Abstract
Seasonality affects the morphological and functional characteristics of reproductive cells, which makes the natural reproduction of dairy goats possible only in certain seasons. Cryopreservation of sperm, as a part of assisted reproductive technologies, provides their flexibility, which increases the chances of increasing the number of livestock. However, it can cause changes in the morphological and functional characteristics and genetic material of sperm. Therefore, the aim of this study was to determine seasonal changes in the viability, motility, and DNA fragmentation level of native and cryopreserved goat sperm. The experiment was conducted using ejaculates of sexually mature male goats of the Saanen breed, obtained in breeding and nonbreeding seasons. To detect the effect of seasonal differences of seminal plasma on the characteristics of cryopreserved sperm, cells were cryopreserved in ejaculate and following isolation. The results of the study showed that the motility of fresh ejaculate sperm in the breeding season was higher than in the non-breeding season (p ≤ 0.05). Cryopreservation led to a decrease in the number of motile sperm of ejaculate and the selected fraction of sperm in the non-breeding season, and the selected fraction of cells in the breeding season (p ≤ 0.05). When comparing the viability and integrity of sperm DNA, there was a significant decrease in all groups in the non-breeding season compared to the breeding season (p ≤ 0.05). It was found that the DNA fragmentation rate of goat ejaculate sperm in the breeding season after cryopreservation did not change compared to the fresh sample, while that in cryopreserved sperm of the selected fraction of the same season increased (p ≤ 0.05). Cryopreserved spermatozoa of the non-breeding season in the ejaculate and isolated fraction had an increased rate of DNA fragmentation compared to the baseline before cryopreservation. Thus, it can be concluded that the composition of semen liquid varies depending on the breeding season, which affects its cryoprotective properties against spermatozoa during the freezing of ejaculate. Therefore, it is recommended to collect whole ejaculate by freezing in autumn and early winter to improve the effectiveness of artificial insemination using the cryopreserved sperm of male goats of the Saanen breed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Most farm animals are characterized by seasonality in reproduction (Chemineau, 2007; Dias, 2020). For example, for temperate goats, the breeding season is in the fall and the kids are born in the spring (Fatet, 2011). It is also known that changes in the seasons affect the morphological and functional characteristics of the sperm of bulls, horses, goats, and sheep (Johnson, 2008; Wrench, 2010; Gamboa, 2010; Suliman, 2020; Arrebola, 2017; Belkadi, 2017). Therefore, the reproduction of such farm animals is naturally possible only during a certain season of the year, which is economically unprofitable (Johnson, 2008). The development of assisted reproductive technologies (ART), namely artificial insemination allowed us to increase productivity and use the best genetic material for breeding, regardless of the season (De Vries, 2005). The cryopreservation of reproductive cells is an important and necessary step in the implementation of ART in animal husbandry since it allows for the accumulation of sperm with the best characteristics and their use throughout the year: divide ejaculate into several sperm doses and effectively carry out artificial insemination, create a cryobank of gametes of valuable animal breeds, and exchange samples between farms (Shcherbak, 2008; Bailey, 2003; Barbas, 2009; Kopeika, 2019). However, cryopreservation can cause changes in the morphological and functional characteristics of sperm: reduce cell motility and viability due to damage to the plasma and membrane of the acrosome, abnormalities of ultrastructural elements of cells, increase DNA fragmentation rate, and reduce the incidence of pregnancy (Pavlovych, 2020; Crespo, 2020; Yurchuk, 2021). As a result of DNA damage, gene function may be impaired, leading to the blockage of embryonic development at various stages of ontogeny. At present, the question about the rate of DNA fragmentation of goat sperm in different breeding seasons and possible changes of this indicator after cryopreservation of sperm/remains open.
The aim of the study was to determine seasonal changes in the viability, motility, and rate of DNA fragmentation of native and cryopreserved goat sperm.
MATERIALS AND METHODS
The study used the ejaculate of five sexually mature male goats of the Saanen breed (Capra hircus hircus), which were raised on the Tatiana 2011 farm (the village of Usivka, Kyiv oblast, Ukraine). All manipulations with animals were carried out in compliance with the norms approved by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1987), the Law of Ukraine “On the Protection of Animals from Cruelty” (Ukraine, 2006) and the decision of the Committee on Bioethics of the Institute for Problems of Cryobiology and Cryomedicine (National Academy of Sciences of Ukraine) (Protocol no. 1 of January 28, 2021). Ejaculate samples (n = 110) were obtained every 2 weeks during the breeding season (September–December, n = 40) and non-breeding (January–August, n = 70) seasons using an artificial vagina (Minitube, Germany) and an estrous goat to attract males.
Immediately after ejaculation, sperm concentration, motility, and viability were determined. The number of cells was counted using a Makler chamber (Sefi Medical Instrument, Israel) under an AmScope B120C light microscope (AmScope, United States). Sperm were considered motile at a rate of more than 50 μm per sec. Cell viability was determined based on the color of the eosin-nigrosin stain (Magapor, Spain) (Agarwal, 2016). A drop of semen and a drop of dye were applied to the slide. The smear was then made and dried. Cells that excluded the dye from the cytoplasm were considered viable (no staining), and cells stained pink were considered nonviable. Staining was assessed in 200 cells in each sample and viability was expressed as a percentage.
Whole ejaculate and isolated sperm fraction were cryopreserved. For this, the ejaculate was diluted with a HEPES buffer (WASH, IVF Bioscience, United Kingdom).
The isolated sperm fraction was obtained by centrifugation the ejaculate for 10 min at 325 g. The supernatant was then removed and 100 μL of the HEPES buffer (WASH, IVF Bioscience, United Kingdom) was added to the precipitate.
The freezing of the ejaculate and isolated sperm was performed using a two-stage method (Jimenez-Rabadan, 2013) with no modifications. The cryoprotectant based on the HEPES buffer with the addition of 10% glycerol (BASF, Germany) and 20% egg yolk was prepared. To do this, the isolated ejaculate fraction was gradually mixed with the cryoprotective medium in a ratio of 1 : 1, and the final concentration of sperm was 200 million/mL. The sperm suspension with the cryoprotectant was transferred to 0.25 mL cryostraws (Minitube, Germany), equilibrated for 15 min at room temperature (+25°C), then cooled for 2.5 h at +5°C and 15 min in liquid nitrogen vapors at a distance of 4 cm, followed by immersion in liquid nitrogen. The samples were heated in a water bath at a temperature of +37°C for 30 s. The cryoprotectant was removed by centrifugation, after which the HEPES buffer (WASH, IVF Bioscience, United Kingdom) was added to the precipitate and the motility, viability, and rate of fragmentation of sperm DNA were assessed. After warming up, the viability was assessed by staining with eosin.
Determination of the DNA fragmentation rate was performed using the Halosperm kit (Halotech, Spain) according to the manufacturer’s protocol. The principle of determination is based on the SCD method (sperm chromatin dispersion) (Fernandez, 2005). Sperm were immobilized in an agarose gel on a glass slide, treated with an acid solution for DNA denaturation, and then with a buffer for lysis of membranes and proteins. Next, after fixation in an ethanol solution, the samples were stained with an eosin and thiazine solution to visualize dispersed DNA loops. Sperm with fragmented DNA had very small or no halos of dispersion, while sperm with low rate of fragmentation released DNA loops that form large halos. The samples were visualized under an AmScope B120C light microscope (AmScope, United States) and the number of sperm with whole and damaged DNA was counted.
Each of the obtained ejaculates was divided into four parts, which consisted of the following groups: 1A—fresh ejaculate of the breeding season; 2A—isolated from the ejaculate fraction of sperm; 3A—cryopreserved ejaculate; 4A—cryopreserved isolated fraction of sperm. Groups 1B to 4B were divided according to the principle of groups 1A to 4A, with the difference that the ejaculates of the animals were obtained in the non-breeding season.
Statistical data processing was performed using the Origin 8.5 software (OriginLab Corporation, United States). Data were presented as mean ± standard deviation. A nonparametric test was used to compare the two samples, and the Mann–Whitney U test was used to compare samples with an abnormal distribution; the difference was considered significant at p ≤ 0.05.
RESULTS AND DISCUSSION
The concentration of sperm of goats in the breeding season was (3.09 ± 0.69) × 109 mL, and that in non-breeding season was (1.64 ± 0.87) × 109 mL.
The motility of sperm of group 1A was (70.43 ± 11.01)%, which was significantly higher (p ≤ 0.05), that in group 1B was (52.22 ± 17.16)% (Fig. 1). The same trend was observed in groups 2A and 2B. Cryopreservation led to a significant reduction in the number of motile sperm of groups 3A, 4A, and 4B (p ≤ 0.05). The smallest number of motile cells was recorded in group 4B.
Sperm motility of male goats of different study groups. *—The difference is significant compared to the corresponding group of the breeding season; #—the difference is significant compared to the corresponding group 1 of the same season; ^—the difference is significant compared to the corresponding indicator of group 2 of the same season, p ≤ 0.05.
When comparing the viability of sperm, its decrease was noted in all groups of the non-breeding season compared to the breeding season (p ≤ 0.05) (Fig. 2). We did not observe a decrease in cell viability in groups 2A and 2B. The largest changes in the studied indicator occurred in groups 3B and 4B.
Viability of sperm of male goats of different study groups. *—The difference is significant compared to the corresponding group of the breeding season; #—the difference is significant compared to the corresponding group 1 of the same season; ^—the difference is significant compared to the corresponding indicator of group 2 of the same season, p ≤ 0.05.
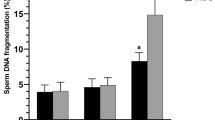
A study of the rate of DNA fragmentation of fresh ejaculates of goats in different breeding seasons showed a significant difference between groups (Figs. 3, 4). Thus, this indicator of group 1B increased by 2.7 times compared to group 1A (p ≤ 0.05). As in the study of motility and viability, a decrease in the number of cells with whole DNA was found in all groups of the non-breeding season compared to the breeding season. It was found that the DNA fragmentation rate of sperm of group 3A did not change significantly, while it increased by almost 30% in group 4A. DNA damage of spermatozoa of groups 3 and 4B increased significantly to 5.66 ± 1.90 and (7.18 ± 3.46)%, respectively (p ≤ 0.05).
Rate of DNA fragmentation of sperm of male goats of different study groups. * —The difference is significant compared to the corresponding group of the breeding season; #—the difference is significant compared to the corresponding group 1 of the same season; ^—the difference is significant compared to the corresponding indicator of group 2 of the same season, p ≤ 0.05.
DNA fragmentation in fresh ejaculate may be related to natural factors, such as seasonality, animal age, and cell metabolism (Gonzalez-Marin, 2012; Hamilton, 2019). In the breeding season, which most often occurs during the cool season, the testes are not affected by exogenous thermal factors that can adversely affect spermatogenesis (Crespo, 2020). It is shown that thermal radiation at the stage of spermatogenesis leads to the impairment of the proper formation of disulfide bonds of sperm protamines (Hamilton, 2018), which is the reason for their easier denaturation in turn. As a result, DNA loses proper compaction and integrity (Love, 1999). Moreover, DNA decompaction makes it more vulnerable to the action of nucleases, which break it down into fragments (Sotolongo, 2005). Heat shock also leads to the destruction of sperm, during which reactive oxygen species are formed (Ball, 2001). Reactive oxygen species cause oxidative stress, which leads to DNA fragmentation in turn (Crespo, 2020).
In our study, seasonality affected several major characteristics of sperm. During the breeding season, the concentration of sperm in the ejaculate, their motility, viability, and DNA integrity were higher compared to those of the non-breeding season. Such data are typical for other animals, such as horses (Gamboa, 2010; Crespo, 2020), camels (Al-Bulushi, 2018; Elsharnoby, 2021), deer (Martinez-Pastor, 2005), bison (Krishnakumar, 2015), and buffaloes (Shahback, 2020). At the same time, a group of scientists (Garcia-Macias, 2006) found the opposite seasonal dependence of the rate of DNA fragmentation in sheep, red deer, and brown bears. The authors explain this by the fact that chromatin is less condensed in the breeding season, probably due to enhanced spermatogenesis and accelerated transit of sperm through the ducts of the epididymis. Therefore, the seasonal dependence of sperm DNA integrity is a species-specific feature.
The rates of motility, viability, and DNA fragmentation of cells isolated from ejaculate by centrifugation (groups 2A and 2B) did not differ from those of groups 1A and 1B, respectively. This indicates that the separation of the sperm fraction by a single centrifugation did not lead to significant negative changes in the functional characteristics of sperm of different breeding seasons.
It is known that the structure of chromatin and sperm DNA can be altered or damaged during cryopreservation (Yurchuk, 2021). Damage to the integrity of DNA can be caused by factors such as cytotoxic substances, which, of course, include cryoprotectant solutions, components of sperm dilution and the method of its production, storage conditions, and cold shock during freezing (Gonzalez-Marin, 2012).
The results of our study showed that cryopreservation significantly reduced the integrity of sperm DNA in the non-breeding period (p ≤ 0.05). This is consistent with the findings of scientists who showed the effect of seasonality on the rate of DNA fragmentation of cells cryopreserved and incubated for 2–48 h at 37°C (Lypez-Fernandez, 2011).
The freezing of fresh ejaculate sperm during the breeding season did not increase the rate of DNA fragmentation and reduced sperm motility, in contrast to the selected fraction of gametes of the same season (p ≤ 0.05). On the other hand, cryopreservation of ejaculate or isolated sperm in the non-breeding season led to a decrease in the motility and integrity of DNA. This finding indicates that the seminal fluid in the breeding season has a composition different from the non-breeding season, which provides greater cryoprotective properties, reducing the degree of DNA damage and sperm motility. It has been shown that seminal plasma components can improve sperm cryotolerance. Thus, a group of Czech scientists (Bubenickova, 2020) showed that the seminal plasma of horses contains proteins that can have a positive effect on sperm characteristics after cryopreservation. Similar results were obtained for the cryopreservation of pig sperm (Recuero, 2019) and bulls (Zoca, 2021). Moreover, it has been shown that some components of seminal plasma, including various proteins, can interact with sperm and attach to their surface, thus protecting the sperm membrane from cryo-damage. Those seminal plasma proteins that increased during the breeding season were mainly involved in the regulation of lipid metabolism, prevention of premature capacitance, and protection against cold shock, which increases the susceptibility of gametes to oxidative stress, leading to disruption of the plasma membrane structure, proteins, and DNA (Üstuüner, 2015; Peris-Frau, 2020). The presence of antioxidants in semen has a positive effect on the onset of redox balance (Li, 2017). However, our studies have shown a positive effect of semen depending on the season. Therefore, it is necessary to study which components of the antioxidant system change during the breeding and non-breeding seasons.
Thus, based on our data, we can conclude that the composition of semen varies depending on the breeding season, which affects cryoprotective properties of sperm during ejaculate freezing. Therefore, it is advisable to cryopreserve the ejaculate obtained during the breeding season for further use for artificial insemination and in vitro fertilization regardless of the breeding season.
The aim of further research will be to establish the composition of seminal fluid, which changes between breeding seasons and affects the cryostability of DNA of frozen sperm.
CONCLUSIONS
The main parameters of goat sperm, such as motility, viability, and DNA integrity are seasonally dependent and decrease in the non-breeding season. Cryopreservation significantly increases the rate of DNA fragmentation and reduces the viability and motility of the sperm of male goats in the non-breeding season. Removal of the sperm from the ejaculate led to a decrease in the viability of cryopreserved sperm, regardless of the breeding season. The cryopreservation of sperm obtained during the breeding season with semen leads to the preservation of their motility and DNA integrity. Therefore, to improve the efficiency of artificial insemination using the cryopreserved sperm of male Saanen goats, it is recommended to collect and freeze whole ejaculate in autumn and early winter.
REFERENCES
Agarwal, A., Gupta, S., and Sharma, R., Eosin-Nigrosin Staining Procedure Andrological Evaluation of Male Infertility, Switzerland: Springer-Verlag, 2016, pp. 73–77. https://doi.org/10.1007/978-3-319-26797-5_8
Al-Bulushi, S., Manjunatha, B., de Graaf, S., and Rickard, J., Reproductive seasonality of male dromedary camels, Anim. Reprod. Sci., 2019, vol. 202, pp. 10–20. https://doi.org/10.1016/j.anireprosci.2018.12.013
Arrebola, F. and Abecia, J., Effects of season and artificial photoperiod on semen and seminal plasma characteristics in bucks of two goat breeds maintained in a semen collection center, Vet. World, 2017, vol. 10, no. 5, pp. 521–525. https://doi.org/10.14202/vetworld.2017.521-525
Bailey, J., Morrier, A., and Cormier, N., Semen cryopreservation: Successes and persistent problems in farm species, Can. J. Anim. Sci., 2003, vol. 83, no. 3, pp. 393–401. https://doi.org/10.4141/a03-024
Ball, B., Vo, A., and Baumber, J., Generation of reactive oxygen species by equine spermatozoa, Am. J. Vet. Res., 2001, vol. 62, no. 4, pp. 508–515. https://doi.org/10.2460/ajvr.2001.62.508
Barbas, J. and Mascarenhas, R., Cryopreservation of domestic animal sperm cells, Cell Tissue Banking, 2008, vol. 10, pp. 49–62. https://doi.org/10.1007/s10561-008-9081-4
Belkadi, S., Safsaf, B., Heleili, N., et al., Seasonal influence on sperm parameters, scrotal measurements, and serum testosterone in Ouled Djellal breed rams in Algeria, Vet. World, 2017, vol. 10, no. 12, pp. 1486–1492. https://doi.org/10.14202/vetworld.2017.1486-1492
Bubenickova, F., Postlerova, P., Simonik, O., et al., Effect of seminal plasma protein fractions on stallion sperm cryopreservation, Int. J. Mol. Sci., 2020, vol. 21, no. 17, art. ID 6415. https://doi.org/10.3390/ijms21176415
Chemineau, P., Malpaux, B., Brillard, J., and Fostier, A., Seasonality of reproduction and production in farm fishes, birds and mammals, Animal, 2007, vol. 1, no. 3, pp. 419–432. https://doi.org/10.1017/s1751731107691873
Crespo, F., Quiñones-Pérez, C., Ortiz, I., et al., Seasonal variations in sperm DNA fragmentation and pregnancy rates obtained after artificial insemination with cooled-stored stallion sperm throughout the breeding season (spring and summer), Theriogenology, 2020, vol. 148, pp. 89–94. https://doi.org/10.1016/j.theriogenology.2020.02.032
De Vries, A., Steenholdt, C., and Risco, C., Pregnancy rates and milk production in natural service and artificially inseminated dairy herds in Florida and Georgia, J. Dairy Sci., 2005, vol. 88, no. 3, pp. 948–956. https://doi.org/10.3168/jds.s0022-0302(05)72762-4
Dias, J. and Veloso, C., A influencia do fotoperíodo na reprodução do macho caprino e ovino, Res. Soc. Dev., 2020, vol. 9, no. 10, art. ID e4359108243. https://doi.org/10.33448/rsd-v9i10.8243
Elsharnoby, H., Kandil, O., and Abu-Elnaga, H., Dromedary camel epididymal sperm characteristics at breeding and non-breeding seasons, Al-Azhar Bull. Sci., 2021, vol. 32, no. 1, pp. 1–9. https://doi.org/10.21608/absb.2021.67232.1104
Fatet, A., Pellicer-Rubio, M., and Leboeuf, B., Reproductive cycle of goats, Anim. Reprod. Sci., 2011, vol. 124, nos. 3–4, pp. 211–219. https://doi.org/10.1016/j.anireprosci.2010.08.029
Fernández, J., Muriel, L., and Goyanes, V., Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test, Fertil. Steril., 2005, vol. 84, no. 4, pp. 833–842. https://doi.org/10.1016/j.fertnstert.2004.11.089
Gamboa, S., Rodrigues, A., Henriques, L., et al., Seasonal functional relevance of sperm characteristics in equine spermatozoa, Theriogenology, 2010, vol. 73, no. 7, pp. 950–958. https://doi.org/10.1016/j.theriogenology.2009.11.023
Garcia-Macias, V., Martinez-Pastor, F., Alvarez, M., et al., Seasonal Changes in Sperm Chromatin Condensation in Ram (Ovis aries), Iberian Red Deer (Cervus elaphus hispanicus), and Brown Bear (Ursus arctos), J. Androl., 2006, vol. 27, no. 6, pp. 837–846. https://doi.org/10.2164/jandrol.106.000315
González-Marín, C., Gosálvez, J., and Roy, R., Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells, Int. J. Mol. Sci., 2012, vol. 13, no. 11, pp. 14026–14052. https://doi.org/10.3390/ijms131114026
Hamilton, T. and Assumpção, M., Sperm DNA fragmentation: causes and identification, Zygote, 2019, vol. 28, no. 1, pp. 1–8. https://doi.org/10.1017/s0967199419000595
Hamilton, T., Siqueira, A., Castro, L., et al., Effect of heat stress on sperm DNA: protamine assessment in ram spermatozoa and testicle, Oxid. Med. Cell Longevity, 2018, vol. 2018, art. ID 5413056. https://doi.org/10.1155/2018/5413056
Jiménez-Rabadán, P., Ramón, M., García-Álvarez, O., Maroto-Morales, A., et al., Improved cryopreservation protocol for Blanca-Celtibérica buck semen collected by electroejaculation, Cryobiology, 2013, vol. 67, no. 3, pp. 251–257. https://doi.org/10.1016/j.cryobiol.2013.08.002
Johnson, S. and Jones, R., A stochastic model to compare breeding system costs for synchronization of estrus and artificial insemination to natural service, Prof. Anim. Sci., 2008, vol. 24, no. 6, pp. 588–595. https://doi.org/10.15232/s1080-7446(15)30909-8
Kopeika, E.F., Petrushko, M.P., Piniaiev, V.I., et al., Cryopreservation of reproductive cells and embryos of laboratory, agricultural and wild animals, Probl. Cryobiol. Cryomed., 2019, vol. 29, pp. 3–18. https://doi.org/10.15407/cryo29.01.003
Krishnakumar, S., Whiteside, D.P., Elkin, B., et al., Effect of reproductive seasonality on gamete quality in the North American bison (Bison bison bison), Reprod. Domest. Anim., Zuchthygiene, 2015, vol. 50, no. 2, pp. 206–213. https://doi.org/10.1111/rda.12471
Li, J., Tvarijonaviciute, I., Molina, A., et al., Seminal plasma antioxidants are directly involved in boar sperm cryotolerance, Theriogenology, 2018, vol. 107, pp. 27–35. https://doi.org/10.1016/j.theriogenology.2017.10.035
López-Fernández, C., Johnston, S.D., Gosálbez, A., et al., Seasonal changes in sperm DNA fragmentation of Murciano-Granadina goats: The compelling case for dynamic assessment, Small Ruminant Res., 2011, vol. 100, pp. 50–53. https://doi.org/10.1016/j.smallrumres.2011.05.006
Love, C.C. and Kenney, R.M., Scrotal heat stress induces altered sperm chromatin structure associated with a decrease in protamine disulfide bonding in the stallion, Biol. Reprod., 1999, vol. 60, no. 3, pp. 615–620. https://doi.org/10.1095/biolreprod60.3.615
Martinez-Pastor, F., Guerra, C., Kaabi, M., et al., Season effect on genitalia and epididymal sperm from Iberian red deer, roe deer and Cantabrian chamois, Theriogenology, 2005, vol. 63, no. 7, pp. 1857–1875. https://doi.org/10.1016/j.theriogenology.2004.08.006
Paramio, M.T. and Izquierdo, D., Current status of in vitro embryo production in sheep and goats, Reprod. Domest. Anim., 2014, vol. 49, no. s4, pp. 37–48. https://doi.org/10.1111/rda.12334
Pavlovych, O., Hapon, H., Yurchuk, T., et al., Ultrastructural and functional characteristics of human spermatozoa after cryopreservation by vitrification, Probl. Cryobiol. Cryomed., 2020, vol. 30, no. 1, pp. 24–33. https://doi.org/10.15407/cryo30.01.024
Peris-Frau, P., Soler, A.J., Iniesta-Cuerda, M., et al., Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality, Int. J. Mol. Sci., 2020, vol. 21, no. 8, art. ID 2781. https://doi.org/10.3390/ijms21082781
Recuero, S., Fernandez-Fuertes, B., Bonet, S., et al., Potential of seminal plasma to improve the fertility of frozen-thawed boar spermatozoa, Theriogenology, 2019, vol. 137, pp. 36–42. https://doi.org/10.1016/j.theriogenology.2019.05.035
Shahzad, Q., Waqas, M., Pu, L., et al., Seasonality and photoperiod influence in vitro production of buffalo embryos, Reprod. Domest. Anim., 2020, vol. 55, no. 9, pp. 1115–1123. https://doi.org/10.1111/rda.13749
Shcherbak, O., Trotskii, P., and Zyuzyun, A., Biotekhnolohichni metody oderzhannya i zberihannya hamet silʹsʹkohospodars’kykh tvaryn, in Faktory Eksperymentalʹnoyi Evolyutsiyi Orhanizmiv, Kyiv: Logos, 2008, vol. 5, pp. 382–385.
Sotolongo, B., Huang, T.T.F., Isenberger, E., et al., An endogenous nuclease in hamster, mouse and human spermatozoa cleaves DNA into loop-sized fragments, J. Androl., 2005, vol. 26, no. 2, pp. 272–280. https://doi.org/10.1002/j.1939-4640.2005.tb01095.x
Suliman, Y., Becker, F., Tuchscherer, A., et al., Seasonal variations in quantitative and qualitative sperm characteristics in fertile and subfertile stallions, Arch. Anim. Breed., 2020, vol. 63, no. 1, pp. 145–154. https://doi.org/10.5194/aab-63-145-2020
Üstüner, B., Nur, Z., Alçay, S., et al., Effect of freezing rate on goat sperm morphology and DNA integrity, Turk. J. Vet. Anim. Sci., 2015, vol. 39, pp. 110–114. https://doi.org/10.3906/vet-1407-70
Wrench, N., Pinto, C., Klinefelter, G., et al., Effect of season on fresh and cryopreserved stallion semen, Anim. Rep. Sci., 2010, vol. 119, nos. 3–4, pp. 219–227. https://doi.org/10.1016/j.anireprosci.2010.02.007
Yurchuk, T., Petrushkî, M., Gapon, A., et al., The impact of cryopreservation on the morphology of spermatozoa in men with oligoasthenoteratozoospermia, Cryobiology, 2021, vol. 100, pp. 117–124. https://doi.org/10.1016/j.cryobiol.2021.02.009
Yurchuk, T.O., Pavlovich, O.V., Gapon, G.O., et al., Lipid peroxidation and DNA fragmentation in fresh and cryopreserved spermatozoa of men at different spermatogenesis state, Ukr. Biochem. J., 2021, vol. 93, no. 3, pp. 24–29. https://doi.org/10.15407/ubj93.03.024
Zoca, G.B., Celeghini, E.C.C., Pugliesi, G., et al., Influence of seminal plasma during different stages of bovine sperm cryopreservation, Reprod. Domest. Anim., 2021, vol. 56, no. 6, pp. 872–883. https://doi.org/10.1111/rda.13928
Funding
The work was supported by the National Academy of Sciences of Ukraine within the priority topic 2.2.6.130 “Assessment of the Degree of DNA Fragmentation of Spermatogenic Cells of Different Differentiation Stages as a Mandatory Component of Their Cryopreservation Technology.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
About this article
Cite this article
Bogdaniuk, A.O., Yurchuk, T.O. & Petrushko, M.P. Seasonal Differences in Sperm Characteristics and the Level of DNA Fragmentation in Fresh and Cryopreserved Sperm of Saanen Goats. Cytol. Genet. 56, 410–416 (2022). https://doi.org/10.3103/S0095452722050036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722050036