Abstract
The new agents are needed in treatment of gastric ulcer that have less side effects, adequate efficacy, and no drug interactions. In this study, we aimed to investigate the potential protective effects of humic acid on experimental gastric ulcer. Wistar Albino male rats (n = 48) were randomly divided into 8 groups as follow; Control (without any applications), Humic acid (50 mg/kg), ethanol group (1 mL/rat), and indomethacin group (25 mg/kg). In the treatment groups, both gastric ulcer model and humic acid 50 mg/kg were applied. In addition, famotidine the antiulcer drug was used as positive control. All medications were administered by oral gavage. Levels of ADAM10 and ADAMTS12 in gastric mucosa were determined by ELISA method. Hematoxylin-Eosin (H&E) staining, iNOS, and PCNA immunohistochemical staining were performed for histopathological investigations. Apoptosis was demonstrated by using the TUNEL method. In addition, the levels of inflammatory cytokines (TNF-α, IL-6, IL-10) and caspase-3 gene were determined by qRT-PCR. ADAM10 and ADAMTS12 levels significantly increased in the treatment groups compared to the ulcer groups (p < 0.05). The experimental groups showed mucosal erosion, bleeding, leukocyte infiltration and edema. Treatment with humic acid and famotidine was found to suppress iNOS activity, thereby decreasing proinflammatory activity and preventing damage to the gastric mucosa, while reducing the number of apoptotic cells. IL-6, IL-10, TNF-α and caspase-3 levels were significantly decreased in the treatment groups compared to damaged gastric mucosa. As a result, humic acid may be defined as a potential protective agent with its anti-inflammatory effect in gastric ulcer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Gastritis and gastric ulcer are the most common forms of gastrointestinal diseases that have increasing rate of morbidity and mortality (Chow and Sung, 2009). The main pathogenesis of ulcer is the disruption of the integrity of the gastroduodenal mucosa due to the changes in balance between aggressive and protective/restorative factors. The most important aggressive factors are acid and pepsin formation, while prevention/repair mechanisms of gastroduodenal mucosa are disrupted due to genetic, environmental and infectious factors with the help of aggressive factors and ultimately ulcer occurs (Takeuchi et al., 1991; Pan et al., 2008). Today, proton pump inhibitors, histamine receptor antagonists, anticholinergics and antibiotics are used in combination for the treatment of gastritis and gastric ulcer (Stewartand and Ackroyd, 2011). Medicines for gastric ulcer treatment are mainly used to relieve symptoms, promoting ulcer healing, preventing ulcer relapse, and avoiding complications. So far, two main kinds of treatment methods are used in clinical practice: those for treating hyperacidity and for protecting the gastric mucosa (DeVault and Talley, 2009; Sen et al., 2009; Baraka et al., 2010; Liu et al., 2012; Chang et al., 2015). However, the potential side effects of these drugs, their limited effects and their interactions with other drugs appear to be shortcomings of these medications (Fisher and Le Couteur, 2001; Wedemeyerand and Blume, 2014). Therefore, there is a need for novel medications that have less side effects and less toxic.
For this purpose, herbal medicine applications have gained importance in recent years and have found widespread application areas. Most recent studies about the treatment of gastrointestinal disorders have focused on the potential role of natural medicine due to their availability, better protection, lower cost, and lower toxicity (Lee et al., 2009; Bansal and Goel, 2012). There are many studies about natural molecules such as safranal (Tamaddonfard et al., 2019), Cuphea ignea extract (Mousa et al., 2019), oxyresveratrol (Aziz et al., 2019), Kangfuxin Periplaneta Americana L. extract (Lu et al., 2019), spirulina (Mahmoud and El-Ghffar, 2018), a natural pigment sodium copper chlorophyllin (Lv et al., 2019) and crocin (El-Maraghy et al., 2015) for the treatment of gastric ulcer. In these studies, the agents used in the protection for gastric mucosal damage have anti-inflammatory, antioxidant and antiapoptotic effects, and they have a relevant effects on wound healing.
The ADAM protein family have a-secretase activity and act on inflammation, cell proliferation, angiogenesis and wound healing (Eto et al., 2000; Seals and Courtneidge, 2003). ADAM10 has a wide range of functions, such as ECM (extracellular matrix) degradation, local shedding of various cell surface proteins and affecting cell signaling patterns. ADAM10 mediates the migration of epithelial cells that are critical for wound healing (Lemjabbar and Basbaum, 2002; Yan et al., 2002). It was reported that ADAM10 levels decrease in gastroduodenal mucosa samples taken from patients with gastritis and ulcers (Erin et al., 2018). ADAMTSs are closely related to ADAM proteinases that play a role in ectodomain shedding or activation of various cell surface molecules, including growth factors and adhesion receptors (Seals and Courtneidge, 2003). ADAMTS12 was associated with osteoarthritis of cartilage components due to its ability to disrupt aggrecan and cartilage oligomeric matrix protein (Somerville et al., 2004). Tissues with ADAMTS12 deficiency were found to show a significant increase in several inflammatory parameters at both RNA and protein levels (Kurz et al., 2006). ADAMTS12 plays a protective role in inflammatory pathogenesis (Moncada-Pazos et al., 2012). However, there is no study that reveals the role of ADAM10 and ADAMTS12 in the development of gastric ulcer and the effects of the agents used for treatment on such metalloproteases.
Humic acids have medical importance and are often found in peat, sapropel, mummies and other humus substances (Archarya et al., 1998; Yudina et al., 1998). Humic acids were used as externally applicable drugs for the clinical treatment of diseases such as hematoma, phlebitis, desmorrhexis, myogelosis, arthrosis, polyarthritis, osteoarthritis and osteochondrosis (Laub, 1999). Daily doses of humic substance extract were determined as 0.05, 0.1, 0.15, 0.2, 0.3, 0.4 and 0.5 g/kg for rabbits and mice. The researchers did not observe any morphological and histological changes in animals compared with the control group at all doses (Kel’ginbaev et al., 1973).
In our study, the effects of humic acid at dose of 0.05 g/kg administered to animals were examined, as given in the literature. Studies about ulcers with substances containing humic acid are available in the literature. There are no studies showing the protective effects of humic acid, which is a common area of use, directly on gastric ulcers.
Therefore, in our study; in gastric ulcer models created using ethanol and also indomethacin, the aim was to investigate the protective effects of humic acid with biochemical, histopathological, immunohistochemical and genetic analysis. Another aim of this study was to determine whether the effects of humic acid have a relationship with changes in stomach ADAM10 and ADAMTS12 levels.
MATERIALS AND METHOD
Study Design
Procurement of animals and the experimental stage was carried out in Canakkale Onsekiz Mart University Experimental Research Application and Research Center (COMUDAM). In our study, 48 adult Wistar Albino male rats with the same biological and physiological characteristics were used and kept in standard laboratory conditions (22 ± 1°C, 12 h light/dark cycle). The animals were randomly divided into 8 groups (n = 6). The grouping of subjects was planned as follows. The drugs were administered to the rats orally. Humic acid was obtained from Sigma-Aldrich, CAS Number 1415-93-6.
Group 1: Control group—There was no application other than distilled water, rats were sacrificed at the end of 24 h of fasting and stomachs were removed.
Group 2: Indomethacin group (Indo) Negative control—After 24 h of fasting, 25 mg/kg dose of indomethacin was administered, stomachs were taken after 6 h.
Group 3: Ethanol group (Eth) Negative control—After 24 h fasting, 1 mL/rat dose of absolute ethanol (>99.5%) was given using oral gavage. After 90 minutes, the stomachs were taken.
Group 4: Humic Acid group (Hum)—After 24 h of fasting, 50 mg/kg dose of humic acid was administered. After 90 min, stomachs were taken.
Group 5: Humic Acid + Indomethacin group (Hum + Indo) – Rats were given a dose of 50 mg/kg humic acid after 24 h of fasting. After 5 min, 25 mg/kg of indomethacin was administered and stomach was removed after 6 h.
Group 6: Humic + Ethanol group (Hum + Eth)—Following 24 h of fasting humic acid was administered at a dose of 50 mg/kg. After 120 min, absolute ethanol with a dose of 1 ml/rat (>99.5%) was given and 90 minutes later the stomachs were removed.
Group 7: Famotidine + Indomethacin group (Fam + Indo) Positive control—40 mg/kg famotidine was administered after 24 h of fasting. After 5 min, 25 mg/kg of indomethacin was administered and stomachs were taken after 6 h.
Group 8: Famotidine + Ethanol group (Fam + Eth) Positive Control—After 24 h fasting, 40 mg/kg dose of famotidine was applied. Then 120 min later, 1 mL/rat dose of absolute ethanol (>99.5%) was applied and stomachs were removed after 90 min).
At the end of the experiment, animals were sacrificed under ketamine-xylazine anesthesia by collecting intracardiac blood. The results obtained at the end of the study were compared with famotidine, which is currently used for antiulcer treatment.
Determination of Gastric Ulcer Index
Gastric ulcer index was determined based on a previous study by a researcher unaware of the study groups (Saviç et al., 2011). Gastric mucosal damage scored as follow; 0: no lesions; 0.5: slight hyperemia or ≤5 petechiae; 1: ≤5 erosions ≤5 mm in length; 1.5: ≤5 erosions ≤5 mm in length and many petechiae; 2: 6–10 erosions ≤5 mm in length; 2.5: 1–5 erosions > 5 mm in length; 3: 5–10 erosions > 5 mm in length; 3.5: >10 erosions > 5 mm in length; 4: 1–3 erosions < 5 mm in length and 0.5–1 mm in width; 4.5: 4–5 erosions < 5 mm in length and 0.5–1 mm in width; 5: 1–3 erosions > 5 mm in length and 0.5–1 mm in width; 6: 4 or 5 grade 5 lesions; 7: ≤6 grade 5 lesions; 8: complete lesion of the mucosa with hemorrhage. The mean gastric ulcer index of the groups was obtained by dividing the total score by the number of animals in the group.
Histopathological Analysis
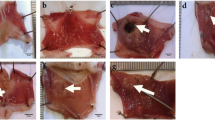
Photographs of the samples were taken for macroscopic examination (Fig. 1). Gastric tissue samples were then fixed in 10% neutral buffered formalin for 48 h. After fixation, paraffin blocks were prepared using routine histological methods. Sections of 5 micrometer thickness were stained with hematoxylin and eosin (H&E). Histopathological lesions were scored on a scale of 0–14 according to the criteria described by (Laine and Weinstein, 1988) and gastric injury score (GIS) was calculated. A total of 1 cm gastric area on each histological section was examined for epithelial cell loss (score: 0–3), edema in the mucosa (score 0–4), hemorrhagic damage (score: 0–4) and the presence of inflammatory cells (score: 0–3). All histological examinations were performed using an Olympus CX41 (Olympus, Japan) light microscopy and image analysis system (Image Analysis Software Camera Gen III, Istanbul, Turkey).
Immunohistochemical Analysis
Inducible nitric oxide synthase (iNOS) and proliferating cell nuclear antigen (PCNA) immunohistochemical markers were used for the avidin biotin complex (Hsu et al., 1981). Primary antibodies (INOS, 1 : 100, NB300-605, Novus Biologicals, Littleton, CO; PCNA, 1 : 100, Abcam 2426, Cambridge, UK) were incubated in the humidified chamber for 60 min. Secondary antibody and streptavidin peroxidase (Ultra Vision Detection System-HRP kit, Thermo Scientific/Lab Vision, Fremont, CA, USA) were used according to the manufacturer’s instructions. In addition, 3-amino-9-ethylcarbazole (AEC) was used as a chromogen for iNOS and PCNA staining to create contrast. Counter-stains were made with Mayer’s hematoxylin. INOS and PCNA positive stained cell numbers were expressed as positive stained cells/mm2 in the stomach tissue for each group.
Apoptosis
Apoptotic cells in the gastric mucosa were determined with the terminal deoxynucleotide transferase dUTP nick-end labeling (TUNEL) method (S7100 ApopTag Plus Peroxidase Onsite, Merck Millipore, Darmstadt, Germany), according to the previous study (Karaboga et al., 2018). Apoptotic cells determined using intense brown nuclear staining were calculated as positive cells/mm2 for each group.
Total RNA, cDNA, and Real-Time PCR (qRT-PCR) Analysis
It was reported in previous studies that cytokine expressions are altered in experimental acute gastric mucosal damage. IL-6, IL-10, TNF-α and Casp-3 gene expressions were determined in stomach tissue. At the end of the experimental process, approximately 10–30 mg of stomach tissue samples were taken and homogenized in the homogenizer (RETSCH brand MM 400 model). Total RNA isolation (Ambion PureLink RNA MiniKit) was performed from the obtained homogenates. Then, the concentrations of the samples were equalized by measuring the RNA concentrations and purities with the Nanodrop device. cDNA synthesis (High Capacity cDNA Reverse Transcription Kit) was performed from the RNA samples. PCR conditions are in order; Step 1: 25°C, 10 min; Step 2: 37°C, 120 min; Step 3: 85°C, 5 min. The obtained cDNAs were increased in StepOne (Thermo Scientific, USA) real time PCR in accordance with the Tagman qPCR Mastermix protocol. B-actin was used as housekeeping gene. Gene expressions were determined using Ct values and floor changes were evaluated by the 2–(ΔΔCT) method. The primary ID numbers for IL-6, IL-10, TNF-α and Casp-3 are Rn01410330_m1, Rn01483988_g1, Rn01525859_g, and Rn00563902_m1, respectively.
Biochemical Analysis
ADAMTS12, which is one of the indicators of the inflammatory process, and ADAM10 activities, which are expressed in patients with gastritis, were investigated in rat stomach tissues with the ELISA method. Tissue ADAMTS12 and ADAM10 concentrations were determined in accordance with the manufacturer’s instructions using commercially available enzyme-linked immunosorbent assay kits (ELISA) (Mybiosource Inc., San Diego, CA 92195-3308 USA). The color intensity was read at 450 nm via a multiplate reader. The results were determined in picograms per milliliter. The results that were fully compatible with the standard curve were evaluated comparatively.
Statistical Analysis
The SPSS 22 program was used for the statistical analysis of the values obtained after biochemical evaluations. Firstly, it was seen that group distributions were not normal (Shapiro-Wilk, p > 0.05). One-Way ANOVA Tukey’s test was carried out for Post Hoc Test. The data, where the difference between the groups was p < 0.05, was considered significant. All results were evaluated as mean ± standard deviation.
Data for histological evaluations were evaluated using the SPSS (PASW Statistics 21, SPSS Inc., IL, USA) statistics program. The numerical parameters of the groups were evaluated using a nonparametric test (Kruskal–Wallis) and the significance of the values obtained in the two-way comparison was measured using the Mann–Whitney U test. p < 0.05 value was considered statistically significant.
RESULTS
Macroscopic Review
As can be seen from the photos of the stomach taken for macroscopic inversion, the treatment effect of the humic acid used as the therapeutic agent is visible, while the wound and necrosis areas are visible in the models with gastric ulcer (Fig. 1). Also, gastric ulcer index of groups was expressed in Table 1. Humic acid treatment provided a significant decrease in Hum + Eth and Hum + Indo groups.
Histopathological and Immunohistochemical Analysis Findings
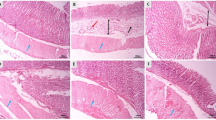
Microphotographs of H&E-stained gastric histopathological examination are presented in Fig. 2. Microscopic examination revealed normal gastric structure in the control group (Fig. 2a). Mucosal erosion, bleeding, leukocyte infiltration and edema were seen in the indomethacin and ethanol groups (Figs. 2b, 2c). Pretreatment with humic acid and famotidine resulted in a marked decrease in CBS in the gastric mucosa. The GIS scores of the groups are presented in Table 1. The data obtained as a result of immunohistochemical analysis show immunohistochemical iNOS activation in the gastric mucosa, as shown in Fig. 3. Ethanol and indomethacin administration increased gastric iNOS expression. Treatment with humic acid and famotidine suppressed iNOS activity; thereby reducing proinflammatory activity and preventing damage to the gastric mucosa. The distribution of iNOS-positive cell numbers belonging to the groups is shown in Table 1. Anti-PCNA labeled gastric mucosa sections are given in Fig. 4. In the control group, PCNA was found to be highly expressed and it was significantly reduced in the ethanol and indomethacin groups. Pretreatment with humic acid and famotidine increased PCNA activity in gastric mucosa. Treatment with humic acid and famotidine increased the number of PCNA-positive cells, accelerating epithelial regeneration and mucosal healing. PCNA-positive cell numbers in the gastric mucosa of the groups are shown in Table 1.
Microscopic examination of H&E-stained gastric mucosa. Control group had normal histological structure (a). Histopathological changes such as bleeding (star), epithelial loss (arrow) and infiltration (arrowhead) were observed in indomethacin (b) and ethanol groups (c). The humic acid group (d) was similar to the control group. Hum + Indo (e) and Hum + Eth (f) had attenuated histopathological changes. Pre-treatment with famotidine retained the structure of the gastric mucosa (g and h) (Magnification; 200×, scale bar; 100 μm).
Apoptosis in the control group was observed as several apoptotic cells seen as nuclei drowning in the gastric mucosa (Fig. 5). The number of apoptotic cells in the ethanol group increased significantly compared to the control group. Pretreatment with humic acid and famotidine reduced the number of apoptotic cells in the gastric mucosa. The apoptotic cell numbers for the groups are shown in Table 1.
Apoptotic examination of gastric tissue with the TUNEL method. There were several TUNEL positive cells in the control and humic acid groups (a and d). Indomethacin and ethanol applications caused an increase in the number of apoptotic cells (b and c). Pretreatment with humic acid and famotidine resulted in a marked reduction in the number of apoptotic cells (d, f, g and h) (magnification; 200×, scale bar 100 μm, plywood; hematoxylin).
Gene Expression Findings
In groups where ulcers were induced by applying indomethacin and ethanol, cytokine expressions were increased compared to control (p < 0.05). Figure 6 shows that humic acid administered for therapeutic purposes reduces cytokine gene expressions. Humic acid has similar effect on genes as famotidine.
Gene expression levels of IL-6 (a), IL-10 (b), TNF-a (c) and Casp-3 (d) in all groups of rat gastric tissue. After normalizing the mRNA level with P-Actin, it showed a 2–(ΔΔCT) relative expression. One-way ANOVA was used to compare gene expression levels between groups and results were evaluated according to the post hoc Tukey’s test. a: different from Indo and Eth groups, b: different from Hum, Hum + Indo, Hum + Eth, Fam + Indo and Fam + Eth groups c: different from Fam + Indo group, d: different from Hum, Fam + Indo and Fam + Eth groups, e: different from Hum + Indo, Hum + Eth and Fam + Eth groups, f: different from Hum, Hum + Indo, Hum + Eth groups g: different from Fam + Indo group. p < 0.05 statistically different.
Biochemical Analysis Findings
Results are given as mean ± standard deviation (pg/ml). When ADAMTS12 levels are examined, the ADAMTS12 level decreased significantly in the ethanol and indomethacin groups compared to the control group (p = 0.003). Similarly, ADAM10 levels were decreased in rats with gastric mucosal damage caused by ethanol and indomethacin (p = 0.000). However, when only the humic acid-treated group is evaluated, the results seem to be close to controls. The results are statistically significant compared to the damaged group (p < 0.05). Famotidine is a commercially available H2 receptor blocker used for the treatment of ulcers. The reason we used famotidine as a positive control is that, it proves the effectiveness of humic acid by comparison. When the results are examined, humic acid has similar effects to famotidine (Figs. 7, 8).
DISCUSSION
With this study, the following findings were firstly revealed in gastric ulcer models created with both ethanol and indomethacin. (a) Humic acid has a protective effect on stomach damage at the macroscopic and histopathological level, (b) humic acid suppresses iNOS and apoptosis and increases PCNA activity, (c) humic acid reduces TNF-α and IL-6 levels that are proinflammatory cytokines in the gastric mucosa, while it increases IL-10 levels which is a member of anti-inflammatory cytokines, (d) humic acid increases ADAM10 and ADAMTS12 levels, and (e) these protective effects of humic acid are as strong as famotidine.
In addition to this data, it was also revealed for the first time that (f) ADAMTS12 was also present in gastric mucosa so ADAMTS12 levels were determined in healthy, gastric ulcer models and humic acid treated groups. These results show us that humic acid has potential for wound healing with its anti-inflammatory and antiapoptotic properties, as well as affecting ADAM10 and ADAMTS12 levels in experimental gastric ulcer models.
Gastric ulcers have widespread morbidity and mortality in worldwide. High concentrations of alcohol disrupt the bicarbonate release mechanism by overcoming the gastric mucosal barrier and act against hydrogen ions to cause acute gastric mucosal lesions characterized by mucosal bleeding. Alcohol also stimulates acid secretion (Peterson et al., 1986).
Non-steroidal anti-inflammatory drugs (NSAIDs) affect the amount of stomach acid, integrity of the mucosal barrier, bicarbonate and glutathione levels, and mucosal blood flow rate through inhibition of prostaglandin synthesis (Flower, 2003). The pathogenesis of symptomatic peptic ulcer disease caused by repeated exposure to NSAIDs is basically the result of systemic (absorption) inhibition of gastrointestinal mucosal cyclo-oxygenase (COX) activity. Even the administration of intravenous or intramuscular NSAIDs can cause stomach or duodenal ulcers in animals and humans (Estes et al., 1993).
The side effects of the drugs used for the treatment of gastric ulcers are few but have led to the trials of some active substances in this area (Muazzam et al., 2015). Examples of molecules used for this purpose and understood to have beneficial effects include Rubus crataegifolius (black raspberry, RF), Ulmus macrocarpa (elm, UL) and Gardenia jasminoides (cape jasmine, GJ) (Park et al., 2019), Jinlingzi powder and extracts (Zhao et al., 2019), Cuphea ignea extract (Aziz et al., 2019), sodium copper chlorophyllin (Lv et al., 2019), safranal (Mousa et al., 2019) and Spirulina (Mahmoud and El-Ghffar, 2018). In these studies, wound healing, antioxidant, anti-inflammatory and antiapoptotic effects of the substances used were investigated in general. Therefore, in our study, we aimed to determine the wound- protective effects of humic acid at the histopathological level. In addition, we determined the antiinflammatory and antiapoptotic effects at the molecular level. Furthermore, we identified the effects on iNOS, PCNA, ADAM10 and ADAMTS12. Thus, we aimed to understand the mechanisms underlying the protective effect of humic acid in gastric ulcers.
In our study, we used two different methods to create a gastric ulcer model. Both the indomethacin-induced gastric ulcer model and the ethanol ulcer model are widely used in gastric ulcer research in pathophysiological studies and drug therapy studies (Aziz et al., 2019; Mousa et al., 2019; Park et al., 2019). We aimed to determine the protective effect of humic acid with both methods. We also administered famotidine, one of the agents commonly used in the treatment of gastric ulcers, and compared it with humic acid. Thus, it was possible to compare the protective effects of humic acid with a adjuvant agent whose effectiveness was determined for gastric ulcer. When we evaluate the protective effects of humic acid in the ulcer model, first, it turns out to be an effective agent in terms of wound healing. As a matter of fact, humic acid was shown to reduce mucosal erosion, bleeding, leukocyte infiltration and edema in the indomethacin and ethanol groups. In many studies, inflammation was identified as one of the factors responsible for gastric mucosal injury. Leukocyte and macrophage migration to ulcerated or damaged areas leads to the emergence of inflammatory functions (Aziz et al., 2019). The secretion of the proinflammatory cytokines of macrophages migrating to the injury area leads to a hike in the blood flow of ulcerated mucosa and thereby delays wound healing (Lu et al., 2019). Li and his colleagues examined the protective effect of tetrahydrocotyline (THC) in an ethanol-induced gastric ulcer model in mice (Li et al., 2013). Accordingly, it was found that pro-inflammatory cytokine (TNF-α and IL-6) levels and myeloperoxidase (MPO) activity increased and administration of THC at 10 and 20 mg/kg significantly decreased gastric lesions and proinflammatory cytokine levels compared to the ethanol group (Li et al., 2013).
In our study, we observed leukocyte infiltration microscopically in rats with a gastric ulcer model and found that TNF-α and IL-6 gene expressions, which are proinflammatory cytokines, increased in samples obtained from gastric mucosa. This increase in proinflammatory cytokines was inhibited by humic acid and the reduction in leakage of inflammatory cells and focal necrosis was confirmed with histological examination (Figs. 2–5). We also observed that humic acid acts as an anti-inflammatory, with a increasing effect on IL-10 gene expression. IL-10, in turn, acts by selectively blocking the expression of genes encoding proinflammatory cytokines and chemokines while at the same time increasing the expression of anti-inflammatory molecules and thereby promotes gastric ulcer healing (Vinagre et al., 2018).
The findings we obtained are compatible with the findings obtained after the application of Cuphea ignea (Aziz et al., 2019), Jinlingzi powder and its extractive components (Zhao et al., 2019), sodium copper chlorophyllin (El-Maraghy et al., 2015), and hesperidin (Elshozly et al., 2018) which were previously used as preservatives in different gastric ulcer studies.
It was reported that iNOS expressions increases in gastric ulcers, and TNF-α causes this increase (Yildirim et al., 2015). While increased iNOS expression induces NO formation that reacts with superoxides forming peroxynitrites (Lanas, 2018), this was shown to occur in rats treated with hesperidin (Elshozly et al., 2018), which is responsible for gastric mucosal injury. In our study, it was determined that in groups with high TNF-α levels, iNOS activity was also high and humic acid decreased iNOS activity. This suggests that the protective role of humic acid in ulcer healing may also be related to iNOS.
In our study, we observed that humic acid also increased the number of PCNA-positive cells. It can be said that this is a factor that reveals the epithelial regeneration and mucosal healing effect of humic acid. PCNA is known as an important tissue proliferation marker and PCNA activity was reported to be important for gastric mucosal healing (Caldas et al., 2014). Cellular proliferation rises with increasing PCNA reactivity and this is an indicator of ulcer re-epithelialization (Polo et al., 2012). Caldas et al. determined that Hyptis martiusii Benth oil extract increased immunohistochemical PCNA activity in the regeneration zone in rats (Lanas, 2018). Similarly, Elsaed et al. observed that increases in PCNA activation were in parallel to ulcer healing (Elsaed et al., 2015).
Humic acid also showed positive effects on apoptosis in the gastric mucosa. This effect of humic acid was seen both with a decrease in the number of apoptotic cells in TUNEL staining and a decrease in caspase-3 gene expression in the gastric mucosa. Various studies were conducted on agents that reduce apoptosis in stomach ulcers. A few of these studies are listed below, and it can be seen that the findings obtained are in line with our study. Arab et al. examined the effects of antioxidant and anti-inflammatory natural citrus diosmin, which has protective properties against cardiac, hepatic and kidney damage, in a rat model with gastric damage caused by ethanol (Arab et al., 2015). Diosmin reduced caspase-3 activity 2.4 times, which is a reliable indicator of apoptosis, and suppressed cytochrome C (Cyt C) levels by increasing anti-apoptotic Bcl-2 in favor of cell survival, as well as increasing anti-inflammatory IL-10 levels. Antonisamy et al. investigated the protective effects of friedelin from the Azima tetracantha plant in the ethanol-induced gastric ulcer model, and found that the apoptotic index of the group given ethanol was 16.64 times higher than that of the control group, and that the DNA fragmentation in the group pre-treated with friedelin decreased 4.62 times compared to the ethanol group and caspase-3 activity decreased by 4.62 time (Antonisamy et al., 2015). In the applied group, caspase-3 activity was 4.63 times higher, while the caspase-3 activity decreased by 3.62 times in the group given friedelin.
Lv et al. applied micsodium copper chlorophyllin as a preservative to mice with ethanol-induced gastric ulcer model and determined reductions in molecules that support apoptosis (Lv et al., 2019). They said this finally maintained the integrity of the normal gastric mucosal barrier. Another study revealed that activation of caspase-3 increases apoptotic cell death in gastric mucosa (Almasaudi et al., 2016).
When ADAMTS12 levels are examined, the rats with gastric mucosal damage had low levels. The low level of ADAMTS12 in lesion tissue causes the initiation of inflammatory processes. As a result, hematoma and various wounds occur with necrosis in the tissue. In a study by Moncada-Pazos et al. ulcerative colitis and sepsis were induced in transgenic mice without ADAMTS12 (Moncada-Pazos et al., 2012). It was revealed that mice without ADAMTS12 had severe inflammation. Since ADAM10 is also an indicator of inflammation, reductions in gastritis levels were observed in the presence of inflammatory factors, as determined in the literature (Erin et al., 2018). In our study, both ADAMTS12 levels decreased in damaged tissue and ADAM10 levels decreased significantly due to inflammation. In another study, it was stated that ADAMTS12 values played an important role in inflammation processes in arthritis and that their levels decreased significantly (Wei et al., 2014). Oztas et al. found that ADAMTS12 levels decreased significantly compared to controls in their study about preeclampsia and intrahepatic cholestasis of pregnancy (Oztas et al., 2016). In our study, we also showed humic acid decreased inflammation and increased ADAM10 and ADAMTS12 levels in humic acid-treated groups, however, it plays a protective effect at least as famotidine.
This study demonstrated the gastroprotective effect of humic acid using models of ethanol and indomethacin-induced ulcers. Humic acid exerts double beneficial protective effects by suppressing gastric aggressive factors, reactive oxygen species, proinflammatory cytokines, proapoptotic protein and developing gastric cytoprotective factors. The antiulcer activity of humic acid was confirmed by macroscopic and histological examination of the number and severity of ulcers, mucosal edema, epithelial abrasion of mucosal tissue, infiltration of inflammatory cells and bleeding. Consequently, humic acid was proven to have a healing effect on stomach tissues with ulcer and gastric mucosal damage, as well as increasing levels of ADAMTS12 and ADAM10, which are important metalloproteinases; thereby reducing inflammation, as well as decreasing inflammatory cytokine levels determined by gene expression. As seen in histopathological evaluations, humic acid has the protective effect against gastric mucosa damage at least as effectively as famotidine. The use of natural antioxidant products instead of chemical production drugs, which have a lot of side effects, is becoming more common. The results of this study showed that humic acid has potential as an protective agent that can be used for gastric ulcer.
REFERENCES
Acharya, S.B., Frotan, M.H., Goel, R.K., et al., Pharmacological actions of Shilajit, Indian J. Exp. Biol., 1988, vol. 26, pp. 775–777. PMID: 3248832.
Almasaudi, S.B., El-Shitany, N.A., Abbas, A.T., et al., Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats, Oxid. Med. Cell Longev., 2016, vol. 2016, art. 3643824.https://doi.org/10.1155/2016/364382
Antonisamy, P., Duraipandiyan, V., Aravinthan, A., et al., Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms, Eur. J. Pharmacol., 2015, vol. 750, pp. 167–175. https://doi.org/10.1016/j.ejphar.2015.01.015
Arab, H.H., Salama, S.A., Omar, H.A., et al., Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions, PLoS One, 2015, vol. 10, art. e0122417. https://doi.org/10.1371/journal.pone.0122417
Aziz, R.S., Siddiqua, A., Shahzad, A., et al., Oxyresveratrol ameliorates ethanol-induced gastric ulcer viadownregulation of IL-6, TNF-α, NF-ĸB, and COX-2 levels, and upregulationof TFF-2 levels, Biomed. Pharmacother., 2019, vol. 110, pp. 554–560. https://doi.org/10.1016/j.biopha.2018.12.002
Bansal, V.K. and Goel, R.K., Gastroprotective effect of Acacia nilotica young seedless pod extract: role of polyphenolic constituents, Asian Pacif. J. Trop. Med., 2012, vol. 5, pp. 523–528. https://doi.org/10.1016/S1995-7645(12)60092-3
Baraka, A.M., Guemei, A., and Gawad, H.A., Role of modulation of vascular endothelial growth factor and tumor necrosis factor-alpha in gastric ulcer healing in diabetic rats, Biochem. Pharmacol., 2010, vol. 79, pp. 1634–1639. https://doi.org/10.1016/j.bcp.2010.02.001
Caldas, G.F.R., Oliveira, A.R.D.S., Araújo, A.V., et al., Gastroprotective and ulcer healing effects of essential oil of Hyptis martiusii Benth. (Lamiaceae), PLoS One, 2014, vol. 9, art. e84400. https://doi.org/10.1371/journal.pone.0084400
Chang, X., Luo, F., Jiang, X., et al., Protective activity of salidro side against ethanol-induced gastric ulcer via the MAPK/NF-휅B pathway in vivo and in vitro, Int. Immunopharmacol., 2015, vol. 28, pp. 604–615. https://doi.org/10.1016/j.intimp.2015.07.031
Chow, D.K. and Sung, J.J., Non-NSAID non-H. pylori ulcer disease, Best Pract. Res. Clin. Gastroenterol., 2009, vol. 23, pp. 3–9. https://doi.org/10.1016/j.bpg.2008.11.010
DeVault, R.K. and Talley, N.G., Insights into the future of gastric acid suppression, Nat. Rev. Gastroenterol. Hepatol., 2009, vol. 6, pp. 524–532. https://doi.org/10.1038/nrgastro.2009.125
El-Maraghy, S.A., Rizk, S.M., and Shahin, N.N., Gastroprotective effect of crocin in ethanol- induced gastric injury in rats, Chem. Biol. Interact., 2015, vol. 229, pp. 26–35. https://doi.org/10.1016/j.cbi.2015.01.015
Elsaed, W.M., Alahmadi, A.M., Al-Ahmadi, B.T., et al., Gastroprotective and antioxidant effects of fluvoxamine on stress-induced peptic ulcer in rats, J. Taibah Univ. Med. Sci., 2018, vol. 13, pp. 422–431. https://doi.org/10.1016/j.jtumed.2018.04.010
Elshazly, S.M., Abd El Motteleb, D.M., et al., Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats, Chem. Biol. Interact., 2018, vol. 291, pp. 153–161. https://doi.org/10.1016/j.cbi.2018.06.027
Erin, N., Türker, S., Elpek, Ö., et al., ADAM proteases involved in inflammation are differentially altered in patients with gastritis or ulcer, Exp. Ther. Med., 2018, vol. 15, pp. 1999–2005. https://doi.org/10.3892/etm.2017.5619
Estes, L.L., Fuhs, D.W., Heaton, A.H., et al., Gastric ulcer perforation associated with the use of injectable ketorolac, Ann. Pharmacother., 1993, vol. 27, pp. 42–43. https://doi.org/10.1177/106002809302700111
Eto, K., Puzon-McLaughlin, W., Sheppard, D., et al., RGD-independent binding of integrin α9β1 to the ADAM-12 and -15 disintegrin domains mediates cell–cell interaction, J. Biol. Chem., 2000, vol. 275, pp. 34922–34930. https://doi.org/10.1074/jbc.M001953200
Fisher, A.A. and Le Couteur, D.G., Nephrotoxicity and hepatotoxicity of histamine H2 receptor antagonists, Drug Saf., 2001, vol. 24, pp. 39–57. https://doi.org/10.2165/00002018-200124010-00004
Flower, R.J., The development of COX2 inhibitors, Nat. Rev. Drug Discov., 2003, vol. 2, pp. 179–191. https://doi.org/10.1038/nrd1034
Hsu, S.M., Raine, L., and Fanger, H.X., Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures, J. Histochem. Cytochem., 1981, vol. 29, pp. 577–580. https://doi.org/10.1177/29.4.6166661
Karaboğa, İ., Ovalı, M.A., Yılmaz, A., et al., Gastroprotective effect of apricot kernel oil in ethanol-induced gastric mucosal injury in rats, Biotech. Histochem., 2018, vol. 93, pp. 601–607. https://doi.org/10.1080/10520295.2018.1511064
Kel’ginbaev, N.S., Sorokina, V.A., Stefanidu, A.G., et al., Treatment of long tubular bone fractures with Mumie Assil preparations in experiments and clinical conditions, Eksp. Khir. Anesteziol., 1973, vol. 18, pp. 31–35. PMID: 4271714.
Kurz, T., Hoffjan, S., and Hayes, M.G., Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci, J. Allergy Clin. Immunol., 2006, vol. 118, pp. 396–402. https://doi.org/10.1016/j.jaci.2006.04.036
Laine, L. and Weinstein, W.M., Histology of alcoholic hemorrhagic “gastritis”: a prospective evaluation, Gastroenterology, 1988, vol. 94, pp. 1254–1262. https://doi.org/10.1016/0016-5085(88)90661-0
Lanas, A., Role of nitric oxide in the gastrointestinal tract, Arthritis Res. Ther., 2008, vol. 10, pp. 1–6. https://doi.org/10.1186/ar2465
Laub, R.J., Process for preparing synthetic soil-extract materials and medicaments based thereon, US Patent no. CA2278759A1, 1999.
Lee, J.H., Lee, D.U., and Jeong, C.S., Gardenia jasminoides ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats, Food Chem. Toxicol., 2009, vol. 47, pp. 1127–1131. https://doi.org/10.1016/j.fct.2009.01.037
Lemjabbar, H. and Basbaum, C., Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells, Nat. Med., 2002, vol. 8, pp. 41–46. https://doi.org/10.1038/nm0102-41
Li, W., Huang, H., Niu, X., et al., Protective effect of tetrahydrocoptisine against ethanol- induced gastric ulcer in mice, Toxicol. Appl. Pharmacol., 2013, vol. 272, pp. 21–29. https://doi.org/10.1016/j.taap.2013.05.035
Liu, Y., Tian, X., Gou, L., et al., Protective effect of L-citrulline against ethanol-induced gastric ulcer in rats, Environ. Toxicol. Pharmacol., 2012, vol. 3, pp. 280–287. https://doi.org/10.1016/j.etap.2012.04.009
Lu, S., Wu, D., Sun, G., et al., Gastroprotective effects of Kangfuxin against water-immersion and restraint stress-induced gastric ulcer in rats: roles of antioxidation, anti-inflammation, and pro-survival, Pharm. Biol., 2019, vol. 57, pp. 770–777. https://doi.org/10.1080/13880209.2019.1682620
Lv, H., Lin, Y., Liu, P., et al., Protective effects and potential underlying mechanisms of sodium copper chlorophyllin against ethanol-induced gastric ulcer in mice, Acta Biochim. Biophys. Sin., 2019, vol. 51, pp. 925–933. https://doi.org/10.1093/abbs/gmz083
Mahmoud, Y.I. and El-Ghffar, E.A.A., Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation, Biomed. Pharmacother., 2018, vol. 109, pp. 314–321. https://doi.org/10.1016/j.biopha.2018.10.118
Moncada-Pazos, A., Obaya, A.J., and Llamazares, M., ADAMTS-12 metalloprotease is necessary for normal inflammatory response, J. Biol. Chem., 2012, vol. 287, pp. 39554–39563. https://doi.org/10.1074/jbc.M112.408625
Mousa, A.M., El-Sammad, N.M., Sherien, K., et al., Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats, BMC Complement. Altern. Med., 2019, vol. 19, p. 345. https://doi.org/10.1186/s12906-019-2760-9
Muazam, S., Rana, R., Jawed, S., et al., Gastroprotective effect of sagu pearls on diclofenac sodium induced gastric ulcer, J. Islam Int. Med. Coll., 2015, vol. 10, pp. 204–209.
Oztas, E., Ozler, S., Ersoy, A.O., et al., Placental ADAMTS-12 levels in the pathogenesis of preeclampsia and intrahepatic cholestasis of pregnancy, Reprod. Sci., 2016, vol. 23, pp. 475–481. https://doi.org/10.1177/1933719115604730
Pan, J.S., He, S.Z., Xu, H.Z., et al., Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury, World J. Gastroenterol., 2008, vol. 14, pp. 5857–5867. https://doi.org/10.3748/wjg.14.5857
Park, J.U., Kang, J.H., Rahman, M.A.A., et al., Gastroprotective effects of plants extracts on gastric mucosal injury in experimental Sprague–Dawley rats, BioMed. Res. Int., 2019, vol. 2019, art. 8759708. https://doi.org/10.1155/2019/8759708
Peterson, W.L., Barnett, C., and Walsh, J.H., Effect of intragastric infusions of ethanol and wine on serum gastrin concentration and gastric acid secretion, Gastroenterology, 1986, vol. 191, pp. 1390–1395. https://doi.org/10.1016/0016-5085(86)90192-7
Polo, C.M., Moraes, T.M., Pellizzon, C.H., et al., Gastric ulcers in middle-aged rats: the healing effect of essential oil from Citrus aurantium L.(Rutaceae), Evid. Based Complement. Alternat. Med., 2012, vol. 2012, art. 509451. https://doi.org/10.1155/2012/509451
Savić, J.S., Dilber, S.P., Marković, B.D., et al., Docking studies and α-substitution effects on the anti-inflammatory activity of β-hydroxy-β-arylpropanoic acids, Molecules, 2011, vol. 16, pp. 6645–6655. https://doi.org/10.3390/molecules16086645
Seals, D.F. and Courtneidge, S.A., The ADAMs family of metalloproteases: multidomain proteins with multiple functions, Genes Dev., 2003, vol. 17, pp. 7–30. https://doi.org/10.1101/GAD.1039703
Sen, S., Chakraborty, R., De, B., et al., Plants and phytochemicals for peptic ulcer: an overview, Phcog. Rev., 2009, vol. 3, pp. 270–279.
Somerville, R.P., Longpre, J.M., Apel, E.D., et al., ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain, J. Biol. Chem., 2004, vol. 279, pp. 35159–35175. https://doi.org/10.1074/jbc.M402380200
Stewartand, D.J. and Ackroyd, R., Peptic ulcers and their complications, Surgery, 2011, vol. 29, pp. 568–574.
Takeuchi, K., Ueshima, K., Hironaka, Y., et al., Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility, Digestion, 1991, vol. 49, pp. 175–184. https://doi.org/10.1159/000200718
Tamaddonfard, E., Erfanparast, A., Farshid, A.A., et al., Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer, Life Sci., 2019, vol. 224, pp. 88–94. https://doi.org/10.1016/j.lfs.2019.03.054
Vinagre, R.M.D.F., Vinagre, I.D.F., Vilar-e-Silva, A.,et al., Helicobacter pylori infection and immune profile of patients with different gastroduodenal diseases, Arq. Gastroenterol., 2018, vol. 55, pp. 122–127. https://doi.org/10.1590/S0004-2803.201800000-21
Wedemeyerand, R.S. and Blume, H., Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update, Drug Saf., 2014, vol. 37, pp. 201–211. https://doi.org/10.1007/s40264-014-0144-0
Wei, J., Richbourgh, B., Jia, T., et al., ADAMTS-12: a multifaced metalloproteinase in arthritis and inflammation, Mediators Inflamm., 2014, vol. 2014, art. 649718 https://doi.org/10.1155/2014/649718
Yan, Y., Shirakabe, K., and Werb, Z., The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors, J. Cell Biol., 2002, vol. 158, pp. 221–226. https://doi.org/10.1083/jcb.200112026
Yildirim, F.I.A, Uyanik, Ö., Özyogurtcu, H., et al., Aggravating effect of atorvastatin on indomethacin-induced gastricinjury: focus on PGE2, TNF-a, neutrophils, and iNOS, Prostaglandins Other Lipid Mediators, 2015, vol. 121, pp. 53–62. https://doi.org/10.1016/j.prostaglandins.2015.07.002
Yudina, N.V., Pisareva, S.I., and Saratikov, A.S., Antiulcerogenic activity of phenol. compounds of peat, Khim. Rastit. Syr’ya, 1998, vol. 4, pp. 29–32. NII Article ID (NAID) 10024717260.
Zhao, X., Li, J., Meng, Y., et al., Treatment effects of jinlingzi powder and its extractive components on gastric ulcer induced by acetic acid in rats, Evid. Based Complement. Alternat. Med., 2019, vol. 2019, art. 7365841. https://doi.org/10.1155/2019/7365841
Funding
This study was supported by the Scientific Research Commission of Canakkale Onsekiz Mart University with the project named “Investigation of the Effects of Humic Acid in Different Acute Gastric Ulcer Models in Rats” coded TSA-2018-2592.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. Our study was approved by Canakkale Onsekiz Mart University Animal Experiments Local Ethics Committee with decision 2018/05-02.
Highlights
Humic acid has a healing effect on stomach damage at the histopathological level, Humic acid suppresses iNOS and apoptosis and increases PCNA activity,
Humic acid reduces TNF-a and IL-6 levels that are proinflammatory cytokines in the gastric mucosa, while it increases IL-10 levels which is a member of anti-inflammatory cytokines, Humic acid increases ADAM10 and ADAMTS12 levels, these healing effects of humic acid are as strong as famotidine.
ADAMTS12 was also present in gastric mucosa for the first time, so ADAMTS12 levels were determined in healthy, gastric ulcer models and humic acid treated groups.
About this article
Cite this article
Şehitoğlu, M.H., Öztopuz, Ö., Karaboğa, İ. et al. Humic Acid Has Protective Effect on Gastric Ulcer by Alleviating Inflammation in Rats. Cytol. Genet. 56, 84–97 (2022). https://doi.org/10.3103/S0095452722010091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722010091