Abstract
In this study, dose-dependent effects of lunularic acid (LA) on some physiological, cytogenetic, biochemical and anatomical parameters were investigated in Allium cepa L. bulbs. For this purpose, physiological parameters to be analyzed experimentally: germination percentage, root length, root number and fresh weight; cytogenetic parameters: micronucleus (MN) frequency, chromosomal aberrations (CAs) and mitotic index (MI); biochemical parameters were determined as catalase (CAT), superoxide dismutase (SOD) activities, malondialdehyde (MDA) level and free proline (Pr) content. In addition, cross-sections were taken from the roots and structural changes in meristem cells were examined. Onion bulbs were divided into four groups as one control and three treatments. The bulbs of the control group were kept in cuvettes containing tap water and the bulbs of the treatment group were kept in cuvettes containing 1, 5 and 10 mM LA for 7 days. LA administrations caused a decrease in all investigated physiological parameter values, an increase in the frequency of MN and CAs, and reduce in MI compared to control group. In addition, LA application caused dose-related increases in CAT and SOD activities and MDA and Pr levels compared to control group. LA application promoted CAs such as sticky chromosome, spindle fiber damage, vagrant chromosome, reverse polarization in root meristem cells. After all LA applications, root anatomical structure changes such as epidermis cell deformations, flattened cell nucleus and unclear transmission tissue were observed and it was determined that these changes reached a maximum at 10 mM LA dose. As a result, it has been understood that high doses of LA promote multi-directional toxicity and the Allium test is a very reliable test in determining this toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Developmental stage of plants is rather a complex process. However, this process is regulated and very well coordinated by the action of small active molecules such as phytohormones. All of plants innately generate hormones that organize growth, development and metabolism, in response diverse environment factors. Hormones are synthesized in parts such as bud, leaf and root of plants. Among these hormones, especially phytohormones are transported to near their synthesis sites or to more distant tissues in order to mediate the molecular, biochemical and physiological reactions of plants under stress or non-stress conditions (Rademacher, 2015; Per et al., 2018). Plant hormones have multiple effects depending on the plant’s developmental stage, target tissue, concentration, water and nutrient uptake, use and storage, and environmental conditions (Spann and Ferguson, 2014). They have been extensively used in agricultural activities such as horticulture and viticulture to stimulate growth in stressful mediums (Rademacher, 2015).
Lunularic acid (LA) is a natural product found in organisms. LA was first isolated from liverwort (Lunularia cruciata) as a somnolence stimulating and growth inhibitory factor. It is found in algae and hepatophyta with few exceptions. However, it could not be detected in vascular plants or mosses. It was determined in 47 genera and 80 species of liverworts. Its function is limited due to LA is localized inside cells (Gorham, 1990). It is evenly distributed between cytoplasm and vacuole, but it does not cumulate in organelles such as chloroplast, mitochondria and peroxisome (Imoto and Ohta, 1985). LA forms both in the soluble fraction of the cell and in association with the cell wall. For this reason, preventers like LA can flow from the plant and having a potential influence on neighbors of the same or different species. The ability of LA to prevent growth of algae and fungi and to retard germination of certain species thinks it could be allelopathic (Schwabe, 1990). Pryce and Kent (1971) stated that LA is an endogenous growth regulator in primitive plants and that ABA performs its function in these plants. Because its dispersion in the vegetable world and functions are similar those of ABA. Also LA has been indicated to have growth-inhibition activity against higher plants. And the two hydroxyl groups on both benzene rings in its structure seemed to be important for this activity (Hashimoto et al., 1988).
Allium is one of the largest monocot plant genera with about 850 species. Allium cepa L. known as onion, is a species belonging to the Amaryllidaceae family and the Allioideae subfamily (Peruzzi et al., 2017). It is one of the oldest cultivated plants used as aroma and vegetable all over the world. Although it is mostly used as a vegetable, it is also widely preferred due to its antidiabetic, antioxidant and antimicrobial effects (Marrelli et al., 2019). On the other hand, it is frequently used as an indicator plant species in scientific research. Because it is a fast, inexpensive and sensitive method, the Allium test is highly preferred in mutagenicity and toxicity studies (Tedesco and Laughinghouse, 2012). The sensitivity of the Allium test is on par with test systems using algae or human lymphocyte cells. The results of many tests using different biological organisms gave similar results with the Allium test (Chaparro et al., 2010). For this reason, it has been approved by the US Environmental Protection Agency (USEPA), the World Health Organization (WHO) and the United Nations Environment Program (UNEP) to be an effective test for genetic monitoring of environmental pollutants (Grant, 1999).
Although there are limited studies on the effects of LA on seed germination and seedling growth in plants, there is no comprehensive study addressing the multifaceted effects of LA in plants. In this study, the multifaceted toxicity induced by different LA doses in A. cepa was investigated with the help of physiological, cytogenetic, biochemical and anatomical parameters.
MATERIALS AND METHODS
Test Material and Chemical
A. cepa bulbs, an indicator species, were used as test material. As the test chemical, LA (CAS number 23255-59-6) was used. LA concentrations were determined according to the detected EC50 dose. The EC50 dose was found to be 5 μM. A dose of 10 μM which double of the EC50 dose, and the lowest dose of 1 μM were preferred.
Measurement of Germination and Growth Parameters
The germination process was carried out in the dark in an incubator at 20°C. Onions of approximately the same size and healthy appearance were selected and divided into four groups (Fig. 1). 20 onions from each test group were placed in sterile containers and allowed to germinate in the incubator for seven days. Onions reaching a root length of 10 mm were considered germinated. At the end of the seventh day, root number and germination percentage of onions were determined, root lengths (mm) and fresh weights (g) were measured. For statistical evaluation, all experiments were arranged in triplicate. Experimental research on plant samples, including the supply of plant material, complies with institutional, national and international guidelines and legislation.
Cytogenetic Analyzes
For cytogenetic studies, 1–1.5 cm long onion root tips were cut with a razor blade and kept in saturated paradichlorobenzene for 4 h. Then, these fractions were fixed in 3/1 ethanol-acetic acid solution and stored in 70% ethanol. Root tips were hydrolyzed in 1 N HCl at 60°C for 17 min. Stained with feulgen for 1.5 h. It was crushed on a slide in a drop of 45% acetic acid and covered with a coverslip (Sharma and Gupta, 1982). Mitosis stages and mitotic abnormalities seen in root tip meristem cells were photographed at 500× magnification with a digital camera mounted on a light microscope. MI was calculated by counting 30 000 cells from each group, and CAs by counting 2000 cells.
Measurement of Antioxidant Enzyme Activity
0.2 g of root tips were weighed and homogenized with 5 mL of 50 mM chilled sodium phosphate buffer (pH 7.8). The homogenates were centrifuged at 10 000 rpm for 20 min and the supernatant obtained was used for internal analysis of SOD and CAT antioxidant enzymes.
To determine the SOD activity in root tip cells, a total volume of 3 mL of reaction mixture (1.5 mL 0.05 M sodium phosphate buffer (pH 7.8), 0.3 mL 130 mM methionine, 0.3 mL 750 μM nitroblue tetrazolium chloride, 0.3 mL 0.1 mM EDTA–Na2, 0.3 mL 20 μM riboflavin, 0.01 mL supernatant, 0.01 mL 4% polyvinylpyrrolidone and 0.28 mL deionized water) was prepared. Then, the reaction was started by keeping the test tube containing this mixture under two 15 W fluorescent lamps for 10 min and the reaction was terminated by keeping the tubes in the dark for 10 min (Beauchamp and Fridovich, 1971). SOD activity was expressed as U/mg FW by measuring absorbance at 560 nm (Zou et al., 2012).
To determine CAT activity in root tip cells, a total volume of 2.8 mL of reaction mixture (0.3 mL of 0.1 M H2O2, 1.5 mL of 200 mM sodium phosphate buffer and 1.0 mL of deionized water) was prepared. The reaction process was initiated with the addition of 0.2 mL of enzyme extract. CAT enzyme activity was measured by monitoring the decrease in absorbance at 240 nm wavelengths as a result of H2O2 consumption and expressed as OD240 nm min/g (Beers and Sizer, 1952).
Measurement of MDA as an Indicator of Lipid Peroxidase
0.5 g of root tips were homogenized with 5% TCA solution in a homogenizer. It was centrifuged at 12 000 rpm at 24°C for 15 min. An equal volume of supernatant and 0.5% TBARS were then transferred to a new test tube and incubated in 20% TCA solution at 96°C for 25 min. At the end of the period, the tubes were placed in an ice bath and centrifuged at 10 000 rpm for 5 min. Absorbance was measured at 532 nm. MDA activity was calculated using the extinction coefficient of 155 M–1 cm–1 and expressed as μmol/g FW (Ünyayar et al., 2006).
Free Proline Measurement
0.5 g of root tips was homogenized in 10 mL of 3% aqueous sulfosalicylic acid. The resulting homogenate was filtered with Whatman no. 2 filter paper. 2 mL of filtrate, 2 mL of acid-ninhydrin and 2 mL of glacial glacial acetic acid were reacted in a test tube at 100°C for 1 h. The reaction was terminated in an ice bath. The reaction mixture was extracted with 4 mL of toluene and stirred vigorously with a stirrer for 15–20 s. The toluene-containing chromophore was aspirated from the aqueous phase, warmed to room temperature and read absorbance spectrophotometrically at 520 nm using toluene for a blank. Proline concentration was determined from a standard curve and determined on a fresh weight basis using the following (Eq. (1)) (Bates et al., 1973).
[(μg proline/mL × mL toluene)/115.5 μg/mol]/[(g sample)/5] = μmoles proline/g of fresh weight material (1).
Anatomical Observations in Root Cells
Roots were washed with distilled water to remove debris from the surface of the root tips. The root tips were placed between the foam material and cross-sections were taken with the help of a sharp razor blade. Sections were stained with 2% methylene blue for 5 min and photographed under a research microscope at 200× magnification (Akgunduz et al., 2020; Macar et al., 2020).
Statistical Evaluation
All data obtained from this study were analyzed with the help of SPSS statistics V 23.0 (2015) package program and expressed as mean value by taking their standard deviations (±SD). Statistical analysis of mean values was determined by Duncan’s multiple range test (DMRT) and p < 0.05 was considered highly significant.
RESULTS AND DISCUSSION
Effect of LA on Physiological Parameters
Figures 2 and 3 show the effects of LA on the physiological parameters in A. cepa. At the end of the 7th day, while the germination percentage of the control group (Group I) was 100%, this value was 74, 48, and 20% at 1, 5 and 10 mM LA levels, respectively. In other words, with the increase in LA doses, the germination percentage was inhibited by 26.5% at 1 mM dose, 52% at 5 mM dose and 80% at 10 mM dose compared to control group (Group I). Similarly, increases in LA doses led to a very serious inhibition on root length, root number and fresh weight of onions. Maximum root elongation was measured as 71.3 mm in control group (Group I) and minimum root elongation was measured as 10.2 mm in Group IV treated with 10 mM LA. Root lengths decreased by 22.6 mm in Group II, 51.9 mm in Group III and 61.1 mm in Group IV compared to control group. While the root number of the control group bulbs was 61.2, this value was 42.8 at 1 mM dose, 27.6 at 5 mM dose and 12.2 at 10 mM dose. At the highest dose (10 mM) of LA application, the number of roots decreased 5 times compared to the control group. While the mean fresh weight of control group (Group I) grown in tap water was 18.9 g, it was calculated as 13.1 g in Group II, 9.5 g in Group III and 4.4 g in Group IV. It was determined that control group (Group I) had maximum fresh weight value and Group IV treated with 10 mM dose of LA dose had minimum fresh weight values. All decreases in physiological parameter values were found to be statistically significant at the p < 0.05 level.
These results are also similar to the results of a limited number of studies investigating the physiological toxicity induced by LA administration in plants. For example, Yoshikawa et al. (2002) reported that LA inhibited seedling growth and germination of cress and lettuce at a dose as low as 1 mM. In addition, they represented that LA inhibited the germination by causing seed dormancy. On the other hand, it is known that LA has an ABA-like effect in plants. Rajaei and Mohamad (2013) observed that ABA application reduced canola seed germination and inhibited root development compared to control.
The decrease caused by LA in physiological parameters can be explained by the fact that LA reduces mitotic cell division with the intake of macro and micro nutrients from onion roots and causes structural damage to root cells. Because it has been reported in the literature that inhibitory compounds, chemicals and metal ions prevents the uptake of water and mineral substances by plant roots (Fan et al., 2021). On the other hand, Harashima and Schnittger (2010) reported that root growth of plants is associated with the elongation level in the differentiation phase and the increase in cell number. Moreover, Andrade et al. (2010) stated that a healthy mitotic division is the key to strong root development. In our study, it was determined that MI decreased proportionally with the increase in LA doses (Fig. 4). Besides, damage such as epidermis cell deformation observed in the roots as a result of LA application is an obvious indicator of the deterioration of the root structure. This is another proof of the inability of the roots to perform their basic function and therefore the decrease in physiological parameters.
Effect of LA on Cytogenetic Parameters
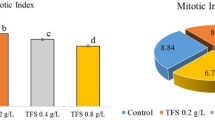
Figures 4 and 5 shows the effects of LA on the cytogenetic parameters such as MI, MN and CAs. MI was calculated as 10.2% in control group (Group I), 7.4% in Group II, 5.9% in Group III and 2.3% in Group IV, the treatment groups. MI in the treatment groups decreased by approximately 27, 42 and 77%, respectively, compared to the control group. These findings show that an increase in LA dose adversely affects MI. Similarly, increases in LA dose also promoted MN formation in root tip meristem cells of bulbs. While MN formation was not observed in control group (Group I), MN formation was observed to increase in LA applied groups and this value was 16.3% in Group II, 37.7% in Group III and 64.9% in Group IV. In Group IV, where the highest dose of LA was administered, the number of MN increased 64.9 times compared to the control group. It has also been shown that LA increases the number of CAs in root tip meristem cells. As a result of microscopic examination, CAs were not observed in the root tip cells of control group (Group I) bulbs, while the number of these abnormalities reached 18.9% at 1 mM dose, 46.7% at 5 mM dose and 71.3% at 10 mM dose. Especially at 10 mM dose of LA, this value increased 71.3 times compared to control group. LA application promoted CAs such as lobated nucleus, nucleus distribution, sticky chromosome, spindle disturbance, bridge and ring chromosome in root meristem cells. It was determined that the decrease in the MI and the increase in the MN and CAs numbers were statistically significant (p < 0.05).
Chromosomal abnormalities induced by LA. Micronucleus = arrow (a, b), lobated nucleus (c), multilobated nucleus (d), nucleus distribution = arrow (e), sticky chromosome (f), spindle disturbance (g, h), anaphase with bridge formations = arrow and ring chromosome = patterned anaphase with vagrant chromosome = arrow (j), telophase with vagrant chromosomes = arrows (k), telophase with reverse polarization = arrow (l). Scale bar = 10 μm.
There is no comprehensive study in the literature about the genotoxicity induced by LA in plants. However, some studies have reported that plant growth promoters can cause cell damage, CAs and mitotic disorders (Ünal et al., 2002; Tütünoğlu et al., 2019). On the other hand, it has been shown that ethylene, an important plant hormone such as LA, inhibits nuclear DNA replication and cell division in pea seedlings and induces programmed cell death at certain phases of the cell cycle in the tobacco TBY-2 cell line (Novikova et al., 2020). It has been determined that low doses of kinetin, a plant hormone that promotes cell division, reduce apoptosis and protect cells from oxidative stress-mediated cell death. However, it has been reported that high doses reduce cell viability and cause in vitro DNA damage (Othman et al., 2016).
It is thought that the decrease in MI value and the increase in MN and CAs numbers of root meristem cells as a result of LA application may be caused by the increase in the amount of reactive oxygen species (ROS) in root cells. Because ROS formed by the effect of LA can cause damage to the structure of microtubules and DNA that play a role in cell division. Many plant hormones are known to produce ROS as part of the mechanism that regulates plant growth and development. In addition, ROS production and accumulation increase during the stress response in plants (Xia et al., 2015). On the other hand, there is also a hormone-dependent activation of ROS production through the activation of NADPH oxidases encoded by respiratory burst oxidase homologous (RBOH) genes in plant genomes (Sagi and Fluhr, 2006).
Effect of LA on Biochemical Parameters
The oxidative stress caused by LA administration is shown in Fig. 6. In parallel with the increase in LA doses, both SOD and CAT activities increased significantly. SOD activity was measured at the lowest level with 87 U/mg FW in the roots of control group (Group I), and at the highest level with 201 U/mg FW in the roots of Group IV. Moreover, SOD activity increased 1.18-fold in Group II, 1.91-fold in Group III and 2.31-fold in Group IV when compared to control group. CAT activity was calculated as 0.9 OD 240 nm min/g FW in the roots of the control group (Group I), and this value was 1.7 OD 240 nm min/g FW in Group II, 2.2 OD 240 nm min/g FW in Group III and 2.8 OD 240 nm min/g FW in Group IV. Increasing doses of LA led to significant increases in MDA and Pr content. While the mean MDA content was 8.8 μmol/g FW in the roots of the control group (Group I), the MDA content in the LA treatment groups increased in a dose-dependent manner. The MDA levels of Group II, III and IV were determined as 11.9 μmol/g FW, 22.5 μmol/g FW and 30.6 μmol/g FW, respectively. While the free Pr content in the roots of control group (Group I) was at the lowest level with 20.6 μmol/g FW, this value reached the highest level with 71.8 μmol/g FW in the roots of Group IV. Moreover, LA administration increased the free Pr content 1.9-fold at 1 mM dose, 2.9-fold at 5 mM dose, and 3.5-fold at 10 mM dose compared to control group.
Reactive oxygen species (ROS), cytotoxic substances with highly deleterious effects, act as intermediate signaling molecules that regulate the expression of genes associated with antioxidant defense systems. Plants contain antioxidant mechanisms to mitigate the damage caused by ROS (Vranova et al., 2002; Srivastava and Singh, 2020) and antioxidant enzymes are an important part of these mechanisms. Among the most important antioxidant enzymes are SOD and CAT (Soares et al., 2010). SOD is the most powerful antioxidant enzyme in cells. It takes part in the first step of the defense developed by the cell against the formed ROS. It catalyzes the conversion of superoxide radicals, which are produced as a by-product of oxygen metabolism, to H2O2. CAT is a tetrameric enzyme commonly found in almost all living organisms exposed to oxygen. It works in interaction with SOD in the cell. It splits the H2O2 created by SOD into H2O and molecular oxygen. It has the ability to convert millions of H2O2 per second (Ighodaro and Akinloye, 2018).
MDA is a three-carbon dialdehyde. It occurs as a result of peroxidation of polyunsaturated fatty acids in the cell membrane. MDA is the end product of lipid peroxidation. Damage to the cell membrane causes an increase in MDA content (Fedina and Benderliev, 2000). Therefore, MDA is a reliable indicator to assess lipid peroxidation due to cell damage (Dikker et al., 2018). It is known that MDA can change DNA and RNA, cause cross-links in proteins and lipids, and suppress genes that play a role in the plant’s response to stress factors. It has also been reported that MDA is carcinogenic and mutagenic (Davey et al., 2005).
Pr is one of the most common osmolytes produced by plants exposed to stress (Ashraf and Foolad, 2007). It is the first product synthesized as a physiological response when plants are exposed to stress factors. In other words, it is an indicator against stress and constitutes the first step of a series of metabolic reactions that activate the plant’s defense mechanism against stress (Ünal, 2019). Pr plays an important role in maintaining osmotic pressure and turgor under stress condition (Munns and Tester, 2008). Pr also stabilizes and protects cell membranes during dehydration (Martinez et al., 2003; Wang and Han, 2009).
It is thought that the increases observed in root SOD and CAT enzyme activities and MDA and Pr levels as a result of LA application may be due to the fact that LA promotes ROS production. Because ROS formed by the effect of LA may cause damage to the membranes of root cells and cause lipid peroxidation resulting in excessive MDA production. It will increase Pr synthesis with SOD and CAT enzymes to inactivate these ROS in the cell. For example, the literature information that plant hormones such as abscisic acid, jasmonic acid and auxin trigger the production of ROS in the roots supports this idea (Kwak et al., 2006; Devireddy et al., 2021). On the other hand, Yao et al. (2020) reported that exogenous ABA (0.5, 1, 10, 100 ve 200 μM) application in Platycladus orientalis (Chinese thuja) seedlings increased SOD, CAT activities and Pr content by modulating ROS metabolism. In addition, some researchers have determined that Pr actively participates in the clearance process of ROS formed by toxic agents and protects cells from oxidative damage (Boughalleb et al., 2020; Matysik et al., 2002).
Effect of LA on Anatomical Parameters
The changes induced by the LA application at different doses in the anatomical structure of the root are shown in Table 1 and Fig. 7. No change was observed in the anatomical structure of the roots of the control group. LA application caused damage such as unclear vascular tissue, flattened cell nucleus and epidermis cell deformation in root anatomy. In addition, an increase in the severity of the damage was detected, depending on the applied LA dose.
Although there is no comprehensive study in the literature investigating the changes induced by LA application in root anatomy, there are some studies investigating the changes caused by other plant growth regulators. For example, Küplemez and Yildırım (2020) reported that high-dose and long-term applications of plant growth regulators such as benzylaminopurine and naphthalene acetic acid caused deformation in the phloem and xylem region and narrowed the stem diameter of lentil (Lens culinaris) seedlings. On the other hand, there are many studies conducted in recent years reporting that different toxic agents cause damage such as epidermis cell deformation, cortex cell deformation, flattened cell nucleus, cortex cell wall thickening, necrosis and indistinct vascular tissue in A. cepa root anatomy (Aydın et al., 2022; Kalefetoğlu Macar et al., 2022; Sipahi Kuloğlu et al., 2022).
It is thought that the damage such as epidermis cell deformation and flattened cell nucleus observed in root meristem cells as a result of LA application may be caused by the defense mechanisms developed by the plant to prevent LA from entering the cell. Because, in microscopic examinations, a significant increase was observed in the number of epidermis cells of the roots exposed to LA doses compared to the control group. This cellular increase acts as a barrier that reduces the entry of LA into the cell. This increase also increases the contact of epidermis cells with each other and creates a mechanical pressure. Due to this pressure, deformities may occur in the epidermis cells and their nucleus. The information that plants can develop some chemical (synthesis of terpenoids, alkaloids, metalloproteins and phenolic compounds) and morphological defense mechanisms (increase in the number of epidermis and cortex cells, thickening of the cortex cell wall) to reduce the entry and effects of toxic agents into the cell supports this idea (Mithöfer and Maffei, 2017; Çavuşoğlu et al., 2021). Another reason for the deformation in the root epidermis cells may be the deterioration of the basic structure of the cell membrane. Because when LA is absorbed by the roots, the epidermis tissue is affected first and lipid peroxidation is observed in these cells. As a result, the membrane structure deteriorates and cell deformation may occur. Penetration of LA into the cell may cause a change in intracellular pressure and in this case may cause flattening of the cell nucleus. On the other hand, changes in the volume and shape of the cell nucleus may also result from the deterioration of DNA double helix structure, DNA volume, and nuclear protein concentration (Dahl et al., 2008; Dauer and Worman, 2009).
CONCLUSIONS
In this study, the multiple toxic effects of externally applied LA at different doses were investigated in A. cepa, a eukaryotic indicator organism. It has been determined that LA has a toxic effect by causing changes in all physiological, cytogenetic, biochemical and anatomical parameters examined, depending on the dose. While LA decreased germination percentage, root elongation, fresh weight and MI, it caused increases in MN and CAs numbers, MDA and Pr levels, and SOD and CAT enzyme activities. LA application also promoted different types of chromosomal and anatomical damage in root meristem cells.
Today, there are dozens of natural and synthetic growth regulators that play a role in plant growth and development. Research has focused on the most well-known growth regulators such as abscisic acid, ethylene and gibberellin. There is no comprehensive study in the literature investigating the toxicity induced by excessive doses of LA in plants. The studies carried out focused more on a single parameter. This is the first study to address the toxic effects of LA on eukaryotic plant cells with the help of multiple parameters. For this reason, we believe that it will make an important contribution to the literature and will shed light on future studies.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this article.
REFERENCES
Akgündüz, M.C., Çavuşoğlu, K., and Yalçın, E., The potential risk assessment of phenoxyethanol with a versatile model system, Sci. Rep., 2020, vol. 10, pp. 1209–1218. https://doi.org/10.1038/s41598-020-58170-9
Andrade, L.F., Davide, L.C., and Gedraite, L.S., The effect of cyanide compounds, fluorides, aluminum, and inorganic oxides present in spent pot liner on germination and root tip cells of Lactuca sativa, Ecotoxicol. Environ. Saf., 2010, vol. 73, pp. 626–631. https://doi.org/10.1016/j.ecoenv.2009.12.012
Ashraf, M. and Foolad, M.R., Roles of glycine betaine and proline in improving plant abiotic stress resistance, Environ. Exp. Bot., 2007, vol. 59, pp. 206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Aydın, D., Yalçın, E., and Çavuşoğlu, K., Metal chelating and anti-radical activity of Salvia ofcinalis in the ameliorative effects against uranium toxicity, Sci. Rep., 2022, vol. 12, p. 15845. https://doi.org/10.1038/s41598-022-20115-9
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water stress studies, Plant Soil, 1973, vol. 39, pp. 205–207. https://doi.org/10.1007/BF00018060
Beauchamp, C. and Fridovich, I., Superoxide dismutase: improved assays and an assay applicable to acrylamide gels, Anal. Biochem., 1971, vol. 44, pp. 276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Beers, R.F. and Sizer, I.W., Colorimetric method for estimation of catalase, J. Biol. Chem., 1952, vol. 195, pp. 133–139.
Boughalleb, F., Abdellaoui, R., Mahmoudi, M., and Bakhshandeh, E., Changes in phenolic profile, soluble sugar, proline, and antioxidant enzyme activities of Polygonum equisetiforme in response to salinity, Turk. J. Bot., 2020, vol. 44, pp. 25–35.
Çavuşoğlu, D., Yalçın, E., Çavuşoğlu, K., Acar, A., and Yapar, K., Molecular docking and toxicity assessment of spirodiclofen: protective role of lycopene, Environ. Sci. Pollut. Res., 2021, vol. 28, no. 40, pp. 57372–57385. https://doi.org/10.1007/s11356-021-14748-y
Chaparro, T.R., Botta, C.M., and Pires, E.C., Biodegradability and toxicity assessment of bleach plant effluents treated anaerobically, Water Sci. Technol., 2010, vol. 62, pp. 1312–1319. https://doi.org/ 389https://doi.org/10.2166/wst.2010.944
Dahl, K.N., Ribeiro, A.J., and Lammerding, J., Nuclear shape, mechanics, and mechanotransduction, Circ. Res., 2008, vol. 102, pp. 1307–1318. https://doi.org/10.1161/CIRCRESAHA.108.173989
Dauer, W.T. and Worman, H.J., The nuclear envelope as a signaling node in development and disease, Dev. Cell, 2009, vol. 17, pp. 626–638. https://doi.org/10.1016/j.devcel.2009.10.016
Davey, M.W., Stals, E., Panis, B., Keulemans, J., and Swennen, R.L., High-throughput determination of malondialdehyde in plant tissues, Anal. Biochem., 2005, vol. 347, no. 2, pp. 201–207. https://doi.org/10.1016/j.ab.2005.09.041
Devireddy, A.R., Tschaplinski, T.J., Tuskan, G.A., Muchero, W., and Chen, J.G., Role of reactive oxygen species and hormones in plant responses to temperature changes, Int. J. Mol. Sci., 2021, vol. 22, no. 16, p. 8843. https://doi.org/10.3390/ijms22168843
Dikker, O., Şahin, M., Atar, S., and Bekpınar, S., Examination of oxidative stress markers in women with postmenopausal osteoporosis, Turk. J. Osteoporosis, 2018, vol. 24, no. 1, pp. 15–20. https://doi.org/10.4274/tod.71501
Fan, X., Zhou, X., Chen, H., Tang, M., and Xie, X., Cross-talks between macro-and micronutrient uptake and signaling in plants, Front. Plant Sci., 2021, vol. 12, p. 663477. https://doi.org/10.3389/fpls.2021.663477
Fedina, I.S. and Benderliev, K.M., Response of Scendesmus incrassatulus to salt stress as affected by methyl jasmonate, Biol. Plant, 2000, vol. 43, pp. 625–627. https://doi.org/10.1023/A:1002816502941
Gorham, J., Phenolic compounds other than flavonoids from bryophytes, in Bryophytes: their Chemistry and Chemical Taxonomy, Zimsmeister, H.D. and Mues, R., Eds., Oxford, 1990, pp. 171–200.
Grant, W.F., Higher plant assays for the detection of chromosomal aberrations and gene mutations—a brief historical background on their use for screening and monitoring environmental chemicals, Mutat. Res., 1999, vol. 426, pp. 107–112. https://doi.org/10.1016/s0027-5107(99)00050-0
Harashima, H. and Schnittger, A., The integration of cell division, growth, and differentiation, Curr. Opin. Plant Biol., 2010, vol. 13, no. 1, pp. 66–74. https://doi.org/10.1016/j.pbi.2009.11.001
Hashimoto, T., Tori, M., and Asakawa, Y., A highly efficient preparation of lunularic acid and some biological activities of stilbene and dihydrostilbene derivatives, Phytochemistry, 1988, vol. 27, pp. 109–113. https://doi.org/10.1016/0031-9422(88)80599-5
Ighodaro, O.M. and Akinloye, O.A., First line defence antioxidants – superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid, Alexandria J. Med., 2018, vol. 54, no. 4, pp. 287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Imoto, S.A. and Ohta, Y., Intracellular localization of lunularic acid and prelunularic acid in suspension cultured cells of Marchantia polymorpha, Plant Physiol., 1985, vol. 79, pp. 751–755. https://doi.org/10.1104/pp.79.3.751
Kalefetoğlu Macar, T., Macar, O., Çavuşoğlu, K., Yalçın, E., and Yapar, K., Turmeric (Curcuma longa L.) tends to reduce the toxic effects of nickel (II) chloride in Allium cepa L. roots, Environ. Sci. Pollut. Res., 2022, vol. 29, no. 40, pp. 60508–60518. https://doi.org/10.1007/s11356-022-20171-8
Küplemez, H. and Yildirim, M.U., Effects of cytokinin and auxin on plant development and vascular tissues in Lens culinaris, Commagene J. Biol., 2020, vol. 4, no. 1, pp. 16–21. https://doi.org/10.31594/commagene.704271431
Kwak, J.M., Nguyen, V., and Schroeder, J.I., The role of reactive oxygen species in hormonal responses, Plant Physiol., 2006. vol. 141, no. 2, pp. 323–329. https://doi.org/10.1104/pp.106.079004
Macar, O., Macar, T.K., Çavuşoğlu, K., and Yalçın, E., Protective effects of anthocyanin-rich bilberry (Vaccinium myrtillus) extract against copper (II) chloride toxicity, Environ. Sci. Pollut. Res., 2020, vol. 27, no. 2, pp. 1428–1435. https://doi.org/10.1007/s11356-019-06781-9
Marrelli, M., Amodeo, V., Statti, G., and Conforti, F., Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities, Molecules, 2019, vol. 24, pp. 119–136. https://doi.org/10.3390/molecules24010119
Martinez, C.A., Maestri, M., and Lani, E.G., In vitro salt tolerance and proline accumulation in Andean potato (Solanum spp.) differing in frost resistance, Plant Sci., 2003, vol. 116, pp. 117–184.
Matysik, J., Alia-Bhalu, B., and Mohanty, P., Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants, Curr. Sci., 2002, vol. 82, pp. 525–532. https://www.jstor.org/stable/24105959
Mithöfer, A. and Maffei, M.E., General mechanisms of plant defense and plant toxins, Plant Toxins, 2017, pp. 3–24. https://doi.org/10.1007/978-94-007-6464-4_21
Munns, R. and Tester, M., Mechanisms of salt tolerance, Annu. Rev. Plant Biol., 2008, vol. 59, pp. 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Novikova, G.V., et al., Coupling of cell division and differentiation in Arabidopsis thaliana cultured cells with interaction of ethylene and ABA signaling pathways, Life, 2020, vol. 10, no. 2, p. 15. https://doi.org/10.3390/life10020015
Othman, E.M., Naseem, M., Awad, E., Dandekar, T., and Stopper, H., The plant hormone cytokinin confers protection against oxidative stress in mammalian cells, PLoS One, 2016, vol. 11, no. 12, p. e0168386. https://doi.org/10.1371/journal.pone.0168386
Per, T.S., et al., Jasmonates in plants under abiotic stresses: Crosstalk with other phytohormones matters, Environ. Exp. Bot., 2018, vol. 145, pp. 104–120.
Peruzzi, L., Carta, A., and Altinordu, F., Chromosome diversity and evolution in Allium (Allioideae, Amaryllidaceae), Plant Biosyst., 2017, vol. 151, pp. 212–220. https://doi.org/10.1080/11263504.2016.1149123
Pryce, R.J. and Kent, U.K., Lunularic acid, a common endogenous growth inhibitor of liverworts, Planta, 1971, vol. 97, pp. 354–357. https://www.jstor.org/stable/23369226.
Rademacher, W., Plant growth regulators: backgrounds and uses in plant production, J. Plant Growth Regul., 2015, vol. 34, pp. 845–872. https://doi.org/10.1007/s00344-015-9541-6
Rajaei, P. and Mohamad, N., Effect of beta aminobutyric acid (BABA), ABA and ethylene synthesis inhibitor (CoCl2) on seed germination and seedling growth of Brassica napus L., Eur. J. Exp. Biol., 2013, vol. 3, pp. 437–440.
Sagi, M. and Fluhr, R., Production of reactive oxygen species by plant NADPH oxidases, Plant Physiol., 2006, vol. 141, pp. 336–340. https://doi.org/10.1104/pp.106.078089
Schwabe, W.W., Lunularic acid in growth and dormancy of liverworts, in Bryophyte Development in Physiology and Biochemistry, Chopra, R.N. and Bhatla, S.C., Eds., Boca Raton, 1990, pp. 245–257.
Sharma, P.C. and Gupta, P.K., Karyotypes in some pulse crops, Nucleus, 1982, vol. 25, pp. 181–185.
Sipahi Kuloğlu, S., Yalçın, E., Çavuşoğlu, K., and Acar, A., Dose‑dependent toxicity profile and genotoxicity mechanism of lithium carbonate, Sci. Rep., 2022, vol. 12, p. 13504. https://doi.org/10.1038/s41598-022-17838-0
Soares, A.M.S., Souza, T.F., Jacinto, T., and Machado, O.L.T., Effect of methyl jasmonate on antioxidative enzyme activities and on the contents of ROS and H2O2 in Ricinus communis leaves, Braz. J. Plant Physiol., 2010, vol. 22, pp. 151–158. https://doi.org/10.1590/S1677-04202010000300001
Spann, T.L. and Ferguson, L., Commercial production of container grown Citrus trees, in Citrus Production Manual, University of California Agriculture and Natural Resources Press, Ferguson, L. and Grafton Cardwell, E., Eds., 2014, p. 433.
Srivastava, A.K. and Singh, D., Assessment of malathion toxicity on cytophysiological activity, DNA damage and antioxidant enzymes in root of Allium cepa model, Sci. Rep., 2020, vol. 10, pp. 1–10. https://doi.org/10.1038/s41598-020-57840-y
Tedesco, S.B. and Laughinghouse, I.V.H.D., Bioindicator of genotoxicity. The Allium cepa test, J. Environ. Contam., 2012, pp. 138–156.
Tütünoğlu, B., Aksoy, Ö., Özbek, R., and Uçkan, F., The effects of gibberellic acid on Allium cepa root tip meristematic cells, Biol. Plant., 2019, vol. 63, pp. 365–370. https://doi.org/10.32615/bp.2019.042
Ünal, Z., Morphological, physiological, and biochemical effects of some abiotic stresses on proline-supported citrus rootstocks, MSc Thesis, Akdeniz Univ. Inst. Sci., 2019, pp. 1–127.
Ünal, M., Palavan Ünsal, N., and Tüfekci, M.A., Role of putrescine and its biosynthetic inhibitor on seed germination root elongation and mitosis in Hordeum vulgare L., Bull. Pure Appl. Sci. Bot., 2002, vol. 21, pp. 33–38.
Ünyayar, S., Çelik, A., Çekic, F.O., and Gözel, A., Cadmium-induced genotoxicity, cytotoxicity, and lipid peroxidation in Allium sativum and Vicia faba, Mutagenesis, 2006, vol. 21, pp. 77–81. https://doi.org/10.1093/mutage/gel001
Vranova, E., Inzé D., and Van Breusegen, F., Signal transduction during oxidative stress, J. Exp. Bot., 2002, vol. 53, pp. 1227–1236.
Wang, X.S. and Han, J.G., Changes in proline content, activity, and active isoforms of antioxidative enzymes in two alfalfa cultivars under salt stress, Agric. Sci. China, 2009, vol. 8, pp. 431–440. https://doi.org/10.1016/S1671-2927(08)60229-1
Xia, X.J., et al., Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance, J. Exp. Bot., 2015, vol. 66, no. 10, pp. 2839–2856. https://doi.org/10.1093/jxb/erv089
Yao, X., et al., Exogenous abscisic acid modulates reactive oxygen metabolism and related gene expression in Platycladus orientalis under H2O2-induced stress, Russ. J. Plant Physiol., 2020, vol. 67, pp. 85–93. https://doi.org/10.1134/S1021443720010264
Yoshikawa, H., Ichik, Y., Sakakibara, K.D., Tamura, H., and Suiko, M., The biological and structural similarity between lunularic acid and abscisic acid, Biosci. Biotechnol. Biochem., 2002, vol. 66, pp. 840–846. https://doi.org/10.1271/bbb.66.840
Zou, J., Yue, J., Jiang, W., and Liu, D., Effects of cadmium stress on root tip cells and some physiological indexes in Allium cepa var. agrogarum L, Acta Biol. Cracov., Ser. Bot., 2012, vol. 54, pp. 129–141. https://doi.org/10.2478/v10182-012-0015-x
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct thisparticular research were obtained.
Author information
Authors and Affiliations
Contributions
All authors (Dilek Çavuşoğlu, Kürşat Çavuşoğlu, Kültiğin Çavuşoğlu, Emine Yalçin) contributed to the study conception and design. All authors read and approved the final manuscript.
All authors contributed equally to this work.
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects. The authors confirm that the manuscript has been read and approved by all authors. The authors declare that this manuscript has not been published and is not under consideration for publication elsewhere.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Çavuşoğlu, D., Çavuşoğlu, K., Çavuşoğlu, K. et al. Identification of Cyto- and Genotoxic Effects of Lunularic Acid in Allium cepa L. Root Tip Meristem Cells. Cytol. Genet. 58, 178–189 (2024). https://doi.org/10.3103/S009545272402004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S009545272402004X