Abstract
While global mammalian diversity is collapsing due to increased poaching and habitat loss, evidence-based conservation in protected areas is often regarded as a panacea. Tiger reserves in India set an example, where annual camera trap monitoring is conducted for understanding the trends in the tiger population. However, less is known about other co-predators and their prey species that occur in the same area. The fundamental hindrance being the absence of individual pelage pattern within these species (i.e. unique pattern on the body), as well as the absence of species-specific monitoring. As a result, there is a demand in techniques that can avail maximum biodiversity information from the existing monitoring protocols. Here, we conducted camera trapping in Periyar Tiger Reserve to evaluate spatiotemporal overlaps within different carnivores, and between prey-predators. Camera trapping was conducted at high resolution (2 km2) for 30 days at 253 locations that yielded 6092 photographs of 18 mammals. Their temporal overlap was estimated using ‘overlap’ R package, while the spatial association was estimated using ‘co-occur’ package. Three large-ranging top predators (tiger, leopard and dhole) were found to have activity peaks segregated temporally. Relationship of these predators with their prey species highlighted the role of body sizes, where largest predator (tiger) had higher overlap with large-bodied prey (gaur and sambar), while small-bodied predator (leopard and dhole) overlapped small-bodied prey (barking deer and wild pig). Results highlight the importance of large-sized prey in conserving the tiger densities of this region. However, selectively conserving only large-bodied prey can have repercussions on other sympatric carnivores, who require different body-sized prey species. Our results have implications for all protected areas in the tropical developing countries, which are mostly smaller in area with species-centered conservation agenda. We highlight the importance of considering species-specific carrying capacity of all co-predators in the region, to optimally conserve the prey-base through habitat restoration, so as to maximize biodiversity conservation within a limited area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, natural areas are fragmented and isolated by anthropogenic modifications that eventually leads to a dichotomy of the natural and human landscape. Large mammals, particularly carnivores, that require larger natural areas are forced to restrict to these isolated small protected areas that lead to their demographic decline (Weber and Rabinowitz 1996; Sanderson et al. 2006 ). If they disperse out of protected areas, they often come in proximity to human land-use, which triggers conflict with humans in most of the cases (Treves and Karanth 2003; Banerjee et al. 2013). Thus, there have been massive efforts for conserving these large carnivores by maintaining their evolutionarily viable populations in protected areas connected by habitat corridors (Ripple and Beschta 2012; Jhala et al. 2019). As a consequence, most of the large carnivores are left predominantly in the protected areas, where they are dependent, as well as influence, on the occurrence of other mammals (e.g. Foster et al. 2013; Lynam et al. 2013; Singh and MacDonald 2017; Karanth et al. 2017). While the recovery of these carnivores might positively benefit the native ecosystem (e.g. Ripple and Beschta 2012), artificially inflating their densities without understanding the existing carrying capacity can be detrimental for other species (Kumar et al. 2019). The mechanism involved in the coexistence of sympatric species, and their dominance over other subordinate species in the guild, is fundamental to community ecology (Krebs 1973) and has immense importance in conserving larger biodiversity in smaller areas.
How sympatric species use time and space to optimize their resource selection while reducing competition; and what is the proportion of spatio-temporal overlap between predators, co-predators, and their prey species? These are amongst the many crucial questions in community ecology Ridout and Linkie (2009). Addressing these questions has provided information on multi-species interaction and has guided actions to conserve maximum biodiversity, in smaller areas. Prey-predator relations have been best explained by activity and concurrence models and has guided in conservation interventions for increasing the carrying capacity of flagship carnivores (e.g. Karanth and Sunquist 2000; Eriksen et al. 2011). In certain cases, activity pattern studies demonstrated how a subordinate or small body-sized species might avoid temporal overlap with dominant species to reduce the potential competition (Lynam et al. 2013; Droge et al. 2016; Karanth et al. 2017). As a consequence of its conservation implications, species co-occurrence has been extensively studied using different techniques in different ecosystems.
Activity patterns of terrestrial vertebrates have been studied using radio telemetry Karanth and Sunquist (2000), direct observations (Schaller 1967; Johnsingh 1983); and has advanced with usage of camera trap surveys (Ramesh et al. 2012; Karanth et al. 2017; Noor et al. 2017; Shankar et al. 2020). For species that are rare and/or elusive, camera trap survey offers a wider and easier way to assess their occurrence and activity pattern (Rowcliffe et al. 2014). While camera trap-based studies have been vastly used across different taxa and regions (Yasuda 2004; Rovero et al. 2010; Bridges and Noss 2011), their utility has not reached its potential in tropical forests of developing countries. The tropical rain forest has one of the highest levels of species diversity and endemism but is less studies due to their cryptic, shy and secretive behaviour Ridout and Linkie (2009).
Western Ghats mountain chains lining the west coast of peninsular India harbours a wide range of habitats and elevation gradient (0–2,800 MSL) (Pascal 1988). The southern Western Ghats is considered as the centre of biodiversity hotspot in the Western Ghats, which has a rich forest harbouring diverse fauna (Ramachandra and Suja 2006; Shameer et al. 2019; Raman et al. 2020), and one of the genetically unique tiger sub-population in the wild (Jhala et al. 2015). While many studies have addressed the activity-based relation in tiger and its prey species in the Northern Western Ghats (Ramesh et al. 2012; Karanth et al. 2017), there is a dearth of information for the Southern Western Ghats. Tigers and elephants in this region have undergone severe poaching episodes in the past (Ramakrishnan et al. 1998). There are around 98 (range: 86–109) tigers occupying 7842 km2 of the Southern Western Ghats, mainly in the four tiger reserves (Parambikulam, Annamalai, Periyar and Kalakad-Mundanthurai) (Jhala et al. 2015). The present rate of infrastructure development and land-use changes are changing this habitat rapidly and pushing most of the large wild mammals to protected areas. To sustain this wide array of wildlife, it is crucial to monitor the occurrence, activity and interactions of different mammals in the protected areas of this region.
In India, tiger reserves are mandated to conduct annual camera trapping to monitor the population trend of tigers (Jhala et al. 2015). However, most of the camera trap species lack pelage pattern to estimate their population by identifying individuals (e.g. mark capture-recapture), and camera trapping are not always intensive to model precise occurrence pattern (e.g. multi-species occupancy) Chandler and Royle (2013). More importantly, advance patterns on species occupancy and densities require camera traps optimally capturing every species in consideration. This is not always true, as the camera traps are set to monitor tigers and its co-predators and might hence fail to account the occurrence of other species that use non-camera trapped areas. This necessitates the requirements of models that can operate on limited data available on an array of species and is not significantly affected by detection bias. In the present study, we use activity pattern and spatial association of different species to serve this demand of easy to derive pattern for managers seeking the information of camera trapped species. We conducted a camera trapping survey in the Periyar Tiger Reserve and subsequently evaluated the activity pattern, activity overlap and spatial association of 18 mammals in this area. We further tested two hypotheses (1) the sympatric carnivores in this area (tiger, leopard and dhole) segregated on a temporal or spatial scale to avoid active competition, and (2) there is a significant overlap in the activity patterns of these predators with their prey, which is proportional to the body mass of prey. Understanding the pressure that wildlife managers face maximizing biodiversity inside protected areas, study results hold immense implications for understanding ecological settings that maximize wildlife occurrence in protected areas of tropical developing countries.
Study area
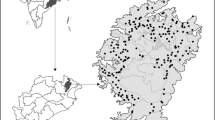
We did this study in the Periyar Tiger Reserve (PTR), which is situated in the Cardamom Hills and Pandalam Hills of the Southern Western Ghats (9.2989° – 9.6195° N and 77.4182° − 76.9367° E) in the Kerala state of India. The PTR falls in the warm humid tropical zone with the temperature between 15 and 31 °C and rainfall from 2000 to 3000 mm. The terrain is highly undulating, with altitude ranging from 100 to 2019 m a.s.l. High rainfall and warm tropical climate in the region has resulted in one of the densest evergreen and semi-evergreen forests with a diverse array of floral assemblage (Sasidharan 1998). The major vegetation types are southern moist wet tropical forest, southern hilltop evergreen forest, west coast tropical evergreen forest, west coast semi-evergreen forest, southern moist mixed deciduous forest, southern semi-moist deciduous forest and grasslands Champion and Seth (1968). There is a large water body inside the PTR formed as a result of the construction of the Mullaperiyar dam on the Periyar River, which further adds to the habitat variability and subsequent biodiversity of the Reserve.
The habitat variability and resource diversity have resulted in exceptionally rich biodiversity (Myers et al. 2000) in PTR. The reserve is home to 1,985 species of flowering plants, 67 species of mammals, 323 birds, 48 reptiles, 48 amphibians, 45 fishes and 262 butterflies (Shukla and Babu 2013; Das et al. 2015; Kalesh and Sreehari 2015; Shameer et al. 2019). It holds one of the source populations of Royal Bengal tiger Panthera tigris tigris and Asian elephant Elephas maximus and was declared as the tenth Tiger Reserve of India in 1978, and Elephant Reserve in 1991. The reserve encompasses 925 km2, of which 881 km2 is notified core zone or critical tiger habitat and the remaining 44 km2 is the buffer zone. The PTR and its surrounding area are designated as Tiger Conservation Landscape TCL 64 of regional importance (Sanderson et al. 2006).
The land surrounding the Reserve is dominated by human settlements and linear infrastructures. The surroundings are intensively farmed, mostly as cash-crop plantations for tea, rubber, cardamom and coffee. In Southern part of the PTR, is the famous Shrine of Sabarimala that accommodates a pilgrim visitation of 20–30 million every season, spaced over a short period of 60 days. PTR has a human population of 225,000 within 2 km buffer of the reserve, who partially or completely dependent on the natural resources of PTR (World Bank 1996; KFD 2003; Sharma et al. 2004). Human land-use makes the landscape unsustainable for many large mammals in this landscape, who while using areas outside tiger reserve are known to damage crops and human property, thereby resulting in conflict with people (e.g. Gubbi 2006; Bhaskaran 2013; Mathur et al. 2015; Kaushik and Mungi 2015). Minimum human density and heterogenous forested habitat inside the PTR could have resulted in higher mammalian diversity. This provides a unique opportunity to assess the overlaps and segregation of spaces and activity by different mammals, with an emphasis on relation amongst large sympatric carnivores (tiger, leopard and dhole) (henceforth, predators), and the relation of these predators with their common prey species (sambar, gaur, barking deer, wild pig and mouse deer).
Data collection and methodology
Camera trapping was conducted from 17 to 2017 to 17 November 2016. The study area was divided into 253 grids of 2 km2 size. Within each grid cell, we recorded locations of animal trails, scats and scent, and locations with previous sightings or photo-captures of study species. Based on this reconnaissance survey, trapping locations were identified and marked using Garmin GPS etrex30 with WGS84 as a standard datum. A pair of high definition passive trail cameras (Cuddeback 20MP colour scouting camera, model C1) was placed at the trapping locations at a height of 30–60 cm from the ground, facing opposite to each other in each of these grids (Fig. 1) (Jhala et al. 2011). The cameras were set up in photo mode with a delay of one second between pictures. Camera trapping was carried out using 506 camera traps and each camera was operational for consecutive 30 days. Each pair of camera traps is considered as a single unit, thereby making a total trapping effort of 7,590 trap-nights. Camera traps were deployed and regularly monitored by the aid of trained forest staff of the Tiger Reserve.
Photographs capture through camera traps had information on time and date of species captured. These times of capture were considered as a random sample of activity of the species so that the likelihood of getting a photograph increases in proportion to how active the species is at that time of day (Ridout and Linkie 2009). To ensure the independence of photographs captured of the same species in the same location the capture was considered as a single record if photographed within ≤ 30 minutes (Linkie and Ridout 2011). But, in the case of individually identifiable species (e.g. tiger, leopard), a capture was considered as independent if consequent photographs within 30 minutes were of different individuals.
First, we used a non-parametric kernel density estimation approach for visualizing the activity pattern of each species (Ridout and Linkie 2009). In the second step, we measured overlap between two species pairs of these estimated distributions with a coefficient of overlapping Δ that ranges from 0 (no overlap) to 1 (complete overlap) (Ridout and Linkie 2009). The overlap is defined as an area under the curve that is formed by taking the minimum of the two density functions at each time point. From the three alternative ways of estimating Δ i.e. Δ1, Δ4, Δ5, we choose Δ1 in case of small sample size and Δ4 for more than 75 samples (Ridout and Linkie 2009). For this analysis, we used the ‘overlap’ package (Meredith and Ridout 2014) in the R platform (R Development Core Team 2019). We used 10,000 bootstrap samples to obtain percentile 95% confidence interval (CI) of Δ1 and Δ4. To test the study hypothesis of sympatric competition, we assessed the overlap in tiger - leopard, tiger - dhole, leopard - dhole and tiger + leopard - dhole. To test the hypothesis on the relation in predator and their preferred prey, we compared the activity overlap of tiger - sambar/gaur vs. tiger - other prey species; leopard - sambar/gaur/barking deer/mouse deer vs. leopard - other prey species; and dhole - barking deer/mouse deer/wild pig vs. dhole - other prey species.
To test for statistically significant pairwise patterns of species co-occurrence, we used a probabilistic model for analyzing species co-occurrence (Veech 2013). This model allows us to understand the probability of two species co-occurring (P) at a frequency less than Plt (or greater than Pgt) than the frequency if they were distributed independently of one another. The model is based on calculating Pj, the probability that two species co-occur at exactly j sites. It helps us understand if the species associations in a guild are negative (if Plt < 0.05), positive (if Pgt < 0.05) or random process. For this analysis site-wise matrix of species presence, (“1” if present) absence (“0” if absent) were prepared, in which rows were filled with species names and columns with site details. We used the ‘co-occur’ package (Griffith et al. 2016) in program R (R Development Core Team 2019) for our analysis.
Results
From 253 camera traps location consisting of 7320 trap nights, a total of 6092 photographs were recorded. In total, 2573 photographs were captured during the daytime (0600 to 1800 h) and 3519 during the nighttime (1800 to 0600 h). In total, 30 species were camera trapped including carnivore, herbivore and omnivore mammals, primates, reptiles and Galliformes. For our study, we removed the captures of domestic animals, primates, Galliformes and reptiles and retained 18 mammal species (Figs. 2 and 3). These mammals had various trophic positions and body sizes, including large apex predators (e.g. tiger, leopard) and mega-herbivores (e.g. elephant, gaur), and extended from endangered to least concerned species (Table 1).
Activity patterns of all study species in the study area were derived (Fig. 4). Tiger and leopard showed a diel activity pattern. Small predators were predominantly nocturnal except jungle cat which showed diel activity pattern and Nilgiri marten, striped necked mongoose, which had diurnal activity pattern.
Temporal activity estimated using the camera trapped derived activity pattern of different mammals in Periyar Tiger Reserve. Horizontally from the top left corner: tiger, gaur, jungle cat, Indian crested porcupine, leopard, sambar deer, leopard cat, stripe-necked mongoose, dhole, wild pig, small Indian civet, brown mongoose, sloth bear, barking deer, brown palm civet, Nilgiri marten, elephant, mouse deer, Nilgiri marten
We further assessed activity overlap and spatial association for testing the study hypotheses. Spatial association of 153 species pairs were analyzed for understanding the co-occurrence pattern from 253 sites. The analysis resulted in 37 positive associations (Pgt < 0.05), 7 negative associations (Plt < 0.05), 109 had random association (Online resource 1).
When the activity overlap and spatial association between the three large predators (tiger, leopard and dhole) were compared (Fig. 5), a moderate activity overlap was observed between tiger– leopard (0.63 with 95% bootstrap confidence interval (0.58–0.69)) and tiger–dhole (0.57(0.52–0.63)) but had no spatial association (Plt = 0.58, Pgt=0.53), (Plt = 0.6, Pgt=0.51) respectively. Leopard and dhole had similar moderate activity overlap (0.65(0.60–0.70)) and an insignificant positive association (Plt = 0.91, Pgt=0.14). When the activity of tiger and leopard were added into one activity pattern and compared with that of the dhole’s, the similar moderate overlap was observed as the above comparisons (0.66(0.61–0.71)).
The overlap in predators (tiger, leopard and dhole) and their prey (sambar, gaur, barking deer, mouse deer and wild pig) revealed species-specific spatial associations and activity overlaps (Fig. 6). Tiger had higher activity overlap with gaur (0.87(0.83–0.92)), sambar (0.83(0.78–0.88)), and lesser overlap with barking deer (0.61(0.54–0.66)) and wild pig (0.62(0.56–0.69)) and mouse deer (0.51(0.46–0.56)). Spatially, the only significant positive overlap of the tiger was with the gaur (Pgt < 0.001). Leopard had maximum activity overlap with wild pig (0.71(0.66–0.76)), barking deer (0.60(0.54–0.66)), Indian gaur (0.58(0.54–0.63)) and sambar deer (0.57(0.52–0.62)), and less overlap with mouse deer (0.40(0.35–0.44)); and had significant positive spatial association with gaur (Pgt < 0.05), barking deer (Pgt < 0.005) and mouse deer (Pgt < 0.005). Dhole had maximum activity overlap with wild pig (0.71(0.66–0.76)), barking deer (0.60(0.54–0.66)) and lesser overlap with Indian gaur (0.58(0.54–0.63)), sambar deer (0.57(0.52–0.62)) and mouse deer (0.16(0.13–0.19)). It had significant positive spatial association with barking deer (Pgt < 0.001) and gaur (Pgt < 0.05).
Discussion
Our study shows preliminary indication towards a trophic dependent spatio-temporal association between different mammals in a tropical forest. We used this association pattern to elucidate hypotheses on niche-segregation of large sympatric carnivores and niche-overlap of predators with their prey species.
Tiger, leopard and dhole are the three top predators in the order of their body weight (Table 1). Due to their dietary overlap in this region (Karanth and Sunquist 1995), niche segregation in terms of either space-use or activity of these three species has been previously recorded (Harihar et al. 2011; Karanth and Sunquist 2000). We observed that tiger and leopard have higher activity overlap as reported from previous studies (Karanth and Sunquist 1995; Ramesh et al. 2012) but we did not find any spatial association. Tiger and dhole had moderate activity overlap and no spatial association. When the activity of tiger and leopard was combined to compare with dhole, it indicated that dhole was more active when the cumulative activity of tigers and leopards was least, thus indicating niche-segregation on the axis of time in these three sympatric predators in this region. An insignificant positive spatial association was observed in leopard and dhole. This could be because, tiger hunt large-bodied nocturnal prey, while leopard and dhole prey on medium-sized diurnal mammals, thus having higher overlap. Studies in the tropical Indian forests have reported spatial niche-segregation of these sympatric carnivores in terms of their density (Kumar et al. 2019). And as our study only considered presence and absence, we see a scope of improving our hypothesis by considering density-based spatial segregation of these sympatric carnivores. While there was no significant spatial association within these predators, it could mostly be due to their larger ranging size, wherein they require a larger area with abundant prey. But owing to the comparative low prey availability within the tiger reserve (Jhala et al. 2011, 2015) and higher human disturbances around the area, these predators best co-occurred in the tiger reserve while temporally avoiding each other. This attests our first hypothesis that the sympatric large carnivore mammals have potential segregation in the PTR.
While the top predators segregated amongst themselves, their activity and spatial distribution are known to be governed by prey availability (Johnsingh 1983; Karanth and Sunquist 2000). Our analysis to test the overlap in prey and predators revealed a hierarchical relationship that can be explained by the body sizes of predator and their prey. The largest predator of the area, tiger, had higher spatial and temporal overlap with larger-bodied prey (gaur and sambar). The second large predator, the leopard, had higher spatio-temporal overlap with small-sized prey (barking deer and wild pig). Dhole had higher spatio-temporal overlap with varied body-sized prey (gaur, wild pig and barking deer), which could be explained by its group hunting behaviour that avails chances to prey on larger as well as smaller-bodied prey Karanth and Sunquist (2000). Thus, we accept the second study hypothesis that there is a potential spatio-temporal overlap in the predators and prey, based on their body mass. The density of sambar was 3.7/km2 and gaur was 1.6/km2 in the tiger reserve, while prey densities are known to be relatively lower in outside the protected area (Jhala et al. 2015). Habitat specific density estimates of prey species in other tiger reserves have helped in the past to assist habitat management and restoration (Awasthi et al. 2016) and can guide similar evidence-based actions in PTR as well. Understanding the unique genetic variability present in the tiger population in this area, it is essential to maintain a prey-base that can sustain a stable tiger population, as well as for leopard and dholes, whose genetic importance in this area is not documented. Hence, it is important to understand the habitat-specific density estimates of all prey species in the park and surrounding, and then estimate the carrying capacity of the top predators by also considering the potential segregation shown in this study. As the habitat of PTR and its surrounding is known to be threatened by invasive plants (Mungi et al. 2019, 2020), site-specific habitat restorations could be prioritized so as to enhance the natural densities of prey species of varied body sizes, which will maximize the carrying capacity of sympatric carnivores in the area.
Present results suggest potential niche-segregation in sympatric top predators and prey-predator overlap with regards to their body weight. Though our results from primary evidence confirm these hypotheses, we highlight the limitation of our interpretation in the light of the correlative indices of occurrence used in this study. There is a scope to use detection corrected probability of occurrence and validate these hypotheses (e.g. Bhattacharya et al. 2012; MacKenzie et al. 2018). Secondly, a spatio-temporal overlap amongst these species is known to be more significant when density is considered as a parameter (e.g. Karanth et al. 2017; Lamichhane et al. 2019; Kumar et al. 2019). Hence, using density-based indices of spatio-temporal overlap can further refine these findings. An extended period of camera trapping across different season might also reveal an enhanced understanding of the dynamic associations within different species, in contrast to the current pattern that was observed during a 30 days’ window. However, as our objective was to highlight the utility of simpler statistical packages in assessing the occurrence and co-occurrence pattern of camera trapped species, we see the implied methods enhancing our understanding of species co-occurrence using relatively simpler indices. We see our study advancing the understanding of multi-species occurrence and association in protected areas that are periodically monitored, particularly areas where systematic monitoring is not always feasible. For example, tiger reserves in India are mandated to conduct camera trapping every year to monitor the population of the tiger; however, the buffer regions of tiger reserves and the corridors are hardly systematically monitored. We see our study being used in these and such systems to understand changes in the activity and occurrence pattern of the species.
Management implications
Despite being a preliminary and suggestive indication towards spatio-temporal overlap and segregation of sympatric predators and their prey species, we importantly lend inference to maximize biodiversity conservation in small protected areas in a megadiverse developing country. In India, conservation actions for doubling the tiger numbers since 2006 have resulted in active conservation measures in and around tiger reserves (Yumnam et al. 2014; Dutta et al. 2016). This has also resulted in increased tiger population in the Periyar landscape (Jhala et al. 2015, 2019). This population increase is dependent on prey-base available in different habitat types of an area. Our results suggest that tiger shows spatio-temporal overlap with gaur and sambar that holds the key to actively manage habitats so that optimal densities of these prey species can be conserved in these habitats, which will in-turn optimize the carrying capacity of tigers. Subsequently, dholes segregate temporally from tigers and leopards, to potentially avoid the competition. Thus, only considering increasing tiger population might limit the capacity to conserve this species, which is dependent on moderate-sized prey species like the barking deer and mouse deer. Furthermore, we add to the existing evidence that suggests only conserving apex predator, can have consequences on other sympatric carnivores, who can face a cascading effect (Kumar et al. 2019). Hence, a key to maximize the biodiversity conservation in this area is to understand the hierarchical dependence and segregation of different species and holistically assess an inclusive conservation plan for the spectrum of available mammalian fauna. While many other protected areas in megadiverse countries are centered towards conserving an umbrella species, they hold disproportionately higher biodiversity as compared to outside areas (Gray et al. 2016; Ghosh-Harihar et al. 2019). Through our study, we demonstrate that it is pertinent to consider the interdependence of different species to maximize the biodiversity conservation in protected areas.
References
Awasthi N, Kumar U, Qureshi Q, Pradhan A, Chauhan J, Jhala Y (2016) Effect of human use, season and habitat on ungulate density in Kanha Tiger Reserve, Madhya Pradesh, India. Reg Environ Chang 16(S1):S31–S41. https://doi.org/10.1007/s10113-016-0953-z
Banerjee K, Jhala YV, Chauhan KS, Dave CV (2013) Living with lions: the economics of coexistence in the Gir forests, India. PLoS One 8(1):e49457. https://doi.org/10.1371/journal.pone.0049457
Baskaran N (2013) An overview of Asian elephants in the Western Ghats, southern India: Implications for the conservation of Western Ghats ecology. J Threat Taxa 5(14):4854–4870. https://doi.org/10.11609/JoTT.o3634.4854-70
Bhattacharya T, Bashir T, Poudyal K, Sathyakumar S, Saha GK (2012) Distribution, occupancy and activity patterns of goral (Nemorhaedus goral) and Serow (Capricornis thar) in Khangchendzonga biosphere reserve, Sikkim, India. Mamm Study 37(3):173–181. https://doi.org/10.3106/041.037.0302
Bridges AS, Noss AJ (2011) Behavior and activity patterns. In: O’Connell AF, Nichols JD, Karanth KU (eds) Camera traps in animal ecology. Springer, Tokyo, pp 57–69
Champion HG, Seth SK (1968) A revised survey of the forest types of India. Forest ecology. Manager of Publications, Delhi
Chandler RB, Royle AJ (2013) Spatially explicit models for inference about density in unmarked or partially marked populations. Ann Appl Stat 7(2):936–954. https://doi.org/10.2307/23566419
Das S, Sreehari R, Rajkmar KP (2015) Report on the Amphibians of Periyar Tiger Reserve. Periyar Tiger Conservation Foundation, Thekkady
Dröge E, Creel S, Becker MS, M’soka J (2016) Spatial and temporal avoidance of risk within a large carnivore guild. Ecol Evol 7(1):189–199. https://doi.org/10.1002/ece3.2616
Dutta T, Sharma S, McRae BH, Roy PS, DeFries R (2016) Connecting the dots: mapping habitat connectivity for tigers in central India. Reg Environ Chang 16(1):53–67. https://doi.org/10.1007/s10113-015-0877-z
Eriksen A, Wabakken P, Zimmermann B, Andreassen H, Arnemo J, Gundersen H, Storaas T (2011) Activity patterns of predator and prey: A simultaneous study of GPS-collared wolves and moose. Anim Behav 81:423–431. https://doi.org/10.1016/j.anbehav.2010.11.011
Foster VC, Sarmento P, Sollmann R, Tôrres N, Jácomo AT, Negrões N, Fonseca C, Silveira L (2013) Jaguar and puma activity patterns and predator-prey interactions in four Brazilian biomes. Biotropica 45:373–379. https://doi.org/10.1111/btp.12021
Ghosh-Harihar M, An R, Athreya R, Borthakur U, Chanchani P, Chetry D, Price TD (2019) Protected areas and biodiversity conservation in India. Biol Conserv 237:114–124. https://doi.org/10.1016/j.biocon.2019.06.024
Gray C, Hill S, Newbold T, Hudson L, Börger L, Contu S, Scharlemann J (2016) Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat Commun 7:12306. https://doi.org/10.1038/ncomms12306
Griffith DM, Veech JA, Marsh CJ (2016) Co-occur: probabilistic species co-occurrence analysis in R. J Stats Softw 69:1–17. https://doi.org/10.18637/jss.v069.c02
Gubbi S (2006) Tiger habitats and integrated conservation and development projects: a case study from Periyar Tiger Reserve, India. Dissertation, Durrell Institute for Conservation and Ecology, Canterbury
Harihar A, Pandav B, Goyal S (2011) Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J Appl Ecol 48:806–814. https://doi.org/10.1111/j.1365-2664.2011.01981
Jhala YV, Gopal R, Sinha PR, Qureshi Q (2011) Status of the tigers, co-predators and prey in India. National Tiger Conservation Authority and Wildlife Institute of India, New Delhi
Jhala YV, Qureshi Q, Gopal R (2015) The status of tigers in India 2014. National Tiger Conservation Authority, New Delhi and Wildlife Institute of India, Dehradun
Jhala YV, Qureshi Q, Nayak AK (eds) (2019) Status of tigers, co-predators and prey in India 2018. Summary Report. National Tiger Conservation Authority and Wildlife Institute of India, New Delhi
Johnsingh AJT (1983) Large mammalian prey-predators in Bandipur. J Bombay Nat Hist Soc 80:1–57
Kalesh S, Sreehari R (2015) Butterflies of periyar tiger reserve. Periyar Tiger Conservation Foundation, Thekkady
Karanth KU, Sunquist ME (1995) Prey selection by tiger, leopard and dhole in tropical forests. J Anim Ecol 64:439–450. https://doi.org/10.2307/5647
Karanth KU, Sunquist ME (2000) Behavioural correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagahole. India J Zool 250:255–265. https://doi.org/10.1111/j.1469-7998.2000.tb01076.x
Karanth UK, Srivathsa A, Vasudev D, Puri M, Parameshwaran R, Samba Kumar N (2017) Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc R Soc B: Biol Sci 284:20161860. https://doi.org/10.1098/rspb.2016.1860
Kaushik M, Mungi N (2015) Human–wildlife interactions and management of invasive alien species. Curr Sci 108(6):1039
KFD (2003) India eco-development project. Kerala Forest Department, Thekkady
Krebs JR (1973) Behavioral aspects of predation. In: Bateson PPG, Klopfer PH (eds) Perspectives in ethology. Springer, Boston, pp 73–111
Kumar U, Awasthi N, Qureshi Q, Jhala YV (2019) Do conservation strategies that increase tiger populations have consequences for other wild carnivores like leopards? Sci Rep 9(1):14673. https://doi.org/10.1038/s41598-019-51213-w
Lamichhane BR, Leirs H, Persoon GA, Subedi N, Dhakal M, Oli BN, Malla S (2019) Factors associated with co-occurrence of large carnivores in a human-dominated landscape. Biodivers Conserv 28(6):1473–1491. https://doi.org/10.1007/s10531-019-01737-4
Linkie M, Ridout MS (2011) Assessing tiger– prey interactions in Sumatran rainforests. J Zool 284:224–229. https://doi.org/10.1111/j.1469-7998.2011.00801.x
Lynam AJ, Jenks KE, Tantipisanuh N, Chutipong W, Ngoprasert D, Gale GA, Leimgruber P (2013) Terrestrial activity patterns of wild cats from camera-trapping. Raffles Bull Zool 61(1):407–415
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2018) Occupancy estimation and modeling - inferring patterns and dynamics of species occurrence. Academic Press, London
Mathur VB, Bist SS, Kaushik M, Mungi NA, Qureshi Q (2015) Management of human-wildlife interaction and invasive species in India. Wildlife Institute of India, Dehradun
Meredith M, Ridout M (2014) Overlap: Estimates of coefficient of overlapping for animal activity patterns. R package version 0.2.4. http://www.CRAN.R-project.org/package=overlap
Mungi NA, Kaushik M, Mohanty NP, Rastogi R, Johnson JA, Qureshi Q (2019) Identifying knowledge gaps in the research and management of invasive species in India. Biologia 74(6):623–629. https://doi.org/10.2478/s11756-018-00186-8
Mungi NA, Qureshi Q, Jhala YV (2020) Expanding niche and degrading forests: Key to the successful global invasion of Lantana camara (sensu lato). Glob Ecol Conserv 23:e01080. https://doi.org/10.1016/j.gecco.2020.e01080
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Noor A, Mir ZR, Veeraswami GG, Habib B (2017) Activity patterns and spatial overlap of sympatric mammals in the moist temperate forest of the Kashmir Himalaya, India. Folia Zool 66(4):231–241. https://doi.org/10.25225/fozo.v66.i4.a4.2017
Pascal JP (1988) Wet evergreen forests of the Western Ghats of India. Ecology, structure, floristic composition and succession. Institut français de Pondichéry, Pondichéry
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, 2.15.2 ed. http://www.R-project.org
Ramachandra TV, Suja A (2006) Sahyadri: Western ghats biodiversity information system. In: Ramakrishnan N (ed) Biodiversity in Indian scenarios. Daya Publishing House, New Delhi, p 22
Ramakrishnan U, Santosh JA, Sukumar R (1998) The population and conservation status of Asian elephants in the Periyar Tiger Reserve, southern India. Curr Sci 74(2):110–113
Raman S, Shameer TT, Sanil R, Usha P, Kumar S (2020) Protrusive influence of climate change on the ecological niche of endemic brown mongoose (Herpestes fuscus fuscus): a MaxEnt approach from Western Ghats, India. Model Earth Syst Environ 6:1795–1806. https://doi.org/10.1007/s40808-020-00790-1
Ramesh T, Kalle R, Sankar K, Qureshi Q (2012) Spatio-temporal partitioning among large carnivores in relation to major prey species in Western Ghats. J Zool Lond 287:269–275. https://doi.org/10.1111/j.1469-7998.2012.00908.x
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14:322–337. https://doi.org/10.1198/jabes.2009.08038
Ripple WJ, Beschta RL (2012) Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol Conserv 145(1):205–213. https://doi.org/10.1016/j.biocon.2011.11.005
Rovero F, Tobler M, Sanderson J (2010) Camera trapping for inventorying terrestrial vertebrates. Manual on field recording techniques and protocols for all taxa biodiversity inventories and monitoring. In: Samyn Y, Vandenspiegel D, Degreef (eds) ABC Taxa, Brussels, Belgium, pp 100–128
Rowcliffe JM, Kays R, Kranstauber B, Carbone C, Jansen P (2014) Quantifying levels of animal activity using camera trap data. Methods Ecol Evol 5:1170–1179. https://doi.org/10.1111/2041-210X.12278
Sanderson EW, Forrest J, Loucks C, Ginsberg J, Dinerstein E, Seidensticker J, Leimgruber P, Songer M, Heydlauff A, O’Brien T, Bryja G, Klenzendorf S, Wikramanayake E (2006) Setting priorities for the conservation and recovery of wild Tigers: 2005–2015. The Technical Assessment, Wildlife Conservation Society, World Wildlife Fund, Smithsonian, and Save the Tiger Fund, Washington, D.C
Sasidharan N (1998) Studies on the flora of Periyar Tiger Reserve. Kerala Forest Research Institute, Thrissur
Schaller GB (1967) The deer and the tiger. University Chicago Press, Chicago
Shameer TT, Ramesh B, Easa PS (2019) Recent records of rusty spotted cat from southern Western Ghats, India. CAT News 70:12–14
Shankar A, Salaria N, Sanil R, Chackaravarthy SD, Shameer TT (2020) Spatio-temporal association of fishing cats with the mammalian assemblages in the East Godavari mangrove delta, India. Mamm Study 45.https://doi.org/10.3106/ms2020-0015
Sharma A, Kabra A, Kinhal GA, Panwar HS, Sharma MK, Upadhyay S, Mohan S, Upadhyay V (2004) Lessons learned from eco-development experiences in India. Peace Foundation, New Delhi
Shukla RR, Babu NVT (2013) Tiger conservation plan, core and buffer. Periyar Tiger Reserve and Periyar Tiger Foundation, Thekkady
Singh P, Macdonald DW (2017) Populations and activity patterns of clouded leopards and marbled cats in Dampa Tiger Reserve, India. J Mammal 98:1453–1462. https://doi.org/10.1093/jmammal/gyx104
Treves A, Karanth KU (2003) Human-carnivore conflict and perspectives on carnivore management worldwide. Biol Conserv 17(6):1491–1499. https://doi.org/10.1111/j.1523-1739.2003.00059.x
Veech JA (2013) A probabilistic model for analysing species co-occurrence. Glob Ecol Biogeogr 22(2):252–260. https://doi.org/10.1111/j.1466-8238.2012.00789.x
Weber W, Rabinowitz A (1996) A global perspective on large carnivore conservation. Biol Conserv 10:1046–1054. https://doi.org/10.1046/j.1523-1739.1996.10041046.x
World Bank (1996) India Eco Development Project. The World Bank, Washington
Yasuda M (2004) Monitoring diversity and abundance of mammals with camera traps: A case study on Mount Tsukuba, central Japan. Mamm Study 29:37–46. https://doi.org/10.3106/mammalstudy.29.37
Yumnam B, Jhala YV, Qureshi Q, Maldonado JE, Gopal R, Saini S, Fleischer RC (2014) Prioritizing tiger conservation through landscape genetics and habitat linkages. PLoS One 9(11):e111207. https://doi.org/10.1371/journal.pone.0111207
Acknowledgements
We thank Kerala forest department, Field director Periyar Tiger reserve, Deputy director Periyar west divisions, range forest officers, front line staff, forest watchers and other members of Periyar Tiger conservation foundation. We thank National Tiger Conservation Authority and Wildlife Institute of India for providing the sampling protocols and facilitating the training for camera trapping. We also thank Neelakhandan madhavan for providing high quality photographs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
ESM 1
(XLSX 20.8 KB)
Rights and permissions
About this article
Cite this article
Shameer, T.T., Mungi, N.A., Ramesh, B. et al. How can spatio-temporal overlap in mammals assist in maximizing biodiversity conservation? A case study of Periyar Tiger Reserve. Biologia 76, 1255–1265 (2021). https://doi.org/10.2478/s11756-020-00645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00645-1