Abstract
The polymorphism of beta-amylase isoenzymes in spring common wheat was studied among varieties cultivated under the conditions of the Northern Trans-Urals. Four zymotypes were identified among the 41 accessions. A, C, and A* isozymes differed most frequently, the share of which, respectively, was 64.6 ± 7.3, 22.0 ± 3.7, and 12.2 ± 3.7%. During 2011–2013, the aggregating ability of grain proteins was studied using -S-S-bonds between groups of varieties carrying variants of beta-amylase A and C. It was established that the presence of isoenzyme C promoted a greater aggregation of grain polypeptides in comparison with beta-amylase A carriers but did not affect the formation of yield. Differences in beta-amylase did not affect the falling number associated with amylase activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The main part of the beta-amylase of cereal grain is in a bound form. During seed germination, this enzyme is released as a result of the proteolytic action of one or several SH-proteases [1]. Accordingly, this leads to a decrease in its molecular weight by 5000 Da compared with beta-amylase extracted by dithiothreitol. By isozyme composition, this wheat enzyme has certain intraspecific polymorphism [2–5]. It has been established that the occurrence of different beta-amylase isoenzymes in winter cultures is not the same. Thus, zymotypes A, B, and C among winter wheat varieties of Ukraine and Russia had the following distribution values, respectively: 51.7 ± 4.7, 30.7 ± 3.8, and 11.9 ± 2.5% [5]. In addition to these variants of the enzyme, five more beta-amylase zymotypes were found. Their occurrence varied within 0.6–3.4%. Beta-amylase polymorphism in spring wheat cultures has not been studied. Considering the ability of beta-amylase to aggregate using disulfide bonds, the role of its various electrophoretic variants in the formation of the quality of winter common wheat grain’s protein part was previously evaluated [5–7]. At the same time, the question on the role of isoenzymes of this enzyme in spring common wheat grain’s formation of quality remains open.
The goals of the study were the assessment of beta-amylase isoenzymes’ distribution in the spring common wheat culture of the Northern Trans-Urals and the influence of hereditary enzyme variants on the formation of grain quality and yield indices.
MATERIALS AND METHODFS
As plant material, a set of 41 varieties of spring common wheat that were zoned or created in Western Siberia was used. During 2011–2013, grain quality indices related to the protein and carbohydrate part of the caryopsis as well as yield under the conditions of Tyumen were investigated.
The content of crude gluten in the grain was determined by the manual method (GOST 31699-2012), and the quality of gluten was determined by gluten strain gage. The activity of amylolytic enzymes according to the falling number value was determined using a Hagberg-Perten device (GOST 27676 ISО 5529: 1992). The amount of disulfide bonds of the protein complex was determined by the method of Netsvetaev et al., 2009 [8]. The content of total nitrogen was obtained by the Micro-Kjeldahl method, and the content of crude protein was obtained by recalculation: N × 5.7.
The following electrophoretic methods of analysis were used to identify the isoenzymes of this enzyme: isoelectrofocusing [2, 3], SDS electrophoresis [4], and electrophoresis in a Tris-glycine system of a polyacrylamide gel at pH 8.3 [5]. For the study of beta-amylase isoenzymes, the last variant of this enzyme was used.
Vertical polyacrylamide gel electrophoresis was performed on 190 × 105 × 1-mm plates. For this purpose, a device [9] manufactured at the Institute of Selection and Genetics of the National Academy of Agrarian Sciences of Ukraine (Odessa) that allowed the formation of two gel plates of corresponding sizes was used. Electrophoresis of individual grains was used to determine the homogeneity or heterogeneity of the variety for amylase isozymes. For this purpose, three to five individual caryopses from each sample were analyzed. β-amylase was isolated from the endosperm of individual mature grains that had been crushed with pliers in advance. In test tubes with crushed caryopses, 250 μL of 3% Na2SO3 solution was added and left overnight. After grinding the grains in test tubes with a stainless-steel rod, the resulting suspension was centrifuged for 4 min at 10 000 rpm. Then, 10 μL of the supernatant from each were taken into clean tubes and 10 μL of a solution containing 2% β-mercaptoethanol, 40% sucrose, and 0.03% bromophenol blue were added to the tubes. The extract (5 μL) was applied to the starting cells of the gel. The electrophoresis conditions and the composition of the gel are similar to those previously described for barley [10]. Electrophoresis was carried out at 300 V. The separation was stopped after the release of 1.5 dye labels (1.5 h). Incubation, staining, and designation of common wheat’s beta-amylase variants was carried out in accordance with the previously described method [5].
For the assessment of the selection of the variety’s ancestor in the selection process based on the occurrence of β-amylase isoenzymes, χ2 was used. The difference in the means was estimated by Student’s criterion [11, 12]. Evaluation of isoenzymes’ frequency of occurrence was carried out according to C. Lee [13]. Analysis of variance was carried out in the StatNov program (VIUA).
RESULTS AND DISCUSSION
In the analysis of beta-amylase isoenzymes in varieties of spring common wheat of Western Siberia, four variants of enzymes were identified: A, A*, C, I (Fig. 1).
Zymyotypes of 2β-amylase occurring among the varieties of spring common wheat in Western Siberia: 1, Novosibirskaya 15; 2–4, Novosibirskaya 29; 5–7, Novosibirskaya 31; 8–10, Melodiya; 11–13, Serebristaya; 14–17, Svirel; 18–20, Aviada; 21–23, Gerakl; 24–26, Margarita; 27–29, Tyumenskaya 29; 30–32–OmGAU-90; 33–34, ShTRU-051911.
The list of varieties carrying one or another isoenzyme of beta-amylase is presented in Table 1.
Among the 41 varieties of wheat, five were heterogeneous (12.2%). The remaining studied forms were samples representing homozygous populations for genetic factors determining β-amylase isoenzymes. Considering the presence of heterogeneous wheat varieties by β-amylase isoenzymes and knowing their frequency, we can estimate in which generation of self-pollination the selection of the parent plant occurred during the selection process. Existence of a heterogeneous variety suggests that the parent plant was heterozygous for this qualitative trait. Thus, it is known that the ratio of homozygotes to heterozygotes for alleles of one locus was close to 1 : 1 in F2, and this ratio was 3 : 1 in F3 and 7 : 1 in F4. The results of such an assessment are presented in Table 2.
The data of Table 2 show that the selection of a parent plant in the breeding process of Russian varieties of spring common wheat occurs not in the second generation of self-pollination but in the third or fourth generations (p, respectively, >0.05 and >0.90).
The percentage of different beta-amylase zymotypes' occurrence among spring varieties is not the same. The zymotype A predominated and its share was higher than 64% (Table 3). The next most common is the zymotype C, which is higher than 20%. Approximately 1% of the occurrence was composed by the zymotype I. Isoenzyme A* (12.2%) occupied the third place by distribution. In general, a significant excess of the occurrence of zymotype A (Table 3) over the other isoenzymes may indicate a more intensive selection under the conditions of the Northern Trans-Urals in favor of this variant of β-amylase.
It is characteristic that β-amylase also dominates in the winter wheat cultures of Ukraine and the European part of Russia [5]. At the same time, the second most common distribution in this case was the zymotype B, the percentage of which was 30.7 ± 3.8. As can be seen (Table 3), this phenotype was not found in the spring wheat culture of the Northern Trans-Urals. Type C in winter culture composed 11.9 ± 2.5%, which was significantly lower (t = 2.26; p > 0.95) in comparison with its occurrence in varieties of spring wheat (Table 3). Consequently, the spring wheat of the Northern Trans-Urals differs in the range of occurrence of β-amylase variants in comparison with the winter wheat varieties of Ukraine and the European part of Russia.
Taking into account the previously found conjugacy of β-amylase isoenzymes with grain quality indices in winter wheat culture [6, 7], variants A and C of this enzyme were evaluated for their ability to aggregate in the endosperm protein complex and association with gluten deformation index (GDI). The 3 years results for the spring culture under the conditions of Tyumen are presented in Table 4.
As can be seen, the differences between the selected groups of varieties in the number of -S-S- bonds in the flour were low in 2011. In 2012, differences in this index between carriers of variants C and A were significant (Table 4). Characteristically, in the period under study, varieties that had type C β-amylase had higher aggregative ability due to disulfide bonds compared with the group carrying variant A. The same trend was observed in the following year, but the differences were insignificant in this case. In general, in assessing the presented data using for 3 years analysis of variance, the differences in the number of disulfide bonds between the selected groups confirmed their significance (Table 4). The differences in the physical properties of gluten (GDI) over the 3-year period between the studied groups of varieties were within the experimental error. During 2012 and 2013, there was a tendency for improvement in the quality of gluten in favor of the wheat groups with variant C. It is characteristic that the conditions of the year had a leading influence on the variation of the indices of the aggregating capacity of the protein complex and the physical properties of gluten. Thus, the effect of the year on these quantitative traits determined more than 90% of variability, while the genotype of β-amylases determined approximately 2%. At the same time, random deviations were less than 1%, which indicates the significance of the variety’s influence on the manifestations of these traits. Consequently, β-amylase isoenzymes affect the aggregative ability of the protein complex of spring wheat grain. A type C enzyme variant has a higher ability to form intermolecular -S-S-bonds of the grain’s protein complex as compared with isoenzyme A. It should be noted that the data presented in Table 4 on the quality of gluten between the selected groups of varieties are not convincing. Minor differences in the quality of gluten may be associated with the use of the standard method of washing gluten and the limited sample. In particular, it is known [14–16] that environmental conditions have a significant effect on the GDI index in this case, and this increases the error of experiment. At the same time, it is known that the modified method of washing out gluten described by Netsvetaev et al. [14–16] reduces the influence of environmental variation and increases the influence of heredity in the formation of the gluten deformation index (GDI). Taking into account the comments presented, the quality of gluten of 95 varieties of common wheat of the 2017 harvest (competitive variety trial-17) was investigated in accordance with the modified method of its washing, and the amount of disulfide bonds in the grain protein was determined. As a result, a significant correlation between these indices (r) equal to –0.314*** (n = 95; p < 0.001) was obtained. In this case, the correlation between the number of disulfide bonds in the flour and grain protein was 0.683*** (n = 95; p < 0.001).
Thus, β-amylase isoenzymes affect the aggregative ability of common wheat grain’s protein complex. The type C variant of this enzyme has a higher ability to form intermolecular -S-S-bonds of the protein complex of the grain as compared with isozyme A and it is capable of improving the physical properties of gluten.
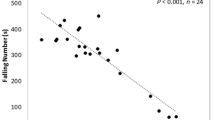
The falling number is an index characterizing the state of the carbohydrate-amylase complex of the grain. It characterizes the resistance of wheat to the germination of grain on the root. This is due to the activity of α-amylase, under the action of which starch is hydrolyzed with the formation of water-soluble substances: dextrins and sugars. Taking into consideration that β-amylase is also involved in the hydrolysis of starch, the selected groups of varieties were investigated according to this index (Table 5).
The presented results indicate that these variants of the enzyme have the same effect (if any) on the falling number.
In 2011, the yield of samples with β-amylase of type C was 4.63 ± 0.20 t/ha (n = 8), and that with the type A isoenzyme was 4.71 ± 0.13 (n = 24). The differences in yield between the studied groups of varieties were not significant, t = 0.349; p > 0.05. Analysis of the yield of variety groups divided according to β-amylase isoenzymes in 2012 confirmed the previous values of these indices. Thus, the yield of varieties with a C variant of this enzyme was 0.81 ± 0.06 t/ha, and that with the zymotype A was 0.91 ± 0.03 t/ha (t = 1.629; p > 0.05). Similar results were also obtained from the results of 2013. In this case, a group of varieties with the C isoenzyme yielded a grain yield of 4.73 ± 0.23 t/ha, and that with variant A yielded a grain yield of 4.52 ± 0.14 t/ha. Differences of 0.21 t/ha are insignificant (t = 0.812; p > 0.05).
CONCLUSIONS
In general, as a result of the presented data’s estimation for 3 years using analysis of variance, the following results were obtained: the group of varieties with the enzyme variant C provided a yield of 3.39 t/ha, and that with variant A was 3.38 t/ha (LSD0.95 = 0.43 t/ha) Thus, the separated groups of varieties did not differ in grain productivity due to the genes controlling the synthesis of isoenzymes A and C. The variation in yield between varieties in this case was solely due to environmental variability over the years, which is expressed by 99.84% (error is 0.16%) value of contribution to this indicator.
Thus, β-amylase isozymes A and C are predominant in the culture of spring wheat of the Northern Trans-Urals. Variant C of this enzyme has a higher ability to form intermolecular -S-S-bonds of the grain’s protein complex as compared to isozyme A and is able to improve gluten properties. Improving the quality of spring common wheat’s grain by the introduction of type-C β-amylase into the culture, which can improve the quality of the protein, will not affect the grain productivity of new wheat varieties. Differences in the falling number between samples with A and C isozymes were not detected.
REFERENCES
Sopanen, T. and Lauriere, C., Release and activity of bound β-amylase in a germinating barley grain, Plant Physiol., 1989, vol. 89, pp. 244–249. doi 0032-0889/ 89/89/0244/06/$01.00/0
Joudrier, P.M. and Bernard, M., Responsability du genome D sur certaines isozymes β-amylase du grain de ble tender, Ann. Amelior. Plantes, 1977, vol. 27, p. 35.
Rybalka, A.I., The Quality of Wheat and Its Improvement, Kiev: Logos, 2011.
Gupta, R.B., Shepherd, K.W., and MacRitchie, F., Genetic control and biochemical properties of some high molecular weight albumins in bread wheat, J. Cer. Sci., 1991, vol. 13, no. is. 3, pp. 221–235.https://doi.org/10.1016/S0733-5210(09)80002-7
Netsvetaev, V.P., Akinshina, O.V., Bondarenko, L.S., and Motorina, I.P., The β-amylase polymorphism of winter common wheat grains, Russ. J. Genet., 2012, vol. 48, no. 2, pp. 146–151. https://doi.org/10.1134/S10227-9541202010X
Netsvetaev, V.P., Akinshina, O.V., and Bondarenko, L.S., Use of the common winter wheat homozygous population for genetic analysis of beta-amylase and evaluation of its aggregation ability, Russ. J. Genet., 2014, vol. 50, no. 11, pp. 1156–1160. https://doi.org/10.1134/S1022795414110106
Netsvetaev, V.P., Nerubenko, O.E., Bondarenko, L.S., Akinshina, O.V., et al., Heterogeneity of the wheat variety as the basis for its improvement in the primary seed production, Achievements Sci. Technol. Agro-Industrial Complex, 2017, vol. 31, no. 6, pp. 43–46.
Netsvetaev, V.P., Lutenko, O.V., Pashchenko, L.S., and Popkova, I.I., Sedimentation methods and evaluation of the quality in common wheat gluten, Sci. Bull. Bel. Gos. Univ., Ser.: Nat. Sci. (Belgorod), 2009, vol. 11 (66), no. 9 (1), pp. 56–64.
Poperelya, F.A., Asyka, Yu.A., and Lazarev, Yu.D., The device for electrophoresis of proteins in the gel, Author. Witness USSR, 1991, no. 1 682 899.
Netsvetaev, V.P., Position of the β-amylase locus, Bmy 1, on barley chromosome 4, Cytol. Genet., 1993, vol. 27, no. 5, pp.74–78.
Rokitsky, P.F., Biological Statistics, Minsk: Vysheyshaya Shkola, 1973.
Rokitsky, P.F., Introduction to Statistical Genetics, Minsk: Vysheyshaya Shkola, 1974.
Li, Ch.Ch., First Course in Population Genetics, Pacific Grove, California: The Boxwood Press, 1976.
Netsvetaev, V.P., Motorina, I.P., and Petrenko, A.V., Comparison of methods for determining the quality of grains gluten in common wheat by the device IDK-1, Rep. RAAS, 2005, no. 4, pp. 14–16.
Netsvetaev, V.P., Motorina, I.P., and Petrenko, A.V., Modification of the method for determining the quality of wheat gluten on an IDK-1 device, Sci. Bull. Bel. Gos. Univ., Ser.: Nat. Sci. (Belgorod), 2006, no. 23/4 (3), pp. 141–144.
Netsvetaev, V.P., Ryzhkova, T.A., and Tretyakov, M.Yu., Quality of Common Wheat: Genetics and Breeding. Monograph, Belgorod: Otchiy Kray, 2015.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by V. Mittova
About this article
Cite this article
Akhtariyeva, M.K., Kozelets, Y.O., Filippova, Y.M. et al. Beta-Amylase Isozymes in Spring Common Wheat and Their Role in the Aggregation of Grain Proteins. Cytol. Genet. 53, 294–299 (2019). https://doi.org/10.3103/S0095452719040029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452719040029