Abstract
The expedience of using oocytes with morphological dysmorphism in assisted reproduction technology programs is debatable. This study aimed to estimate morphological and molecular cytogenetic characteristics of enlarged oocytes and to predict results of their further fertilization. Among 774 oocytes retrieved from 80 patients, 83 (11%) cells were enlarged. Morphometric analysis showed the diameter of the ooplasm to be (176.67 ± 1.76) μm and the diameter with ZP (200.8 ± 4.24) μm. Among these giant oocytes, 47 (56.6%) were at the metaphase II stage, 26 (31.3%) at the metaphase I stage, and 10 (12.1%) at the prophase I stage. The retrieved giant oocytes were characterized by dysmorphism of endo- and exocytoplasmic structures. Among 47 cells at the metaphase II stage, 40 (85.1%) oocytes had two polar bodies, three (6.4%) oocytes had one polar body, and four (8.5%) oocytes had a fragmented polar body. The assessment of the presence of meiotic spindle by polarization microscopy showed that 39 (83%) oocytes had two meiotic spindles, 3 (6.4%) oocytes had one meiotic spindle, and 5 oocytes (10.6%) had no visualized meiotic spindles despite the presence of polar bodies. After fertilization of 47 oocytes at the metaphase II stage, pronuclei were detected in 42 (89.4%) cells, including 9 (21.4%) oocytes with two pronuclei, 27 (63.3%) oocytes with 3 pronuclei, and 6 (15.3%) oocytes with four pronuclei. The molecular cytogenetic analysis demonstrated that the embryos obtained after fertilization of giant oocytes had a polyploid chromosome set number. Thus, the results of our study showed the inexpediency of using giant oocytes for further fertilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Female gametes are unique cells whose main function is fertilization and support of early embryonic development since it is the oocyte that supplies the future embryo with proteins, energy substrates, and cytoplasmic organelles, which are involved in the activation of the embryonic genome (Rienzi, 2012).
After the luteinizing hormone (LH) surge, oocytes undergo nuclear and cytoplasmic maturation. Nuclear maturation depends on the formation and maintenance of the structure of the meiotic spindle, which requires centrosomal proteins, such as the nuclear mitotic apparatus and γ-tubulin, and other regulatory proteins (Reader, 2017). Cytoplasmic maturation involves the rearrangement of organelles and the final storage of mRNA, proteins, lipids, and transcription factors required during fertilization and early embryogenesis (Conti, 2018). Recent studies have shown that some of the key structures required for proper early human oogenesis are Balbiani bodies, whose composition was described to include a range of structures such as the large RNA–protein granule, centrioles, endoplasmic reticulum, Golgi complex, annular lamellae, and mitochondria. They are formed in oogonia in the complex at the perinuclear position, interact with the cytoskeleton, in particular with actin microtubules, and participate in the formation of oocyte polarity (Heim, 2014). Also, the error-free work of these structures in the ooplasm eliminates damaged mitochondria (Bilinski, 2017). At the same time, the malfunctioning of Balbiani bodies is reflected in the divergence of chromosomes, their distribution, and ploidy (Elkouby, 2017). Balbiani bodies are regulated by the Macf1a gene whose mutations disrupt early oogenesis and affect the formation of the embryo and the body as a whole (Escobar-Aguirre, 2017).
A mature female gamete consists of Zona pellucida (ZP), glycoprotein membrane, ooplasm, nucleus, and organelles (mitochondria, endoplasmic reticulum, Golgi apparatus). The size of the oocyte varies from 120 to 170 μm, the average volume is 9.05 × 105 μm3. Changes in the morphological characteristics of oocytes can be the result of internal (individual, physiological, or age-related) and external factors (for example, environmental degradation, diseases, treatment using gonadotoxic drugs or assisted reproductive technologies (ART)). Among the factors determined by ART, one can mention the induction of superovulation, which allows for obtaining a cohort of mature oocytes, in contrast to the natural menstrual cycle of women (Bosch, 2015; Wang, 2015). However, this can lead to changes in morphological characteristics, impaired nuclear and cytoplasmic maturation and, as a consequence, result in abnormalities of embryos and their poor implantation (Balaban, 2006).
Cryopreservation of oocytes is an important component of ART programs. On the one hand, its effectiveness depends on the initial state of morphological, functional and genetic characteristics of oocytes and, on the other hand, cryopreservation itself can be a factor in morphological and genetic changes in gametes (Yurchuk, 2019; Buderatska, 2020).
Therefore, the development of a system for assessing the morphological characteristics of oocytes is necessary to predict the genetic integrity, the success of cryopreservation and fertilization of oocytes, the further development of embryos in vitro, and their ability to be implanted (Buderatska, 2017). In embryological protocols, oocytes are usually sorted according to the degree of maturity after isolation: morphological characteristics of ooplasm, polar bodies (PT), and ZP. Particularly significant are oocytes that differ from the cohort of extracted oocytes by increased sizes and the abnormal number of polar bodies (Machtinger, 2011; Rosenbusch, 2012; Lehner, 2015).
The volume of the oocyte cytoplasm is considered to be proportional to the amount of organelles and accumulated metabolites that affect oocyte quality, suggesting that larger egg cells have better quality (Reader, 2017). At the same time, some authors do not recommend the use of enlarged oocytes for fertilization because these cells have a diploid set of chromosomes (Rosenbusch, 2008). Thus, the question of the expedience of using giant oocytes in ART programs remains open. The aim of our work was to evaluate the morphological and molecular cytogenetic characteristics of enlarged oocytes and to predict results of their use in ART.
MATERIALS AND METHODS
Embryological parameters of ART infertility treatment cycles were analyzed in women in whom giant oocytes were aspirated. The mean age of women was 34.9 ± 4.2 years. After aspiration of the follicles, the cumulus-oocyte complexes were transferred from the follicular fluid to the Global Total for Fertilization medium (Life Global, United States). After oocyte denudation using 80 IU/mL hyaluronidase (Life Global, United States), cells were examined for the presence of meiotic spindles by the Oosight polarization system (Hamilton Thorne, United States) using a Nikon Eclipse Ti–U microscope (Japan) with Narishige micromanipulators (Rosenbusch, 2012).
Prior to fertilization by intracytoplasmic sperm injection (ICSI), oocytes were cultured in the above-mentioned medium in atmosphere of 6% CO2 (Petrushko, 2018). Enlarged (giant) oocytes were examined under an inverted microscope at 400× magnification (Olympus IX-71, Japan). Their diameter was measured and polar bodies and meiotic spindles were counted. After fertilization, the embryos were cultured in the Global Total medium (Life Global, United States).
On the fifth or sixth day of development, trophectoderm cell biopsy was carried out for preimplantation genetic testing of embryos for aneuploidy (PGT-A) by fluorescent in situ hybridization (FISH).
Cytogenetic analysis of unfertilized oocytes was carried out according to the Tarkovsky–Dyban method.
Statistical processing of results was done in the Statistica 6.0 software.
RESULTS AND DISCUSSION
From 80 patients who received infertility treatment by ART, 774 oocytes were retrieved, including 83 (11%) enlarged cells (Fig. 1).
According to morphometric analysis, the diameter of the ooplasm was (176.67 ± 1.76) μm, and the diameter of the oocyte with ZP was (200.8 ± 4.24) μm. Thus, giant oocytes were 38% larger than normal female gametes, which have diameters of 127.17 ± 4.67 and (146.22 ± 3.25) μm, when measuring the ooplasm and the oocyte with ZP, respectively. This increase was due to the larger diameter of the oolemma, since ZP sizes were (24.13 ± 3.4) μm for giant oocytes and (19.05 ± 2.2) μm for normal oocytes, which was not statistically significant.
N. Balakier et al. reported a slightly larger average diameter of giant human oocytes when measuring oocytes with ZP, 147.73 mm (Balakier, 2002).
During aspiration of follicles in women aged 22–35, 36–39, and over 40 years, 48 (58%), 21 (25%), and 11 (17%) giant oocytes were retrieved, respectively. Thus, giant oocytes were significantly more likely to occur in younger women, which is consistent with data from other researchers [16].
Among 83 giant oocytes, 47 (56.6%) cells were in the metaphase II stage, 26 (31.3%) cells were in the metaphase I stage, and 10 (12.1%) cells were in the prophase stage of the first meiotic division and were characterized by the presence of two germinal vesicles (GV) (Fig. 2).
Researchers of oocyte diploidy have found that it is associated with cytoplasmic immaturity (Almeida, 1993). Experiments on mouse oocytes emphasized the importance of synchrony between nuclear and cytoplasmic maturation for the correct sequence of events after fertilization. In particular, impaired synthesis of proteins involved in spindle formation and cytokinesis may be the reason for the formation of oocytes with diploid metaphase II (Soewarto, 1995). In human oocytes, defects in maturation can be caused by various parameters: physiological, hormonal, genetic, or environmental. In a specific case of unfertilized oocytes, in vitro additional factors associated with the ART procedure, namely, stimulation modes and temperature fluctuations, cytoplasmic dysmorphisms, and disturbances in the organization of microtubules, may also affect the kinetics of maturation and fertilization. All these data have a significant impact on the understanding and diagnostics of various forms of infertility (Pellestor, 2002).
The extracted giant oocytes were characterized by dysmorphism of endo- and exocytoplasmic structures. Nine (10.8%) gametes had a distinct oval shape, refractive bodies in the cytoplasm were found in 12 (14.5%) cells, and the granular region of the endoplasmic reticulum was found in 15 (18.1%) oocytes. Combinations of these features were characteristic of 47 (56.6%) oocytes.
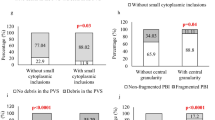
Among 47 giant oocytes at the metaphase II stage, 40 (85.1%) cells had two polar bodies in the perivithelin space (Fig. 3a), 3 (6.4%) oocytes had one polar body, and four cells (8.5%) had fragmented polar body. In 39 (82.9%) oocytes, polarization microscopy visualized two spindles (Fig. 3b); in 3 (6.4%) oocytes, one meiotic spindle; in 5 (10.7%) oocytes, no spindles were visualized, although polar bodies were present.
(a) An oocyte increased in size, oval shape, contains two polar bodies; (b) the same oocyte contains two meiotic spindles (visualization using the polarizing system Oosight (Hamilton Thorne, United States); (c) a zygote with three pronuclei; (d) blastocyst 6AA on the sixth day of cultivation; (e) biopsy of trophectoderm cells; (f) trophectoderm cell nuclei, the combination of hybridization signals corresponds to the triploid set of the studied chromosomes.
After fertilization of 47 oocytes at the metaphase II stage, pronuclei were detected in 42 (89.4%) cells. In these, two pronuclei were visualized in 9 oocytes (21.4%), three pronuclei in 27 (63.3%) oocytes, and four pronuclei in 6 (15.3%) oocytes.
It is believed that, with multiple meiotic spindles, any particular pronucleus or nucleus may be not complete but be a fragment of the initial chromosome set (Escobar-Aguirre, 2017). In such cases, after fertilization, three pronuclei are visually observed, although triploidy is not diagnosed.
Cytogenetic analysis of four of the five unfertilized oocytes revealed that the cells contained 46 chromosomes (a diploid set).
From 42 zygotes (Fig. 3c), 11 embryos (26.2%) developed to the blastocyst stage (Fig. 3d); they were characterized by large size, a multilayered trophectoderm, and a large intracellular mass.
Molecular cytogenetic analysis of trophectoderm cells showed that five of seven (71.4%) embryos had a triploid set, and the remaining two (28.6%) had a tetraploid set of chromosomes (Fig. 3f). Detection of triploidy is an expected result of the study since it proves that giant oocytes have a double chromosome set instead of a haploid one. Therefore, fertilization by a single haploid sperm results in a 3n chromosome set in the zygote and the future embryo. A tetraploid set can occur for several reasons, which were investigated by researchers. One of these reasons is the use of a diploid sperm for fertilization. We cannot rule out such a mechanism of embryonic polyploidy, but it should be noted that spermatozoa used for intracytoplasmic injection were selectively screened for normal morphological characteristics (Egozcue, 2002; McFadden, 2002). Also, we cannot know the premeiotic set of chromosomes in an oogonium. There are described cases of tissue mosaicism, including in the ovaries. Some meiotic chromosomal abnormalities may be present in the germ cells before meiosis, but the frequency of this phenomenon is not clearly determined. Oocytes with premeiotic defects significantly increase the level of preimplantation and prenatal mortality. Currently available data suggest that, depending on the mother’s age, up to 40% of chromosomal abnormalities present in oocytes at the end of meiosis I may be due to mosaicism of the generative organs (Delhanty, 2019). Therefore, we cannot say with certainty that giant oocytes carry exclusively a double set of chromosomes. It is the higher level of ploidy that can cause the detection of tetraploid chromosome sets in embryos derived from enlarged female gametes.
Giant oocytes were first described in 1988. It was believed that the reason for the formation of an oocyte with such dysmorphism was the superovulation induction (Mahadevan, 1988). Ovarian follicles in reproductive-age women are characterized by regular first-order oocyte maturation, as a result of which the chromosome set of the gamete becomes haploid during the first meiotic division (Verlhac, 2016). Several hypotheses regarding giant oocytes were put forward. The formation of such abnormal cells is most often associated with cytoplasmic fusion of two oogonia (Balakier, 2002). This phenomenon is also observed in the absence of cytokinesis during mitotic divisions of oogonia, which may occur due to immaturity of the actin-myosin cytokinetic ring or due to its regression (Martin, 2008; Storchova, 2008; Pampalona, 2012).
In addition, endoreduplication in primary oocytes can lead to the formation of mononuclear or binuclear giant metaphase II oocytes (Munné, 1994; Ullah, 2009). The vast majority of these phenomena are caused by malfunctioning of certain genes that regulate processes involved in oogenesis or directly in the chromosomal distribution. Thus, mutations in the genes encoding the proteins actin and myosin are reflected in the improper functioning of the filaments of the spindle and cytokinesis, which may not even be initiated, as in endoreduplication (Brunet, 2011; Yi, 2014; Roeles, 2019). Mutations in the genes of cohesin proteins lead to the fact that the strands do not interact with chromatids, which do not diverge to the poles of the cell or are not segregated into the polar bodies. Moreover, the level of cohesins decreases with age, which often leads to chromosomal abnormalities in the oocyte, including abnormal ploidy (Garcia-Cruz, 2010; Tsutsumi, 2014; Burkhardt, 2016).
Improper functioning of Balbiani bodies also affects the oogenesis and correctness of chromosome distribution not only due to the functions of centrioles but also because of the absence of elimination of abnormal mitochondria, which play an important role in chromosome divergence (Bilinski, 2017). Thus, in these cases, disruptions result in diploid gametes, while “extra” ooplasm increases the size of such cells.
Subsequently, the two haploid sets of chromosomes can combine to form a metaphase II oocyte with a single diploid metaphase plate and the first diploid polar body. After monospermic fertilization, a haploid male pronucleus and diploid female pronucleus are formed. In this case, the second polar body, which contains two sets of chromatids, should be extruded. Based on the exclusive estimation of the number of pronuclei and polar bodies, this oocyte will be assessed as normally fertilized, although the zygote will be triploid. Therefore, determination of the number of pronuclei is not always a predictor of polypoidity of the future embryo. In addition, a mature oocyte may contain two separate chromosome complexes and two haploid first polar bodies. Monospermic fertilization leads to the formation of three pronuclei (Fig. 3c) with extrusion of two polar bodies, each of which has a haploid chromatid set.
The results of our studies confirm the fact that giant oocytes have a polyploid set of chromosomes. Therefore, such a morphological feature of the oocyte as abnormally increased size has prognostic value for determining the expedience of using such cells in ART programs. Since more than half of the gametes obtained in aspiration of follicles have morphological abnormalities that can affect main embryological parameters in different ways, it is important to determine the most significant characteristics, one of which is the cell size.
CONCLUSIONS
The diameter of giant oocytes with ZP was (200.8 ± 4.24) μm, which is 38% larger than the size of normal mature female gametes. Despite the high fertilization rate (89.4%), only 26.2% of embryos developed to the blastocyst stage. Cytogenetic analysis revealed that unfertilized giant oocytes had diploid sets of chromosomes. Molecular cytogenetic analysis showed that embryos obtained after fertilization of giant oocytes had polyploid sets of chromosomes. Thus, the results of our study confirmed the inexpediency of using enlarged oocytes for fertilization and subsequent in vitro cultivation of embryos due to their abnormal sets of chromosomes.
REFERENCES
Almeida, P.A. and Bolton, V.N., Immaturity and chromosomal abnormalities in oocytes that fail to develop pronuclei following insemination in vitro, Hum. Reprod., 1993, vol. 8, no. 2, pp. 229–232. doihttps://doi.org/10.1093/oxfordjournals.humrep.a138028
Balaban, B. and Urman, B., Effect of oocyte morphology on embryo development and implantation, Reprod. Biomed. Online, 2006, vol. 12, no. 5, pp. 608–615. https://doi.org/10.1016/s1472-6483(10)61187-x
Balakier, H., Bouman, D., Sojecki, A., et al., Morphological and cytogenetic analysis of human giant oocytes and giant embryos, Hum. Reprod., 2002, vol. 17, no. 9, pp. 2394–2401. https://doi.org/10.1093/humrep/17.9.2394
Bilinski, S.M., Kloc, M., and Tworzydlo, W., Selection of mitochondria in female germline cells: is Balbiani body implicated in this process?, J. Assist. Reprod. Genet., 2017, vol. 34, no. 11, pp. 1405–1412. https://doi.org/10.1007/s10815-017-1006-3
Bosch, E., Labarta, E., Kolibianakis, E., et al., Super-ovulation induced changes of lipid metabolism in ovaries and embryos and its probable mechanism, PLoS One, 2015, vol. 10, no. 7. e0132638. https://doi.org/10.1371/journal.pone.0132638
Brunet, S. and Verlhac, M.H., Positioning to get out of meiosis: the asymmetry of division, Hum. Reprod. Update, 2011, vol. 17, no. 1, pp. 68–75. https://doi.org/10.1093/humupd/dmq044
Buderatska, N.O. and Petrushko, M.P., Variability of morphological parameters as a prognostic criterion of human oocyte cryopreservation, Morphologia, 2017, vol. 10, no. 4, pp. 18– 22. https://doi.org/10.26641/1997-9665.2016.4.18-22
Buderatska, N., Gontar, J., Ilyin, I., et al., Does human oocyte cryopreservation affect equally on embryo chromosome aneuploidy?, Cryobiology, 2020, vol. 93, pp. 33–36. https://doi.org/10.1016/j.cryobiol.2020.03.002
Burkhardt, S., Borsos, M., Szydlowska, A., et al., Chromosome cohesion established by rec8-cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice, Curr. Biol., 2016, vol. 26, no. 5, pp. 678–685. https://doi.org/10.1016/j.cub.2015.12.073
Conti, M. and Franciosi, F., Acquisition of oocyte competence to develop as an embryo: integrated nuclear and cytoplasmic events, Hum. Reprod. Update, 2018, vol. 24, no. 3, pp. 245–266. https://doi.org/10.1093/humupd/dmx040
Delhanty, J.D., SenGupta, S.B., and Ghevaria, H., How common is germinal mosaicism that leads to pre-meiotic aneuploidy in the female?, J. Assist. Reprod. Genet., 2019, vol. 36, no. 12, pp. 2403–2418. https://doi.org/10.1007/s10815-019-01596-6
Egozcue, S., Blanco, J., Vidal, F., et al., Diploid sperm and the origin of triploidy, Hum. Reprod., 2002, vol. 17, no. 1, pp. 5–7. https://doi.org/10.1093/humrep/17.1.5
Elkouby, Y.M., Jamieson-Lucy, A., and Mullins, M.C., Oocyte polarization is coupled to the chromosomal bouquet, a conserved polarized nuclear configuration in meiosis, PLoS Biol., 2016, vol. 14, no. 1. e1002335. https://doi.org/10.1371/journal.pbio.1002335
Escobar-Aguirre, M., Zhang, H., Jamieson-Lucy, A., et al., Microtubule–actin crosslinking factor 1 (Macf1) domain function in Balbiani body dissociation and nuclear positioning, PLoS Genet., 2017, vol. 13, no. 9. e1006983. https://doi.org/10.1371/journal.pgen.1006983
Garcia-Cruz, R., Brieco, M.A., Roig, I., et al., Dynamics of cohesin proteins REC8, STAG3, SMC1 beta and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes, Hum. Reprod., 2010, vol. 25, no. 9, pp. 2316–2327. https://doi.org/10.1093/humrep/deq180
Heim, A.E., Hartung, O., Rothhamel, S., et al., Oocyte polarity requires a Bucky ball-dependent feedback amplification loop, Development, 2014, vol. 141, no. 4, pp. 842–854. https://doi.org/10.1242/dev.090449
Lehner, A., Kaszas, Z., Murber, A., et al., Giant oocytes in human in vitro fertilization treatments, Arch. Gynecol. Obstet., 2015, vol. 292, no. 3, pp. 697–703. https://doi.org/10.1007/s00404-015-3679-0
Machtinger, R., Politch, J.A., Hornstein, M.D., et al., A giant oocyte in a cohort of retrieved oocytes: does it have any effect on the in vitro fertilization cycle outcome?, Fertil. Steril., 2011, vol. 95, no. 2, pp. 573–576. https://doi.org/10.1016/j.fertnstert.2010.06.037
Mahadevan, M.M., Fleetham, J., Long-Simpson, L., et al., Recovery of a preovulatory binucleate oocyte in a patient following induction of ovulation for in vitro fertilization, J. In Vitro Fert. Embryo Transf., 1988, vol. 5, no. 5, pp. 299–300. https://doi.org/10.1007/BF01132182
Martin, R.H., Meiotic errors in human oogenesis and spermatogenesis, Reprod. Biomed. Online, 2008, vol. 16, no. 4, pp. 523–531. https://doi.org/10.1016/s1472-6483(10)60459-2
McFadden, D.E., Jiang, R., and Langlois, S., Dispermy—origin of diandric triploidy: brief communication, Hum. Reprod., 2002, vol. 17, no. 12, pp. 3037–3038. https://doi.org/10.1093/hum-rep/17.12.3037
Munné, S., Alikani, M., and Cohen, J., Monospermic polyploidy and atypical embryo morphology, Hum. Reprod., 1994, vol. 9, no. 3, pp. 506–510. https://doi.org/10.1093/oxfordjournals.humrep.a138536
Pampalona, J., Frías, C., Genesca, A., et al., Progressive telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells, PLoS Genet., 2012, vol. 8, no. 4. e1002679. https://doi.org/10.1371/journal.pgen.1002679
Pellestor, F., Andréo, B., Arnal, F., et al., Mechanisms of non-disjunction in human female meiosis: the coexistence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes, Hum. Reprod., 2002, vol. 17, no. 8, pp. 2134–2145. https://doi.org/10.1093/humrep/17.8.2134
Petrushko, M.P., Yurchuk, T.O., and Buderatska, N.O., Oolemma invagination of fresh and cryopreserved human oocytes during in vitro fertilization by ICSI, Probl. Cryobiol. Cryomed., 2018, vol. 28, no. 3, pp. 258–265. https://doi.org/10.15407/cryo28.03.258
Reader, K.L., Stanton, J.L., and Juengel, J.L., The role of oocyte organelles in determining developmental competence, Biology (Basel), 2017, vol. 6, no. 3, p. 35. https://doi.org/10.3390/biology6030035
Rienzi, L., Balaban, B., Ebner, T., et al., The oocyte, Hum. Reprod., 2012, vol. 27, suppl. 1, pp. i2–i21. https://doi.org/10.1093/hum-rep/des200
Roeles, J. and Tsiavaliaris, G., Actin-microtubule interplay coordinates spindle assembly in human oocytes, Nat. Commun., 2019, vol. 10, no. 1, p. 4651. https://doi.org/10.1038/s41467-019-12674-9
Rosenbusch, B., Mechanisms giving rise to triploid zygotes during assisted reproduction, Fertil. Steril., 2008, vol. 90, no. 1, pp. 49–55. https://doi.org/10.1016/j.fertnstert.2007.06.031x
Rosenbusch, B., The potential significance of binovular follicles and binucleate giant oocytes for the development of genetic abnormalities, J. Genet., 2012, vol. 91, no. 3, pp. 397–404. https://doi.org/10.1007/s12041-012-0195-x
Soewarto, D., Schmiady, H., and Eichenlaub-Ritter, U., Consequences of non-extrusion of the first polar body and control of the sequential segregation of homologues and chromatids in mammalian oocytes, Hum. Reprod., 1995, vol. 10, no. 9, pp. 2350–2360. https://doi.org/10.1093/oxford-journals.humrep.a136298
Storchova, Z. and Kuffer, C., The consequences of tetraploidy and aneuploidy, J. Cell Sci., 2008, vol. 121, pt. 23, pp. 3859–3866. https://doi.org/10.1242/jcs.039537
Tsutsumi, M., Fujiwara, R., Nishizawa, H., et al., Age-related decrease of meiotic cohesins in human oocytes, PLoS One, 2014, vol. 9, no. 5. e96710. https://doi.org/10.1371/journal.pone.0096710
Ullah, Z., Lee, C.Y., and Lilly, M.A., Developmentally programmed endoreduplication in animals, Cell Cycle, 2009, vol. 8, no. 10, pp. 1501–1509. https://doi.org/10.4161/cc.8.10.8325
Verlhac, M.H. and Terret, M.E., Oocyte maturation and development, F1000Res, 2016, vol. 5. https://doi.org/10.12688/f1000re-search.7892.1
Wang, L.Y., Wang, N., Le, F., et al., Superovulation induced changes of lipid metabolism in ovaries and embryos and its probable mechanism, PLoS One, 2015, vol. 10, no. 7. e0132638. https://doi.org/10.1371/journal.pone.0132638
Yi, K. and Li, R., Actin cytoskeleton in cell polarity and asymmetric division during mouse oocyte maturation, Cytoskeleton (Hoboken), 2012, vol. 69, no. 10, pp. 727–737. https://doi.org/10.1002/cm.21048
Yurchuk, T.O., Buderatska, N.O., Ilyin, I.E., et al., Morphological characteristics of the first polar body of oocytes and results of embryos PGT-A, Morphologia, 2019, vol. 13, no. 4, pp. 50–54. https://doi.org/10.26641/1997-9665.2019.4.50-54
Funding
This work was carried out by the authors without funding from state or nonstate funds or financial institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement of compliance with standards of research involving humans as subjects. All studies were carried out in accordance with the rules of biomedical ethics. To work with oocytes and embryos, written, voluntary, and informed consent of patients was obtained. All manipulations with oocytes and preimplantation embryos were carried out in accordance with the order of the Ministry of Health of Ukraine from September 9, 2013, no. 787 “On Approval of the Procedure for the Use of Assisted Reproductive Technologies in Ukraine” and the European Protocol on Embryo Protection. The research was carried out in accordance with the principles of the Helsinki Declaration of Human Rights, the European Convention of Human Rights and Biomedicine, and the recommendations of ESHRE and ARSM. The studies were approved by the Bioethics Committee of the Institute of Problems of Cryobiology and Cryomedicine of the National Academy of Sciences of Ukraine (minutes no. 7, 2015, and no. 1, 2019).
Additional information
Translated by K. Lazarev
About this article
Cite this article
Petrushko, M.P., Buderatska, N.O., Gontar, J.V. et al. Morphological and Molecular Cytogenetic Characteristics of Giant Human Oocytes. Cytol. Genet. 55, 132–137 (2021). https://doi.org/10.3103/S0095452721020110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721020110