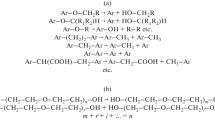Abstract
The colloid-chemical properties of humic acids (HAs) were studied depending on the mechanochemical modification of their structure. An increase in the amount of acidic ionogenic groups and hydrophilic fragments in the composition of modified HAs facilitated a decrease in the excess energy of surface molecules. In an alkaline solution, two types of colloids characterized by a decrease in the energy of adsorption and the constant of adsorption equilibrium were formed as the concentration of HAs was increased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Humic acids (HAs) are natural organic colloids whose structures consist of an aromatic skeleton substituted with oxygen-containing and nitrogen-containing groups and also include polysaccharide and peptide fragments. HA macromolecules have complexing, adsorption, solubilizing, and detoxifying properties, which allow them to play the role of a natural filter in the environment [1, 2]. The ability of HAs to bind toxic substances (pesticides, PAHs, and heavy metals) is due to the structural features of natural colloids [3–7]. The surfactant properties of HAs are determined by the presence of a hydrophobic aromatic skeleton, aliphatic chains, and hydrophilic polysaccharide and peptide fragments and various functional groups, which allow them to enter into ionic, donor–acceptor, and hydrophobic interactions. The dependence of the surface activity and sorption and solubilizing properties of HAs on their structure, dissociation of acidic groups, concentration, pH of aqueous solution, and the nature of the counterion was reported in the literature [8–13].
Due to the complex composition of macromolecules and their stochastic nature, an important problem is to establish the relationship between the surface-active properties of HAs with their structure and state of aggregation. A structural modification of the macromolecule and an assessment of the hydrophobic–hydrophilic balance of the molecular ensemble are required for solving this problem. The mechanical activation (MA) of solid caustobioliths is a method for such modification of the structure of natural HA colloids.
The mechanochemical effect consists not only in an increase in the effective surface area of mixture components and a decrease in diffusion hindrances but also in the chemical transformation of target substances [14, 15]. Skripkina et al. [16] showed the prospects of the mechanochemical treatment of brown coal in the presence of oxidizing alkaline reagents aimed at modifying the structure of HAs in order to increase the amount of phenolic and carboxyl groups.
The purpose of this work was to study the surface-active properties of humic acids isolated from brown coals mechanically activated under oxidizing alkaline conditions.
EXPERIMENTAL
The test materials were humic acids isolated from oxidized brown coal (OBC) from the Chui-Kenul deposit (China) and brown coal (BC) from the Ching-Chai deposit (China), which were characterized by ash contents of 16.7 and 29.9 wt % and moisture contents of 16.8 and 6.7 wt %, respectively.
Coal was preliminarily crushed in a Nossen 8255 disintegrator to a particle size of 1–3 mm (rotational speed of the grinding parts was 3000 rpm). Mechanical treatment (MT) of coal was carried out in an AGO-2 planetary mill in the presence of 8 wt % NaOH (OBC1) and an oxidizing alkaline complex containing 5% NaOH, 2% Na4P2O7, and 3% Na2CO3 ∙ 1.5 ∙ H2O2 (OBC2 and BC2) in the following mode: drum speed, 1820 rpm–1; centrifugal acceleration, 600 m/s2; and time, 2 min. Grinding bodies were steel balls with a diameter of 8–10 mm.
The concentration of acidic ionogenic groups was determined by back potentiometric titration on an Anion 4100 laboratory pH meter (Russia). During the titration of HAs, the ionic strength of solution was maintained at a constant level with a saturated solution of sodium chloride. Three clear inflections were distinguished in the obtained titration curves in ranges of pH 10–11 (phenolic hydroxyls of CArOH), pH 6.5–9.5 (carboxyl groups at the aromatic ring CArCOOH), and pH 2.5–6.5 (carboxyl groups at hydrocarbon chains CAlkCOOH). The equivalence point was calculated using numerical interpolation.
The study of the structural group composition was carried out on a Bruker Avance 400 NMR spectrometer with an operating frequency of 100 MHz for 13С nuclei. A weighed portion of HAs was dissolved in NaOD/D2O.
The surface tension of the solutions was measured on a K20 digital tensiometer (EasyDyne) by the Du Nouy ring method at a temperature of 24°C. The accuracy of measuring the surface tension of solutions was 0.08%. Ionic strength was maintained by adding a 2 M solution of KCl. Working solutions with concentrations from 0.01 to 2.0 g/L were obtained by dissolving HA samples in sodium hydroxide (pH 8).
The critical micelle concentration (CMC) was determined from the inflection point of the curve σ = f(log C) [11].
The standard Gibbs energy ∆\(G_{{\text{M}}}^{0}\) per mole of functional group was calculated using the formula
where CCMC is the concentration of functional groups at the point of critical concentration; R is the universal gas constant (8.314 J mol–1 K–1); and T is temperature, K.
The limiting adsorption at the gas–liquid interface Γmax was determined by plotting the graphic dependence С/Γ = f(С). Adsorption equilibrium constants (Ka, L/g) were calculated based on the experimental isotherms of monomolecular adsorption of HA samples [2, 18].
The surface area S per molecule in the adsorption layer was determined from the formula
where Na is the Avogadro number (6.02 × 1023 mol–1).
The thickness of the adsorption layer δ was calculated using the formula
The free energy of adsorption –ΔGads, kJ/mol, was determined by the formula
where ∆σ = σw – σCMC is the efficiency of reducing surface tension;
σw is the surface tension of water; and
σCMC is the surface tension at CMC.
RESULTS AND DISCUSSION
Humic acids in aqueous solutions can exist in different forms—individual macromolecules, associates, supramolecular structures, and micelles—depending on the pH and concentration of solution [8, 17]. HA associates at pre-micellar concentrations above 5 mg/dm3 are classified as supramolecular structures [18, 19]. The behavior of humic colloids in aqueous solutions depending on the concentration and structure of molecular ensembles has been the subject of a small number of studies, although it determines many processes in ecosystems [8, 18, 19].
The colloidal properties of HAs determine their tendency to concentrate on the water–air interface and, at the same time, orient themselves: the nonpolar part is directed toward the nonpolar phase, and the polar part is directed into water. As a result, the difference between the polarities of the phases decreases; according to the Rehbinder rule, this decreases the surface tension σ. The resulting force directed into the phase decreases due to the increase in the interaction of HAs with water, which leads to a decrease in the excess energy of surface molecules.
The experimental surface tension isotherms shown in Fig. 1 illustrate the dependence on structural changes in HA macromolecules after the MA of coals. For HAs isolated from OBC and BC, the surface tension isotherms were identical (Fig. 1a). A decrease in the value of σ from 70 × 10–3 to 56 × 10–3 kJ/m2 indicated a low surface activity of HAs. At a certain value of the critical HA concentration of 0.17–0.2 g/dm3 in an alkaline solution, colloids of a certain type were formed from HA macromolecules that do not have surface activity. A further increase in the concentration of HAs in the solution did not lead to a decrease in σ at the phase boundary, the values of which remained constant.
Figure 1b shows the σ isotherms of aqueous solutions of HAs isolated from OBC and BC after MA with 8% NaOH and a complex of reagents. The curves of the dependence σ = f(C) exhibited two steps characterized by different values of CMC. If the system contained several surfactants that differed sharply in surface activity, the surface tension isotherm had a stepped shape in some cases, which was explained by the presence of two types of colloids and two values of CMC.
The Gibbs free energy ΔGº and the free energy of adsorption ΔGads per mole of functional groups were calculated after the mathematical processing of experimental σ isotherms of HAs taking into account the functional groups involved in the formation of CMC (Table 1).
The critical concentrations for mechanically treated HA1OBC, HA2OBC, HA1BC, and HA2BC increased by factors of 1.4–1.9 and 7–9 at the first and second stages, respectively, as compared to the CMCs of the initial HAs. The processes of formation of first type colloids in mechanically processed and original HAs were characterized by similar values of the Gibbs energy. The second type of colloids was formed at higher CMC values and lower Gibbs and adsorption energies. A decrease in the adsorption energy was observed in mechanically processed HA samples, as compared to that of the initial HA. The adsorption energy was higher than the Gibbs energy for all of the HA samples, and this fact indicated the predominance of the adsorption process.
Table 2 shows the adsorption parameters of HAs depending on the conditions of mechanical processing. The maximum adsorption of HAOBC and HABC in monomolecular layers per elementary chain unit, which was 0.3 × 10–5 and 0.2 × 10–5 g/m2, respectively, was achieved at solution concentrations of 0.20 and 0.17 g/dm3, respectively. The formation of first type colloids in HA1OBC, HA2OBC, HA1BC, and HA2BC was characterized by a decrease in the value of adsorption Γmax to 0.1 × 10–5 g/m2 in the above concentration ranges. The maximum adsorption of colloids of the second type in mechanically treated HAs reached values similar to those of the HAOBC and HABC samples but at concentrations of 1.05–1.32 g/dm3 in solution.
The area S occupied by a segment of HA1OBC, HA2OBC, HA1BC, and HA2BC molecules at the first stage of colloid formation increased by a factor of 3, as compared with those of HAOBC and HABC. This can be due to an increase in the size of a structural unit due to the replacement of hydrogen atoms by carboxyl and hydroxyl groups, which is in good agreement with the results of the determination of functional groups.
Table 3 shows the concentrations of acidic ionogenic groups in HAs. Carboxyl groups in humic acids isolated from untreated coals partly occurred in the form of stable complexes, which did not completely dissociate in aqueous solution and did not react with sodium hydroxide to exchange for hydrogen ions.
Mechanochemical treatment in the presence of acid–base and redox reagents led to a change in the yield and quality characteristics of the main components of coals. The concentration of hydroxyl and carboxyl groups in humic acids isolated from coals after MA with 8 wt % NaOH and a complex of oxidizing alkaline reagents increased (Table 3).
An increase in the number of polar functional groups promoted the horizontal arrangement of molecules thereby reducing their concentration in the surface layer (Table 2). Because of this, the calculated layer thickness δ for mechanically treated HA samples at the first stage decreased by a factor of 2–3.5.
The close values of the area S occupied by a segment of molecules of mechanically treated HA samples at the second stage of σ isotherms, and those of HAOBC and HABC indicated a decrease in the role of functional groups compared to that at the first stage of colloid formation. As a result, the thickness of the adsorption layer increased (Table 2).
Higher values of the adsorption equilibrium constants for HA molecules in mechanically treated samples were observed at the first step of the σ isotherms, but they were lower by a factor of 1.5–2.0 than those for HAOBC and HABC due to structural differences as a result of mechanochemical modification. The characteristics of the structure of all HA samples were given on the basis of the results of 13C NMR spectroscopy (Table 4). To assess the hydrophobic–hydrophilic balance of the molecular ensemble of HAs, we used the ratio of the concentration of hydrophobic aromatic and aliphatic fragments Ʃ(СAr + СArО + СAlk) to the concentration of hydrophilic fragments Ʃ(CCOOH + СAlkО), the composition of which is dominated by carbohydrates and peptides. The hydrophobic component of the mechanically activated HA molecules decreased in comparison with that of HAOBC and HABC, and this led to a decrease in the Gibbs energy, adsorption energy, and adsorption equilibrium constants.
CONCLUSIONS
It should be noted that humic acids isolated from untreated coals in an alkaline solution form associates with a minimum average particle radius and an increase in the dissociation of acid groups and surface activity at the gas–liquid interface. The resulting colloids are characterized by higher values of adsorption energy and adsorption equilibrium constants in comparison with mechanically processed samples of HAs.
Upon the structural mechanochemical modification of humic acids, the amount of acidic ionogenic groups and hydrophilic fragments, the composition of which is dominated by carbohydrates and peptides, increases; the hydrophobicity of molecules and the resulting force directed inside the phase decreases to facilitate a decrease in the excess energy of surface molecules. With an increase in the concentration of HAs in the solution, the second type of colloids is formed, which are characterized by a decrease in the Gibbs energy, adsorption energy, and adsorption equilibrium constant compared to those of the initial HAs.
REFERENCES
Dmitrieva, E., Efimova, E., Siundiukova, K., and Perelomov, L., Environ. Chem. Lett., 2015, vol. 13, p. 197. https://doi.org/10.1007/s10311-015-0497-3
Gamboa, C. and Olea, A.F., Colloids Surf., A, 2006, vol. 278, p. 241. https://doi.org/10.1016/j.colsurfa.2005.12.026
Mal’tseva, E.V., Filatov, D.A., Yudina, N.V., and Chaikovskaya, O.N., Solid Fuel Chem., 2011, vol. 45, p. 62. https://doi.org/10.3103/S0361521911010071
Perminova, I.V., Grechishcheva, N.Yu., Kovalevskii, D.V., Kudryavtseva, V., Petrosyan, V.S., and Matorin, D.N., Environ. Sci. Technol., 2001, vol. 35, p. 3841. https://doi.org/10.1021/es001699b
Wang, H.B. and Zhang, Y.J., J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2014, vol. 49, p. 78.
Plaza, C., Brunetti, G., Senesi, N., and Polo, A., Anal. Bioanal. Chem., 2007, vol. 386, p. 2133. https://doi.org/10.1007/s00216-006-0844-0
Pankratov, D.A., Anuchina, M.M., Konstantinov, A.I., and Perminova, I.V., Russ. J. Phys. Chem. A, 2019, vol. 93, no. 7, p. 1235. https://doi.org/10.1134/S0036024419070203
Tarasevich, Yu.I., Dolenko, S.A., Trifonova, M.Yu., and Alekseenko, E.Yu., Colloid J., 2013, vol. 75, no. 2, p. 207. https://doi.org/10.1134/S1061933X13020166
Ryabova, I.N., Mustafina, G.A., Akulova, Z.G., and Satymbaeva, A.S., Colloid J., 2009, vol. 71, no. 5, p. 729. https://doi.org/10.1134/S1061933X09050226
Pankratov, D.A., Anuchina, M.M., Borisova, E.M., Volikov, A.B., Konstantinov, A.I., and Perminova, I.V., Russ. J. Phys. Chem. A, 2017, vol. 91, no. 6, p. 1109. https://doi.org/10.7868/S0044453717060206
Terashima, M., Fukushima, M., and Tanaka, S., Colloids Surf., A, 2004, vol. 247, p. 77. https://doi.org/10.1016/j.colsurfa.2017.02.075
El-Bayaa, A.A. and Al-Amir Asmaa, Al-Azhar Bull. Sci., 2017, vol. 28, no. 3, p. 17. https://doi.org/10.21608/ABSB.2017.8174
Linkevich, E.V., Yudina, N.V., and Savel’eva, A.V., Russ. J. Phys. Chem. A, 2020, vol. 94, no. 4, p. 742. https://doi.org/10.1134/S0036024420040093
Mal’tseva, E.V., Shekhovtsova, N.S., Shilyaeva, L.P., and Yudina, N.V., Russ. J. Phys. Chem. A, 2017, vol. 91, no. 7, p .1273. https://doi.org/10.1134/S0036024417070214
Skripkina, T.S., Bychkov, A.L., Tikhova, V.D., and Lomovskii, O.I., Solid Fuel Chem., 2018, vol. 52, no. 6, p. 356. https://doi.org/10.3103/S0361521918060101
Skripkina, T.S., Bychkov, A.L., Tikhova, V.D., Smolyakov, B.S., and Lomovsky, O.I., Environ. Technol. Innovation, 2018, vol. 11, p. 74. https://doi.org/10.1016/j.eti.2018.04.010
Gerke, J., Agronomy, 2018, vol. 8, no. 5, p. 76. https://doi.org/10.3390/agronomy8050076
Lavrik, N.L. and Mulloev, N.U., Khim. Interesakh Ustoich. Razvit., 2006, no. 4, p. 379.
Sutton, R. and Sposito, G., Environ. Technol. Innovation, 2005, vol. 39, no. 23, p. 9009. https://doi.org/10.1021/es050778q
Funding
This work was funded by the Ministry of Science and Higher Education of the Russian Federation and carried out within the framework of a state contract of the Institute of Petroleum Chemistry, Siberian Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
About this article
Cite this article
Yudina, N.V., Savel’eva, A.V. Influence of the Mechanochemical Modification of Humic Acids on the Formation of Colloids in Solution. Solid Fuel Chem. 57, 314–318 (2023). https://doi.org/10.3103/S0361521923050075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521923050075