Table 3, respective kinetic parameters. Curve 3 in Fig. 3 shows the derivative dα/dT plotted using Eq. (4) and calculated kinetic parameters.
Linearized Eq. (1) for the first stage of calcium iodate decomposition; Y is given by formula (2).
Curve 3 satisfactorily describes experiment only up to 470 K. The deviation can be explained by the involvement of water vapor diffusion through the intergranular space.
Note that our values of activation energy E, reaction order n, and pre-exponential factor A are much higher than those for the dehydration of related compounds such as Li2O2⋅H2O, CaC2O4⋅H2O, and NiC2O4⋅2H2O [16]. Nevertheless, the fact that absolute correlation coefficient r is close to unity allows us to consider our results close to true ones. According to the theory of topochemical reactions, n = 1.4 means that a given reaction proceeds in a kinetic mode [17]. So, the first stage of our process can be described as the decomposition of Ca(IO3)2⋅H2O crystals followed by the diffusion of water vapor over the intergranular space.
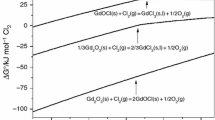
The endothermic process around 690 K is not accompanied by mass change. According to [13], this is the α → β phase transition in calcium iodate. However, after isothermal annealing, no β-Ca(IO3)2 was detected by XRD method. Apparently, this can be due to the reversibility of this phase transition. An endothermic process in the range 770–920 K is accompanied by mass loss (cf. Fig. 1). As can be deduced from Fig. 5, this is the conversion of Ca(IO3)2 iodate into Ca5(IO6)2 periodate. The processed data of the TG curve for this stage are given in Fig. 6. Calculated curve 3 (based on kinetic parameters from Table 1) is seen to comply with experiment (data points 2). However, the presence of an inflection point on the DTG and DSC curves (see Fig. 1) suggests that the process is two-stage. Therefore, the kinetic parameters, calculated for this process as a one-stage one, are formal. Nevertheless, since the absolute correlation coefficient of the model is close to unity, it can be recognized that the kinetic constants adequately describe the process (see Fig. 7). The DTG and DSC curves show that temperature ranges in which these two processes proceed are very close and mutually overlapped. It is difficult to discern these processes in this experiment.
Linearized kinetic Eq. (1) for the third stage of calcium iodate decomposition; Y is defined by formula (2).
According to [18], the decomposition of Ca(IO3)2 includes two stages:
Reaction (a) starts around 823 K and ends near 1043 K. However, the reaction is reversible and is the sum of reactions (c)–(e):
Thus, the two-stage character of our process can be associated with the occurrence of reactions (c)–(e). It is obvious that the process under study must be pressure-dependent.
5 CONCLUSIONS
Thermolysis of commercial calcium iodate—comprising of 44% Ca(IO3)2⋅H2O and 56% Ca(IO3)2 (by weight)—proceeds within the temperature range 300–973 K by the following scheme: (i) dehydration of calcium iodate (413–513 K) and (ii) two-stage decomposition reaction Ca(IO3)2 → 0.2Ca5(IO6)2 + 0.2I2 + 1.8O2 (773–973 K). The calculated kinetic parameters adequately describe experiment. The XRD results for thermolysis products formed (in air) at 523, 723, and 873 K are in good agreement with the literature data.
REFERENCES
Sullivan, K.T., Piekiel, N.W., Chowdhury, S., Wu, C., Zachariah, M.R., and Johnson, C.E., Ignition and combustion characteristics of nanoscale Al/AgIO3: A potential energetic biocidal system, Combust. Sci. Technol., 2011, vol. 183, pp. 285–302. https://doi.org/10.1080/00102202.2010.496378
Wang, H., Jian, G., Zhou, W., De Lisio, J.B., Lee, V.T., and Zachariah, M.R., Metal iodate-based energetic composites and their combustion and biocidal performance, ACS Appl. Mater. Interfaces, 2015, vol. 7, pp. 17363−17370. https://doi.org/10.1021/acsami.5b04589
Guerrero, S.E., Dreizin, E.L., and Shafirovich, E., Combustion of thermite mixtures based on mechanically alloyed aluminum–iodine material, Combust. Flame, 2016, vol. 164, pp. 164–166. https://doi.org/10.1016/j.combustflame.2015.11.014
Wang, S., Liu, X., Schoenitz, M., and Dreizin, E.L., Nanocomposite thermites with calcium iodate oxidizer, Propellants Explos. Pyrotech., 2017, vol. 42, no. 3, pp. 284–292. https://doi.org/10.1002/prep.201600213
Wang, H., Kline, D.J., Rehwoldt, M., and Zachariah, M.R., Ignition and combustion characterization of Ca(IO3)2 based pyrotechnic composites with B, Al, and Ti, Propellants Explos. Pyrotech., 2018, vol. 43, no 10, pp. 977–985. https://doi.org/10.1002/prep.201800041
Wang, S., Schoenitz, M., Grinshpun, S.A., Yermakov, M., and Dreizin, E.L., Biocidal effectiveness of combustion products of iodine-bearing reactive materials against aerosolized bacterial spores, J. Aerosol Sci., 2018, vol. 116, pp. 106–115. https://doi.org/10.1016/j.jaerosci.2017.11.007
Wu, T., Wang, X., Zavalij, P.Y., DeLisio, J.B., Wang, H., and Zachariah, M.R., Performance of iodine oxides/iodic acids as oxidizers in thermite systems, Combust. Flame, 2018, vol. 191, pp. 335–342. https://doi.org/10.1016/j.combustflame.2018.01.017
Liu, X., Schoenitz, M., and Dreizin, E.L., Preparation, ignition, and combustion of magnesium–calcium iodate reactive nano-composite powders, Chem. Eng. J., 2019, vol. 359, pp. 955–962. https://doi.org/10.1016/j.cej.2018.11.091
Brusnahan, J.S., Shaw, A.P., Moretti, J.D., and Eck, W.S., Periodates as potential replacements for perchlorates in pyrotechnic compositions, Propellants Explos. Pyrotech., 2017, vol. 42, no. 1, pp. 62–70. https://doi.org/10.1002/prep.201600084
Manelis, G.B., Nazin, G.M., Rubtsov, Yu.I., and Strunin, V.A., Thermal Decomposition and Combustion of Explosives and Propellants, New York: Taylor and Francis, 2003. https://doi.org/10.1201/9781482288261
Gabdrakhmanov, R.N., Ketov, A.A., and Korzanov, V.S., Study of the thermal behavior of potassium chlorate, bromate and iodate for the production of potassium perchlorate, perbromate and periodate, Nauchn.-Tekh. Vestn. Povolzh’ya, 2012, no. 4, pp. 75–81. https://www.elibrary.ru/item.asp?id=17888460.
Gabdrakhmanov, R.N., Ketov, A.A., and Korzanov, V.S., Investigation of the thermal behavior of potassium iodate and potassium periodate, Vestn. Permsk. Univ., 2013, no. 2 (10), pp. 82–85. https://www.elibrary.ru/item.asp?id=20289864.
Maneva, M., and Koleva, V., Thermal and calorimetric studies of M(IO3)2·6H2O and M(IO3)2·6D2O for M2+ = Ca2+ and Sr2+, J. Therm. Anal., 1992, vol. 38, pp. 2491–2499. https://doi.org/10.1007/bf01974627
Shitole, S.J., Synthesis and characterization of calcium iodate monohydrate crystals grown in silica gel, J. Phys.: Conf. Ser., 2013, vol. 423, 012060. https://doi.org/10.1088/1742-6596/423/1/012060
Kramarenko, V.Yu., Nonisothermal kinetics in the thermal analysis of polymers: 1. Simple reactions, Bull. Nat. Tech. Univ. KhPI, 2013, no. 64 (1037), pp. 64–75. http://repository.kpi.kharkov.ua/bitstream/KhPI-Press/ 5880/1/vestnik_HPI_2013_64_Kramarenko_Neizotermicheskaya.pdf.
Ferapontov, Yu.A., Putin, S.B., Ferapontova, L.L., and Putin, P.Yu., Studying the kinetics of topochemical processes in the nonisothermal mode by the derivatographic method, Bull. Tambov State Tech. Univ., 2009, vol. 15, no. 4, pp. 826–835.
Prodan, E.A., Pavlyuchenko, M.M., and Prodan, S.A., Zakonomernosti topokhimicheskikh reaktsii (Principles of Topochemical Reactions), Minsk: Nauka i Tekhnika, 1976. https://www.twirpx.org/file/2606170/.
Stern, K.H., High Temperature Properties and Thermal Decomposition of Inorganic Salts with Oxyanions, Boca Raton: CRC, 2001, Ch. 9. https://doi.org/10.1201/9781420042344
ACKNOWLEDGMENTS
This work was performed using the set of modern scientific instruments available for multiple accesses at the Tomsk Common Use Center.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 18-03-00875a).
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
About this article
Cite this article
Maksimov, Y.M., Shulpekov, A.M., Avramchik, A.N. et al. Thermolysis of Calcium Iodate: Kinetic Parameters. Int. J Self-Propag. High-Temp. Synth. 30, 40–46 (2021). https://doi.org/10.3103/S1061386221010076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386221010076