Abstract
The structure of Podocotyle atomon metacercariae cysts and their surrounding capsules in the second intermediate hosts Locustogammarus locustoides (Brandt, 1851) and Spinulogammarus ochotensis (Brandt, 1851) was assessed using light and transmission electron microscopy. A single, amorphous layer represents the cyst formed by the parasite. The capsules formed by the host consist of two layers: an electron-light, loose inner layer, and an electron-dark, unstructured outer layer, including a small amount of intact and fragmented host cells. The inner capsule layer may have been formed by the degeneration and transformation of the muscle tissue of amphipods surrounding the larva. The outer layer of the capsule contained deposits of pigment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Trematodies Podocotyle atomon is a widespread parasite of the intestines of marine fish. The second intermediate hosts of these trematodes are various crustaceans; in particular, their metacercariae were revealed in amphipods of at least ten species in the basin of the Sea of Okhotsk [1].
When describing the total preparations of P. atomon metacercariae, it was noted that these parasites are enclosed in two easily detachable envelopes in the body of crustaceans: an inner transparent cyst (formed by the parasite) and an outer-pigmented capsule (formed by the host) [1]. There is no information about the internal structure of the cyst, but there are conflicting data on the capsule. According to Uspenskaya [2], based on the data of light-microscopic study, the capsule consists of several layers of brown pigment and it does not contain cellular elements. In an experiment on infection of various amphipod species, James [3] noted that encystic metacercariae are covered with a thick fibrous envelope. Køie [4] in an experiment with amphipods Gammarus sp., using the scanning electron microscopy method, concluded that the capsule is modified muscle tissue of a crustacean. The aim of this study was the investigation of the structure of the cyst and capsule of metacercariae of P. atomon using light and transmission electron microscopy.
MATERIALS AND METHODS
Metacercariae of P. atomon were sampled from L. locustoides (Brandt, 1851) and S. ochotensis amphipods (Brandt, 1851) collected in the coastal areas of the Nagaev Bay, Sea of Okhotsk. The larvae surrounded by a cyst and a capsule were fixed and embedded in Araldite-Epon in accordance with the standard electron microscopic technique. Semithin sections (1–2 μm) were stained with a mixture of methylene blue and crystal violet and examined in an Olympus CX 41 light microscope. Ultrathin sections were examined in a JEM-1400 PLUS transmission electron microscope (JEOL, Japan).
RESULTS AND DISCUSSION
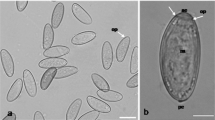
In total 2237 amphipods (853 S. ochotensis and 1384 L. locustoides) were studied, out of which 422 and 466, respectively, were infected. Metacercariae were rarely located in the muscles of the extremities; they were revealed more often in the hemocoel, from which they were freely released during the dissection of the crustacean (Fig. 1a).
(a) Spinulogammarus ochotensis, through the integument of which the metacercariae of Podocotyle atomon, enclosed in pigmented capsules, are visible (indicated by arrows). (b, c) Encapsulated metacercariae isolated from the hemocoel of an amphipod. Arrows show (b) larvae in transparent cysts extracted from capsules, (c) metacercaria, enclosed in a light capsule. (d) Semithin section of the encapsulated metacercaria. The cyst adheres tightly to the larva; therefore, it is not visible in the picture. (e) Ultrathin section of the surface of the metacercaria and surrounding cysts and capsules. ILC—inner layer of the capsule; Ca—capsule; M—metacercaria; OLC—outer layer of the capsule; Cy—cyst. Ruler: (b, c) 1 mm, (d) 50 μm, (e) 10 μm.
Capsule morphology. The capsules with the enclosed metacercariae had an elongated (less often rounded) shape. The number of capsules found in one crustacean varied from one to seventeen. Their color varied from light brown to dark brown, sometimes black (Fig. 1b), but the capsules were light in some cases (Fig. 1c). In one crustacean, capsules of the same type and simultaneously light and colored capsules were found. The length of the elongated capsules was approximately 0.5‒1.0 mm, and the width was 0.2‒0.4 mm, the diameter of the rounded capsules varied from 0.2 to 0.4 mm. In elongated capsules, helminths were located both in the center and closer to one of the edges (Fig. 1d). One capsule usually contained one encysted metacercaria, but 2–4 larvae were often revealed in a common capsule, each larva was enclosed in its own cyst.
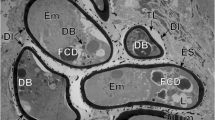
The structure of the capsule wall from both studied amphipod species was similar. In its composition, two distinct layers were visible on semithin sections: (1) the inner layer was loose, light-colored and (2) the outer one was destructed, dark (Figs. 1d, 2). The basis of the inner layer of the capsule was composed by large, elongated strands, from either structure-less, sometimes-fine fibrillar material with low electron density, separated by lighter narrow areas (Fig. 3a). In the structure of this layer, small membrane-like formations and electron-dense bodies resembling lipid droplets were rarely found. The outer layer of the capsule consists of an electron-dense amorphic mass, resembling deposits of pigment (melanin) (Fig. 3b). On some preparations, this layer was thin and weakly expressed (Fig. 1d), while it comprised more than half of the capsule’s wall thickness on others (Fig. 2). It can be assumed that the thickness of the outer layer of the capsule is related to the duration of its formation. If this assumption is correct, then the light capsule with metacercaria is probably at an early stage of the formation of its outer layer, and the pigment is just beginning to be deposited. Fragments of cells were found in the pigment accumulations of the outer layer (Fig. 3b). In some capsules, a small amount of pigment was found inside the contents of the inner layer of the capsule. Small groups of cells with expressed signs of destruction were occasionally found on the periphery of the capsule (Fig. 3d). In these cells, nuclei with large accumulations of heterochromatin along the inner membrane, as well as dense phagolysosomes and membrane bodies, were identified.
Capsule formation is considered the most important adaptation of tissue parasites to the immune response of the host. Unlike other species of opecoelid metacercariae, for example, P. reflexa [5] and Allopodocotyle lepomis [6, 7], whose capsule consists of several layers of host cells, metacercariae of P. atomon are surrounded by a capsule mainly of noncellular material. An electron-light, fine-fibrous material forms the inner layer of the capsule, and the outer one consists of an electron-dense substance resembling melanin, with the inclusion of host cells and/or their fragments. According to Køie [4], metacercariae of P. atomon are localized in the muscles of the dorsal part of amphipods and are encapsulated by material formed by degeneration and transformation of the muscle tissue of the invertebrate adjacent to the parasite. In the future, this area of muscle tissue, together with the larva inside it, is released into the hemocoel. Other species of parasites can cause structural changes, including degenerative changes, in invertebrate muscle cells. For example, in the muscle cells of Aedes aegypti mosquitoes infected with the larvae of the nematodes Brugia pahangi, the nuclei and mitochondria increase in size, and the myofibrils adjacent to the parasites become denser as they grow [8]. A dense layer of dissociated sarcoplasm, including a large number of cell membranes and vesicles [9], surrounds the larvae of the same nematode species in Anopheles quadrimaculatus mosquitoes. More extensive changes in muscle fibers, such as their complete destruction, associated mainly with the migration of mature filaria into the hemocoel of mosquitoes were also described [8]. It was noted that the nuclei and mitochondria of degenerating muscle fibers are destroyed, after which they are removed by macrophages, and myofibrils disintegrate into fragments that fuse and form an amorphous mass. In the composition of the studied capsules of P. atomon, we did not find structural components of muscle tissue, since the material from which they were formed was degenerated. At the same time, the comparison of the ultrafine structure of the destroyed muscle fibers of insects exposed to the invasive action of the aforementioned nematode microfilariae, and the inner layer of the capsule of P. atomon, revealed their similarity. This similarity consisted in the general low electron density and loose organization of the substance forming them. However, additional experimental studies with a phased study of histological sections are needed for the conformation of the hypothesis about the muscular nature of this layer.
The peculiarity of the structure of the outer layer of the P. atomon capsule is the presence of electron-dense substance similar to melanin. It is known that melanization in arthropods is the most important defense mechanism leading to the formation and accumulation of melanin on the surface of pathogenic organisms. In the process of melanogenesis, a number of toxic compounds are formed, which, in some cases, negatively affect the vital activity of parasites/parasitoids [10, 11]. Among invertebrates, the mechanisms of defense against various pathogenic organisms have been studied most fully in insects. Thus, in the hemocoel of A. quadrimaculatus mosquitoes, a layer of melanin is first deposited on the surface of the microfilariae of the nematodes B. pahangi and B. malayi, and then hemocytes, forming several layers, start to precipitate on top of it [12]. Some of these nematodes show signs of destruction. A similar cell-mediated melanization reaction and subsequent encapsulation have been described around degenerated eggs of Asobara tabida and Asobara citri wasps in several Drosophila species [13] as well as around the larvae of Leptopilina heterotoma wasps that died in Drosophila suzukii [14]. Among crustaceans, in particular, amphipods, melanized larvae of various acanthocephalans and trematodes species were found [15–17]. Thus, in Spinulogammarus ochotensis amphipods, the cystacanths of Corynosoma strumosum were surrounded by a solid rim of dark brown matter, presumably melanin, tightly adhering to the surface of the parasite, while the larvae surrounded by the melanotic capsule were dead [17]. In the case of melanization of metacercariae of trematodes, the pigment was often deposited on the surface of the cyst, synthesized by the tegument of the parasite. For example, in Gammarus insensibilis amphipods, four species of microphallid trematodes with different intensities of melanization were found. The authors suggested that the highest pigmentation was characteristic for the species that either was poorly adapted to the host or was the most pathogenic for the latter [16]. However, the study does not indicate the viability of these trematodes. In the investigated parasites, the layer of the putative pigment was located not on the surface or the surrounding cysts but on the surface of the inner layer of the capsule, e.g., it was the outer layer of the capsule.
According to the literature data, completely melanized parasites do not die in all cases, in other words, melanization does not always lead to their death [18]. Moreover, dead parasites are often found without signs of melanization [19] or the pigment can be deposited later on the surface of already dead parasites [20, 21], e.g., already dead tissue undergoes pigmentation. In the latter case, an example is the study of Dedkhad et al. [22], which demonstrated under experimental conditions that degenerated B. malayi nematodes were found 3 h after the infection of mosquitoes in the hemocoel of Anopheles paraliae and in Anopheles lesteri after 4 h. Melanized species of these worms were observed in both mosquito species after 1 h, e.g., 4 and 5 h after infection, respectively. As we showed above, in the studied Podocotyle atomon, the inner layer of the capsule was formed by destroyed tissue. Thus, it can be assumed that the deposition of pigment on the surface of the capsule of Podocotyle atomon in a certain way was associated with the death and degeneration of the material of its inner layer.
Cyst morphology. The cyst structure in Podocotyle atomon from both studied amphipod species was also similar. At the light-microscopic level, the cyst in all cases was characterized by a relatively constant thickness (approximately 8–10 μm) and was intensely stained with histological dyes (Fig. 2). At higher magnification, it appears as a single layer of homogeneous, amorphous material (Fig. 1e). The surrounding capsule sometimes adhered tightly to the cyst, but they were more often separated by “free” space.
The structure and formation of cysts of metacercariae have been studied in a large number of trematode species infecting both vertebrates [23] and invertebrates [24, 25] as a second intermediate host. Depending on the taxonomic affiliation of the trematodes and their hosts, the cysts of metacercariae differ in the number of layers: the latter can vary in structure, thickness, and chemical composition. In the considered P. atomon, as well as in P. reflexa, studied at the histological level [5], the cyst was presented by a single layer formed by a homogeneous substance.
Thus, the results obtained indicate that the capsule surrounding the studied metacercariae of the Podocotyle atomon consists of two layers. In this case, the outer layer was presumably pigmented, while the inner one was formed by degenerated host tissue (possibly muscle tissue). The cyst of the studied metacercariae did not differ in structure from that described for P. reflexa. Further studies are required for the clarification of the structure of the inner layer of the capsule and verification of the hypothesis of melanization of the outer layer of the capsule.
REFERENCES
Orlovskaya, O.M., New information on the life cycles of some species of trematodes of coastal fish of the Northern Okhotsk Sea, in Bioraznoobrazie i ekologiya parazitov. Trudy Tsentra parazitologii (Biodiversity and Ecology of Parasites. Proceedings of the Center for Parasitology), Zinov’ev, S.V., Ed., Moscow: Nauka, 2010, pp. 186–197.
Uspenskaya, A.V., Parazitofauna benticheskikh rakoobraznykh Barentseva morya (Parasite Fauna of Benthic Crustaceans in the Barents Sea), Moscow: Akad. Nauk SSSR, 1963.
James, B.L., Host selection and ecology of marine digenean larvae, Fourth European Marine Biology Symposium, Crisp, D.J., Ed., Cambridge: Cambridge Univ. Press, 1971, pp. 179–197.
Køie, M., On the morphology and life history of Podocotyle reflexa and a comparison of its developmental stages with those of P. atomon (Trematoda, Opecoelidae), Ophelia, 1980, vol. 20, no. 1, pp. 17–43.
Shimazu, T., On two metacercariae on the genus Podocotyle from the shrimp, Pandalus goniurus, from Aniwa Bay, Sakhalin, USSR (Trematoda, Opecoelidae), Bull. Jpn. Soc. Sci. Fish, 1973, vol. 39, no. 5, pp. 481–487.
Lo, S.J., Hall, J.E., Allender, P.A., and Klainer, A.S., Scanning electron microscopy of an opecoelid cercaria and its encystment and encapsulation in an insect host, J. Parasitol., 1975, vol. 61, no. 3, pp. 413–417.
Knowles, E.E. and Hall, J.E., Histopathology of an opecoelid trematode infection in mayfly naiads, J. Invert. Pathol., 1976, vol. 27, no. 3, pp. 351–362.
Beckett, E.B., Histological changes in mosquito flight muscle fibres associated with parasitization by filarial larvae, Parasitology, 1971, vol. 63, no. 3, pp. 365–372.
Chikilian, M.L., Bradley, T.J., Nayar, J.K., and Knight, J.W., Ultrastructural comparison of extracellular and intracellular encapsulation of Brugia malayi in Anopheles quadrimaculatus, J. Parasitol., 1994, vol. 80, no. 1, pp. 133–140.
Glupov, V.V., Slepneva, I.A., and Dubovskii, I.M., Generation of activated oxygen metabolites in the formation of an immune response in arthropods, Tr. Zool. Inst. Ross. Akad. Nauk, 2009, vol. 313, no. 3, pp. 297–307.
Dubovskiy, I.M., Kryukova, N.A., Glupov, V.V., and Ratcliffe, N.A., Encapsulation and nodulation in insects, Invert. Surv. J., 2016, vol. 13, no. 1, pp. 229–246.
Chen, C.C., Further evidence of both humoral and cellular encapsulations of sheathed microfilariae of Brugia pahangi in Anopheles quadrimaculatus, Parasitology, 1988, vol. 78, no. 6, pp. 819–826.
Nappi, A., Poirié, M., and Carton, Y., The role of melanization and cytotoxic by products in the cellular immune responses of Drosophila against parasitic wasps, Adv. Parasitol., 2009, vol. 70, no. 4, pp. 99–121.
Kim-Jo, C., Gatti, J-L., and Poirie, M., Drosophila cellular immunity against parasitoid wasps: A complex and time-dependent process, Front. Physiol., 2019, vol. 10, 603.
Dezfuli, B.S., Simoni, E., Duclos, L., and Rossetti, E., Crustacean-acanthocephalan interaction and host cell-mediated immunity: Parasite encapsulation and melanization, Folia Parasitol., 2008, vol. 55, no. 1, pp. 53–59.
Kostadinova, A. and Mavrodieva, R.S., Microphallids in Gammarus insensibilis Stock, 1966 from a Black Sea Lagoon: Host response to infection, Parasitology, 2005, vol. 131, no. 3, pp. 347–354.
Skorobrekhova, E.M. and Nikishin, V.P., Encapsulation of the acanthocephalan Corynosoma strumosum (Rudolphi, 1802) Lühe, 1904, in the intermediate host Spinulogammarus ochotensis, J. Parasitol., 2019, vol. 105, no. 4, pp. 567–570.
Christensen, B.M., Li, J., Chen, C.-C., and Nappi, A.J., Melanization immune responses in mosquito vectors, Trends Parasitol., 2005, vol. 21, no. 4, pp. 192–199.
Carton, Y., Frey, F., and Nappi, A.J., Inheritance of cellular immune resistance in Drosophila melanogaster, Heredity, 1992, vol. 69, pp. 393–399.
Salt, G., The cellular defense reactions of insects, in Cambridge Monograph in Experimental Biology, London: Cambridge Univ. Press, 1970.
Kobayashi, M., Ogura, N., and Yamamoto, H., Studies on filariasis VIII: Histological observation on the abortive development of Brugia malayi larvae in the thoracic muscles of the mosquitoes, Armigeres subalbatus, Jpn. J. Sanit. Zool., 1986, vol. 37, no. 2, pp. 127–132.
Dedkhad, W., Christensen, B.M., Bartholomay, L.C., Joshi, D., Hempolchom, C., and Saeung, A., Immune responses of Aedes togoi, Anopheles paraliae and Anopheles lesteri against nocturnally subperiodic Brugia malayi microfilariae during migration from the midgut to the site of development, Parasites Vectors, 2018, vol. 11, 528.
Martin, T.R. and Conn, D.B., The pathogenicity, localization, and cyst structure of echinostomatid metacercariae (Trematoda) infecting the kidneys of the frogs Rana clamitans and Rana pipiens, J. Parasitol., 1990, vol. 76, no. 3, pp. 414–419.
Taft, S.J., Cinephotomicrographic and histochemical observations on cercarial penetration and encystment by Plagiorchis sp. in larvae of Chaoborus sp., Trans. Am. Micros. Soc., 1990, vol. 109, no. 2, pp. 160–167.
Galaktionov, K.V., Malkova, I.I., Irwin, S.W.B., Saville, D.H., and Maguire, J.G., The structure and formation of metacercarial cysts in the trematode family Microphallidae Travassos 1920, J. Helminthol., 1997, vol. 71, no. 1, pp. 13–20.
Funding
The study was performed within the framework of the state task “Taxonomic, Morphological and Ecological Diversity of Helminths of Vertebrates in North Asia,” no. AAA-A17-117012710031-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest. The authors declare that they have no conflict of interests.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
ADDITIONAL INFORMATION
ORCID ID: 0000-0002-9497-584X
Additional information
Translated by V. Mittova
About this article
Cite this article
Skorobrekhova, E.M. Structure of the Envelopes Formed around the Metacercariae of the Trematode Podocotyle atomon (Rudolphi, 1802) in the Second Intermediate Host. Moscow Univ. Biol.Sci. Bull. 75, 242–246 (2020). https://doi.org/10.3103/S0096392520040100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0096392520040100