Abstract
Anatomical analysis of root, rhizome, leaf, peduncle and inflorescences has been conducted on Balkan Amphoricarpos taxa, A. neumayerianus (Vis.) Greuter subsp. neumayerianus, A. neumayerianus subsp. murbeckii Bošnjak, A. autariatus Blečić & Mayer subsp. autariatus and A. autariatus subsp. bertisceus Blečić & Mayer using light microscopy (LM) and scanning electron microscopy (SEM), in order to examine the anatomical traits of this genus for the first time. All taxa show similar features. Young adventitious roots share a typical structure. Sclerenchyma fibers are present in the center of older root. On the rhizome cross sections, secondary tissues are noticed with wide parenchyma rays which interrupt a-well developed xylem. Rhizomes show eccentric growth. The leaf blade is amphistomatous, with dorsiventral structure. Crystal druses are found in leaf epidermal and mesophyll cells. The peduncle cross sections is characterized by more or less polygonal shape with medullary collateral vascular bundles arranged in a circle, and a few of them outside of the circle, toward to cortex region. Secretory canals are absent. Involucral bracts and paleae are characterised by the presence of multilayer sclerenchyma in the mesophyll. Inflorescence anatomy shows structures commonly described for Asteraceae members. Densely distributed vermiform (lanate) and glandular biseriate trichomes are present on the peduncle and on both leaf sides, but much more on the abaxial. Anatomical uniformity indicates very close relationships between examined taxa regarding conserve nature of the genome of the genus. Obtained characters contribute to the knowledge of the genus Amphoricarpos anatomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Asteraceae comprises around 1600 – 2000 genera and 24,000 – 30,000 species with global distribution (Funk et al. 2005; Funk and Robinson 2005; Anderberg et al. 2007; Hind 2007). Members of this family are mostly annual or perennial herbaceous plants. Metcalfe and Chalk (1957) noted some particular anatomical traits, which showed to have taxonomic importance within the family, e.g., presence of secretory and laticiferous canals, types of nonglandular and glandular trichomes, occurrence of medullar and cortical vascular bundles and presence of anomalous secondary thickening.
The genus Amphoricarpos Vis. is a member of subtribe Xerantheminae from tribe Cardueae (Herrando-Moraira et al. 2019). The Xerantheminae include unarmed annual or perennial herbs, rarely dwarf shrubs with entire leaves. Besides Amphoricarpos, four genera are included in the subtribe, namely Chardinia Desf., Siebera J. Gay, Xeranthemum L. and Shangwua Yu J. Wang, Raab-Straube, Susanna & J. Quan Liu (Wang et al. 2013; Herrando-Moraira et al. 2019). Molecular-phylogenetic analysis placed Shangwua and Amphoricarpos as successive sister to the rest of genera, while Chardinia was placed as sister to Siebera and Xeranthemum (Wang et al. 2013). Amphoricarpos is formed by heterocarpic perennial mountain chasmophytic plants from the eastern Mediterranean in the Balkans, Anatolia and the Caucasus (Susanna and Garcia-Jacas 2007). Such disjunct distribution indicates that Amphoricarpos taxa most likely belong to the orophytic flora of Tertiary age and thus represents part of an ancient flora which connect Balkans, Asia Minor and Caucasus (Turril 1929).
Amphoricarpos is a genus of complicated taxonomy. Most authors consider that the genus includes five species (Euro+ Med Plantbase, 2019 http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameCache=Amphoricarpos&PTRefFk=7000000): Amphoricarpos autariatus Blečić & E. Mayer, A. elegans Albov, A. exsul O. Schwarz, A. neumayerianus (Vis.) Greuter (Greuter 2003) and A. praedictus Ayasligil & Grierson. In the Balkan Peninsula, populations of Amphoricarpos taxa inhabit crevices and fissures of limestone from central Bosnia to north-west Greece (Blečić and Mayer 1967). The type species A. neumayerianus (Vis.) Greuter from Mt. Orjen was described by Visiani (1844). Murbeck (1891) described a variety with broad leaves from Herzegovina as var. velezenzis Murbeck. Later, Bošnjak (1936) redescribed this taxon as subsp. murbeckii Bošnjak. According to Blečić and Mayer (1967), there are two species distributed on the Balkan Peninsula: A. neumayerianus with long narrow acuminate leaves distributed on the mountain ranges Orjen, Bijela Gora, and Lovćen; and broad-leafed A. autariatus with wider distribution range. They noted that both taxa can be additionally separated by the shape of cypselae, the cypselae wings width and the involucral bracts, especially by the nature of the apex of the involucral bracts. Moreover, Blečić and Mayer (1967) recognized two subspecies within A. autariatus subsp. autariatus Blečić & Mayer with northwestern distribution (Bosnia and Herzegovina and Montenegro) and subsp. bertisceus Blečić & Mayer with a more southeastern range (Montenegro, Macedonia, Kosovo, Albania and northern Greece). On the other hand, some authors have suggested that Balkan Amphoricarpos should be treated as a single species, A. neumayerianus (Caković et al. 2015). For this study, we accept four taxa in the Balkan Amphoricarpos (Bošnjak 1936; Blečić and Mayer 1967).
Some Balkan Amphoricarpos have been the subject of phytochemical (Djordjević et al. 2004, 2006; Cvetković et al. 2014, 2018), biological activity (Atrrog et al. 2008; Jadranin et al. 2013; Gavrilović et al. 2016), morphological (Petit 1997) and molecular and taxonomic studies (Caković et al. 2015). To our knowledge, there are no anatomical investigations of Amphoricarpos from the Balkan complex. Also, there is only one study on the morpho-anatomy of A. elegans (Petit 1997), making Amphoricarpos almost unexplored from an anatomical point of view. In this regard, the main aim of the present study was to conduct anatomical analysis of root, rhizome, leaf, peduncle and inflorescences of A. neumayerianus subsp. neumayerianus, A. neumayerianus subsp. murbeckii, A. autariatus subsp. autariatus and A. autariatus subsp. bertisceus, in order to examine the anatomy of these taxa, as well as to explore important anatomical traits which will contribute to the anatomy of the genus.
Material and methods
Plant material
We examined Balkan Amphoricarpos taxa (Table 1). Parts of root, rhizome, leaf, peduncle and inflorescences from five individuals per taxon were collected from natural populations in Montenegro and Bosnia and Herzegovina during the flowering period (2013) and kept in FAA. Voucher specimens are deposited in the Herbarium (BEOU) of University of Belgrade, Faculty of Biology, Institute of Botany and Botanical Garden “Jevremovac” (Table 1).
Anatomical analysis
Temporary and permanent slides of mature roots, rhizomes, leaves, peduncles and inflorescences of adult plants were prepared. Plant parts were sectioned fresh or fixed (FAA) before preparation for a standard paraffin method (Ruzin 1999). Fresh plant material was hand sectioned with a razor blade and stained with toluidine blue (0.05%, w/v, aqueous) (O’Brien et al. 1964), phloroglucinol-HCl to detect lignified cell walls (Jensen 1962), and Lugol to visualise starch (Johansen 1940). In addition, parts of leaves were cleared by keeping in a mixture of glacial acetic acid and 30% hydrogen peroxide (1:1 v/v) at 60 °C (1 – 5 days). Leaf-blade epidermal prints were made using transparent nail polish and adhesive tape. Paraffin method was applied for preparing cross-sections (8 – 10 μm thick) of middle parts of mature roots, rhizome, leaf, peduncle and inflorescences, as well as longitudinal sections of inflorescenes (8 – 10 μm thick). Sections were double stained in Safranin O (1 %, w/v, 50 % ethanol) and Alcian blue (1 %, w/v, aqueous) and mounted using Canada balsam. The permanent slides are storaged in the Department of Morphology and Systematics of Plants, University of Belgrade, Faculty of Biology. Observations of the obtained microslides were performed on a light microscope Leica DM2000 equipped with a digital camera Leica DFC320 and a computer with the imaging software Leica IM 1000.
Micromorphological methods
Micromorphological analysis was carried out using scanning electron microscopy (SEM). Small parts of dry leaves were sputter-coated with gold for 180 s at 30 mA (BAL-TEC SCD 005), and observed using a JEOL JSM-6460LV electron microscope at an acceleration voltage of 20 kV.
Results
Root
Amphoricarpos taxa are rhizomatous perennial herbs and develop adventitious roots. The cross section is rounded in outline (Fig. 1a–d). Roots show typical structure, with rhizodermis on its surface and parenchyma cortex below, which is a dominant part of the root (Fig. 1a–d). Exodermis, first layer of cortex, is noticed. Parenchyma cells are rich in starch (Fig. 1a, b, d). In the center of the young root parenchyma cells are noticed (Fig. 1b, c), surrounded with xylem, while in the center of the older root sclerenchyma fibers, surrounded with xylem, are visible (Fig. 1a, d). In later stage, roots show secondary structure. Roots lack secretory canals (Fig. 1a–d).
Cross-sestions of the adventitious roots of A. neumayerianus subsp. neumayerianus (a), A. autariatus subsp. autariatus (b), A. autariatus subsp. bertisceus (c) and A. neumayerianus subsp. murbeckii (d). b, c. Young root with parenchyma cells in the center. a-d. Older root with sclerenchyma fibers in the center. Abbreviations: Co = cortex; Pc = parenchyma cells; Rh = rhizodermis; Scf = sclerenchyma fibers; Xy = xylem. Bar = 200 μm
Rhizome
On cross-section, rhizomes are more or less round (Fig. 2a, c, e), or irregular in shape (Fig. 2g). On the rhizome cross-sections secondary tissues are noticed (Fig. 2a, c, g, e) with well developed periderm on its surface and a narrow zone of parenchyma cortex below (Fig. 2b, d, f, h). In the central cylinder, well developed xylem is noticed (Fig. 2b, d, f, h) interrupted by wide parenchyma rays (Fig. 2b, d, f, h). Rhizomes show eccentric growth (uneven growth of xylem rings) (Fig. 2g). The vascular rays are homocellular and mostly multiseriate. A pith, composed of very large parenchyma cells, is noticed in the central region (Fig. 2a, c, e, g). Rhizomes lack secretory canals.
Cross-sestions of the rhizomes A. neumayerianus subsp. neumayerianus (a-b), A. autariatus subsp. autariatus (c-d), A. autariatus subsp. bertisceus (e-f) and A. neumayerianus subsp. murbeckii (g-h). a, c, e, g General rhizome anatomy showing secondary structures and pith. b, d, f, h Detail of the rhizome anatomy showing periderm, parenchyma cortex, secondary phloem, well developed xylem and wide parenchyma rays. Abbreviations: Co = cortex; Pe = periderm; Pi = pith; Pr = parenchyma rays; Sph = secondary phloem; Sxy = secondary xylem. Bar = 500 μm in a, c, e and g; 200 μm in b, d, f and h
Leaf
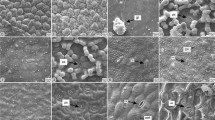
On the leaf cross section, the main vein is heart-shaped with two ribs (Fig. 3a, c, e, g). Adaxial epidermis is one-layered and covered with thick cuticle (Fig. 3b, d, f, h, i). The cells of adaxial epidermis are visibly larger compared to the cells of abaxial epidermis (Fig. 3b, d, f, h). Epidermal cells are irregularly polygonal in shape (Fig. 4a–d). Outer periclinal cell walls are convex and smooth (Fig. 4a–d). Anticlinal cell walls are straight (Fig. 4a–d). The leaf blades are amphistomatous (Fig. 3b, d, f, h). Raised stomata could be noticed, predominantly on abaxial side (Fig. 3b, d, f, h), while their presence on the adaxial side is extremely rare (Fig. 3b). The leaf blade has dorsiventral structure (Fig. 3b, d, f, h). Below adaxial epidermis, two-layered palisade tissue, consisted of large cells rich in chloroplasts, and spongy tissue of several layers of polygonal cells and large intercellular spaces is visible (Fig. 3b, d, f, h). Collateral closed vascular bundles, surrounded by parenchyma tissue, arranged in a row, are noticed in the central leaf blade plane (Fig. 3b, d, f, h). One vascular bundle, or one large and two small vascular bundles are present in the main vein, with a surrounding parenchyma sheath which extended to both epidermises (Fig. 3a, c, e, g). On the adaxial side, subepidermal collenchyma is noticed, whereas on the abaxial side it alternated with chlorenchyma (Fig. 3a, c, e, g). The abaxial epidermis is one-layered and covered with a thinner cuticle compared to adaxial epidermis. Leaves lack of secretory canals.
Cross-sestions of the leaves of A. neumayerianus subsp. neumayerianus (a, b), A. autariatus subsp. autariatus (c, d), A. autariatus subsp. bertisceus (e, f) and A. neumayerianus subsp. murbeckii (g–j). a, c, e, g, i Heart-shaped main vein, with one, or three vascular bundles, and two ribs. b, d, f, h Detail of the leaf anatomy showing amphistomatous structure and raised stomata (arrowheads). i, j Crystals of irregular shape (arrowheads). Abbreviations: Abe = abaxial epidermis; Ade = adaxial epidermis; Pp = palisade parenchyma; Sp = spongy parenchyma; Vb = vascular bundle. Bar = 200 μm in a-b-c-d-e-f-g-h; 100 μm in i-j
Druses (CaOx crystals) of irregular shape can be noticed in both adaxial and abaxial epidermal cells of the leaves (Fig. 3i, j). These crystals are especially visible inside the adaxial epidermal cells (Fig. 3j). They are also observed in some leaf mesophyll cells (Fig. 3i).
Curly, nonglandular trichomes, cover both leaf surfaces, but much more so abaxial side, forming a velvety indumentum (Fig. 5a, b). These trichomes are vermiform (lanate). Glandular trichomes, seen as glandular dots, are also evident on both leaf surfaces, but much more so on the abaxial side (Fig. 5a, b). Glandular trichomes are of the biseriate type (Fig. 6a–c).
Peduncle
Peduncle cross section has irregular polygonal shape (Fig. 7a, c, e, g). One-layered epidermis consists of oval to isodiametric cells is on the surface (Fig. 7b, d, f, h). Below epidermis there is a cortex made up of collenchyma and chlorenchyma, alternately arranged. Prominent ribs contain collenchyma tissue, while chlorenchyma occurs between the ribs (Fig. 7b, d, f, h). The vascular bundles are collateral and arranged in a circle (Fig. 7a, c, e, g) separated from one another by a parenchyma tissue (Fig. 7b, d, f, h). Well-lignified sclerenchyma tissue surrounds each vascular bundle. A few vascular bundles (“cortical” vascular bundles) are positioned outside the circle, near cortex (Fig. 7b, c, f, h). A clearly visible endodermis layer, which contains starch, separates cortex from the central cylinder (Fig. 7h). There is a pith, composed of large parenchyma cells, in the central region of the peduncle (Fig. 7b, d, f, h). Peduncles lack secretory canals.
Cross-sestions of the peduncles of A. neumayerianus subsp. neumayerianus (a, b), A. autariatus subsp. autariatus (c, d), A. autariatus subsp. bertisceus (e, f) and A. neumayerianus subsp. murbeckii (g, h). a, c, e, g. General peduncle anatomy. b, d, f, h Detail of the peduncle anatomy showing epidermis, cortex, medullary vascular bundles and a few “cortical” vascular bundles (arrows). h Endodermis layer with starch separated central cylinder from cortex (arrowhead). Abbreviations: Chl = chlorenchyma; Col = collenchyma; Ep = epidermis; Pi = pith; Vb = vascular bundles. Bar = 200 μm
Inflorescence
The cross and longitudinal sections of the inflorescence show different stages of flowers and fruits development (Fig. 8a–h). Involucral bract has one-layered epidermis, below a one-layer hypodermis, or hypodermis is absent, and a highly developed multilayer sclerenchyma tissue (Fig. 8b, d, f). Below sclerenchyma, a parenchyma tissue with vascular bundles is present. Receptacular bracts or paleae show similar anatomy to involucral bract anatomy (Fig. 8b, d, f). In the early stages of ovule development, stamens with polen grains are observed (Fig. 8g). Ovary is inferior, bicarpelar, syncarpous, and unilocular (Fig. 8b, f, g, h). The ovule is anatropous, unitegmic, and tenuinucellate with basal placentation (Fig. 8g). The outer and inner pericarp epidermis is uniseriated, whereas two regions could be seen in the mesophyll (Fig. 8g).
Inflorescence cross sections (a, c, e, g) and longitudinal sections (b, d, f, h) of A. neumayerianus subsp. neumayerianus (a, b), A. autariatus subsp. autariatus (c, d), A. autariatus subsp. bertisceus (e, f) and A. neumayerianus subsp. murbeckii (g, h). Abbreviations: Cr = crashed cells; En = endosperm; Ep = pericarp epidermis; Et = testa epidermis; Ib = involucral bracts; IC = inner cypsela; OC = outer cypsela; Ova = ovary; Ovu = ovule; Pa = Palea; Pc = Parenchyma cells; Re = receptaculum; Sf = sclerenchyma fibers; Tvb = testa vascular bundles. Bar = 500 μm in a,c,e and g; 200 μm in b,d,f and h
Amphoricarpos taxa are heterocarpic plants: immature inner cypselae, derived from central hermaphrodite florets (Fig. 8b, d, f), and immature outer cypselae, derived from peripheral female florets, can be observed (Fig. 8f, h). Immature fruit contains a pericarp layer, composed of uniseriated epidermis, groups of sclerenchyma fibers and parenchyma cells (Fig. 8d), and testa layer, composed of testa epidermis with lignified cells and crashed cells below (Fig. 8d). The cells of the pericarp parenchyma have more or less the same structure throughout the pericarp and in the mature state they are rich in intercellular space (Fig. 8d). Phytomelanin layer is not observed. The endosperm, which surrounds the embryo, is composed of one to three cell layers (Fig. 8a, d). The mature embryo is axial and occupies the whole seminal chamber (Fig. 8a, d). The embryo axis is straight, and the plumule is poorly differentiated (Fig. 8a). Two plano-convex cotyledons are noticed (Fig. 8a).
Discussion
Amphoricarpos taxa show thickened underground organs, a rhizome, which produces adventitious roots. In general, anatomical studies which included roots and rhizomes were mainly focused on those species that possess some secretory structures and have potential application in pharmacy. Cury and Appezzato-da-Glória (2009) thoroughly analyzed and documented different secretory structures in thickened underground organs (root, xylopodium, underground stem) of six Asteraceae species belonging to the different tribes. Anatomical investigations of underground organs of Cardueae are scarce (Fritz and Saukel 2011a, b), and most studies deal with South American species, especially those which contain secretory ducts and cavities (Melo-de-Pinna and Menezes 2002; Appezzato-da-Glória et al. 2008). Ginko et al. (2016) tested suitability of root and rhizome anatomical characters for taxonomic classification and phylogenetic reconstruction of 59 species from tribes Cardueae and Cichorieae, although selection of taxa was based on pharmaceutical importance. They concluded that most anatomical features showed at least some degree of homoplastic evolution, thus limiting their suitability as phylogenetically informative characters. Fritz and Saukel (2011c) documented the presence of interxylary cork in the roots of two Saussurea DC. species. Interxylary cork originates in the secondary xylem and involves the development of a periderm which separates the originally xylem cylinder. Thereafter, the root is splitting into various strands (Fritz and Saukel 2011c). However, more comparative taxonomic studies of the root/rhizome anatomy of other Cardueae species are needed.
Presence of meristematic endodermis and secretory canals is related to adventitious roots in Asteraceae (Williams 1947; Melo-de-Pinna and Menezes 2002). According to Williams (1947), who studied a number of different plant species, the meristematic endodermis produces tissues between the endodermis and the hypodermis. It was documented that in roots of Ianthopappus corymbosus (Less.) Roque & D.J.N.Hind (Mutisieae, Asteraceae), the meristematic endodermis forms more than 2/3 of the cortex, while the hypodermis, at early differentiation stages, produces the external part of the cortex (Melo-de-Pinna and Menezes 2002). Likewise, in the species of Richterago (Melo-de-Pinna 2000), meristematic endodermis only forms the inner cortex. Also, in Ianthopappus corymbosus, the meristematic endodermis has a main role in the formation of secretory canals (Melo-de-Pinna and Menezes 2002). Moreover, epithelial organization of the secretory canals cells of adventitious roots support the exclusion of I. corymbosus from the genus Richterago (Melo-de-Pinna and Menezes 2002). In the present work, we didn’t notice meristematic endodermis, as we used older adventitious roots for sectioning, where cortex is already developed. Although root secretory canals segregating lipophilic substances are common in Asteraceae (Fahn 1979; Cury and Appezzato-da-Glória 2009; Janaćković et al. 2019), as well as in some Cardueae species (Metcalfe and Chalk 1950; Williams 1947), Amphoricarpos lacks secretory canals in the roots. The same character, lack of root secretory canals, was documented for two related Xeranthemum species (Gavrilović et al. 2019a).
Rhizome, although showing secondary structures, retained primary cortex in the examined Amphoricarpos taxa. Rhizome anatomy could be considered as typical, except for observed eccentric growth. Evans et al. (2012) reported the interesting phenomenon of eccentricity in the stems of Artemisia tridentata Nutt. They showed that some determinate flowering branches growth causes the death of the vascular cambium which surrounds their attachment points on the main stem. This death results in the eccentric growth of the stem. Furthermore, they suggested that this peculiar eccentric growth phenomenon, which is not associated with interxylary cork, supports the hypothesis that A. tridentata descends from an herbaceous ancestor and has evolved secondarily imperfect woodiness. However, eccentric growth documented in rhizomes of Amphoricarpos do not agree with that kind of scenario. Namely, in the Xerantheminae, our favored hypothesis, also based on other results (Garnatje et al. 2004; Barres et al. 2013; Gavrilović et al. 2019a, b), is that the annual habit in Xeranthemum, Chardinia and Siebera is a secondary adaptation to arid climates from mesophilous perennial ancestors. Another evidence came from Omer and Moseley (1981) who studied the vegetative anatomy of Jaumea carnosa (Less.) A. Gray and found very broad parenchymatous areas that develop in the secondary tissues of the rhizome. Omer and Moseley (1981) connected these parenchymatous areas to a slower activity of vascular cambial cells, which forms depressions in the axis. Broad parenchymatous areas were also recorded in examined Amphoricarpos taxa. It was also concluded that adventitious roots in the rhizomes develop from ground tissues near to the primary xylem and from interfascicular regions close to the primary phloem (Omer and Moseley 1981).
Peduncle anatomy of the examined taxa was typical one described for the Asteraceae (Metcalfe and Chalk 1957) and related Xeranthemum species (Gavrilović et al. 2019a). We observed several so called “cortical” vascular bundles outside the circle consisted of medullary vascular bundles. According to Metcalfe and Chalk (1950), the presence of medullar and cortical bundles is important for taxonomy. Makbul et al. (2012) found cortical vascular bundles in the stem of Scorzonera ahmet-duranii Makbul & Coşkunç, but not in the stem of related S. semicana DC., reinforcing the taxonomic importance of these bundles. In another study, Makbul et al. (2016) found small cortical bundles among the main large vascular bundles of Turkish species of Scorzonera. However, they stated that there is no correlation between this feature and the subgeneric or sectional taxonomy (Makbul et al. 2016). Occurrence of cortical and medullar vascular bundles is also documented in Centaurea sadleriana Janka (Luković et al. 2013), Ianthopappus corymbosus (Melo-de-Pinna and Menezes 2002) and Xeranthemum annuum L. (Gavrilović et al. 2019a). Melo-de-Pinna and Menezes (2002) distinguished cortical vascular bundles from leaf traces regarding xylem and phloem position; in the cortical vascular bundles the xylem is outside the phloem, whereas in leaf traces xylem is oriented to the organ axis. But, are these cortical vascular bundles really positioned in the cortex of examined Amphoricarpos taxa? In a simple histochemical test, we confirmed that endodermis, which cells are rich in starch, surrounds all vascular bundles. Moreover, in these bundles, xylem is oriented to the organ axis. Thus, one should check xylem/phloem orientation in the bundles and use term “cortical” with caution, as these bundles are within central cylinder, just outside the circle, although term cortical was freely used throughout literature. Anyhow, presence of these bundles might have taxonomic value, but other related taxa from subtribe Xerantheminae should be examined anatomically.
All the studied taxa lack secretory organs in the peduncle, as was also documented for related Xeranthemum species (Gavrilović et al. 2019a). Most genera of Cardueae have only laticifers or have no secretory organs in the aerial parts, e.g. Xeranthemum, Siebera, Chardinia, Cardopatium Juss. etc. (Dittrich 1996; Gavrilović et al. 2019a). Thus, lack of secretory organs inside the plant body connect Amphoricarpos and Xeranthemum taxa.
The taxonomic value of leaf epidermal characters is very well documented in Asteraceae (Barthlott 1981; Adedeji and Jewoola 2008; da Silva et al. 2014; Karanović et al. 2015; Gavrilović et al. 2019b). Epidermal cells in examined taxa lack ribbed thickenings of outer periclinal cell walls, as was also documented for Amphoricarpos exsul and Shangwua denticulata (DC.) Raab-Straube & Yu J. Wang (Gavrilović et al. 2019b). This feature is most likely associated to the mesophytic, mountane habitats of these species. In all the studied taxa, epidermal cells are polygonal in shape, as also documented for A. exsul. Anticlinal epidermal cell walls in the examined taxa are straight, while those in A. exsul are slightly sinuate (Gavrilović et al. 2019b) and sinuate in A. elegans (Gavrilović et al. 2018).
Although leaf anatomical features are often related with the environment, they are under genetic control and thus have taxonomic value (Anderson and Creech 1975). Presence of thick cuticle, which we documented on the adaxial surface of all examined taxa, might indicate xeromorphy (Anderson and Creech 1975). On the other hand, leaves of studied taxa have dorsiventral leaf structure, which is common in Asteraceae members (Duarte et al. 2011; Oliveira et al. 2011). This trait is particularly important for the phylogeny of the Xerantheminae, as it is conserved in two Xeranthemum species (Gavrilović et al. 2019a) that grow at open, arid habitats. The hypothesis that Xeranthemum species may have originated from mesophylous ancestors is probably true.
Morphology and distribution of crystals are considered genetically controlled by the cell (Meric 2009) and could be useful in taxonomic evaluation of certain taxa. According to Prychid and Rudall (1999) druses are common in dicotyledons. Meric (2008) documented druses in leaves (epidermis and mesophyll) of Conyza canadensis (L.) Cronquist and C. bonariensis (L.) Cronquist. Crystals were found in the epidermal cells of Gleditsia triacanthos L. (Borchert 1984) and in the leaflets of Stylosanthes guianensis (Aubl.) Sw. (Brubaker and Horner 1989). Wu and Kuo-Huang (1997) also recorded druses in the leaf epidermal cells of Artocarpus altilis (Parkinson ex F.A.Zorn) Fosberg and in the mesophyll cells of A. altilis, Cudrania cochinchinensis (Lour.) Yakuro Kudo & Masam., Ficus virgata Reinw. ex Blume and Morus australis Poir. Meric (2009) found druses in the leaf mesophyll layers of Aster squamatus (Spreng.) Hieron. but not in the leaf epidermal cells. In addition, Lersten and Horner (2000) reported that druses in the leaves of Prunus L. species can be useful for the vague taxonomy of this genus. Kuo-Huang et al. (2007) mentioned that druses from the palisade cells of Peperomia glabella (Sw.) A.Dietr. are involved in the photosynthetic process and that the diameter of the crystals was correlate to the light intensity. Moreover, druses provide structural support to the tissues and are involved in Ca regulation (Nakata 2003). In the leaves of related Xeranthemum species, we did not found these crystals (Gavrilović et al. 2019a). Thus, further anatomical investigations of other Amphoricarpos and related taxa from subtribe Xerantheminae are needed for understanding and evaluating taxonomic importance of druses.
Indumentum traits represent valuable characters in taxonomy (Hayat et al. 2009). We have shown here that all of the examined taxa possess hairy peduncles and a woolly indumentum on both leaf surfaces, but it much more densely on the abaxial side. It was shown that most Carduinae taxa possess a woolly indumentum (Häffner 2000). Trichomes could be categorized as unicellular or multicellular, composed of one to a few basal cells and a long filiform terminal cell; rarely, trichomes are large, uniseriate and multicellular (Häffner 2000). Capitate glandular trichomes are found on peduncles and on leaves of all of the studied species, but they are much more numerous on the abaxial leaf surface. In Asteraceae, a widely distributed feature on stems and leaves is short-stalked capitate glandular trichomes, seen as glandular dots (Robinson 2009). Glandular trichomes are often formed by a biseriate peduncle, usually formed by five pairs of cells (Ciccarelli et al. 2007), and a head, formed from one to many cells. Development of glandular trichomes was described in Artemisia annua L. (Duke and Paul 1993; Duke et al. 1994) and in A. campestris subsp. maritima (DC.) Arcang. (Ascensão and Pais 1987). Analysing glandular trichomes of Grindelia pulchella Dunal, Bartoli et al. (2011) recorded that the secretion product is accumulated between the cell wall and the cuticle, which breaks and releases the secretion. It is documented that the secretion is composed of different specialized metabolites, e.g. essential oils, sesquiterpene lactones, pectin-like substances, flavonoids, etc. (Ascensão and Pais 1987, 1988; Pagni et al. 2003; Andreucci et al. 2008; Lusa et al. 2016; Gavrilović et al. 2018). However, glands are absent in some Carduinae s. l. species (Häffner 2000). A dense glandular indument, interpreted as an ancient adaptation to xeric habitats, was found in the genus Phalacrachena Iljin from Siberia (Susanna et al. 2011). On the leaf surfaces of the A. elegans both nonglandular as well as rare glandular capitate trichomes are recorded (Gavrilović et al. 2018).
Some floral anatomical and morphological traits, e.g., pappus form, branchs of the style, morpho-anatomy of the corolla and anthers, are useful for the classification of the taxa within the tribes of Asteraceae (Judd et al. 2002). However, historically Asteraceae taxa represents difficulties for ontogenetic studies (Dadpour et al. 2012), and the papers regarding floral and inflorescence anatomy is rare. Batista and De Souza (2017) examined the floral ontogeny of ten Asteraceae species and showed that flower characters are useful in distinguishing species. Also, Franca et al. (2015) investigated embryology of two Ageratum L. species and confirmed heterogeneity of embryological processes within the family. According to Palser (1975) and Stuessy (2009) embryological characters could be important in perceiving taxonomic relationships among families, genera and taxa. Regarding Xerantheminae, Dadpour et al. (2012) analyzed flower morphology of X. squarrosum Boiss. and found substantial differences in development between female and perfect florets. Also, Harris (1995) in his comprehensive micromorphological work on florets and inflorescences development of 39 Asteraceae taxa, included X. annuum. A well-developed multilayer sclerenchyma present in bracts and palea of examined Amphoricarpos taxa might be considered as a protection layer for the inflorescence. The same bract and palea anatomy was found in Xeranthemum species (Gavrilović et al. 2019a), except for crystals, which were not found on bracts surface of Amphoricarpos. In the Cardueae, the receptacle is often bristly, and numerous bristles are scattered over the receptacle (Bremer 1994). In Amphoricarpos the true bracts form a laciniate keeled tongue, which is more narrow in A. elegans, while in A. neumayerianus it is wider and usually irregularly split lengthwise (Petit 1997). Amphoricarpos are heterocarpic plants, thus produce two types of cypselae, the inner ones from central florets and outer ones from peripheral florets (Bremer 1994). We documented several sclerenchyma fascicles in the pericarp of immature outer cypsela, which is characteristic for Xerantheminae (Lavialle 1912; Petit 1997). We observed radially lengthened and sclerified walls of testa epidermal cells, which were also mentioned by Lavialle (1912) and Petit (1997). Regarding ovary, as earlier documented for the family (Davis 1966; Johri et al. 1992; Gavrilović et al. 2019a), examined taxa have an anatropous ovule with basal placentation. Phytomelanin was observed in some tribes of Asteraceae (Pandey and Singh 1982), although we did not observe such layer in the pericarp. Embryo, cotyledons and endosperm layer are in accordance with description in Häffner (2000). Our data contribute to the knowledge of the inflorescence anatomy of the genus, but further analysis of related species from subtribe Xerantheminae should be carried out.
All examined taxa show similar anatomical features. Adventitious young root shows typical structure. Sclerenchyma fibers are present in the center of older root. On the rhizome cross sections secondary tissues are noticed, with wide parenchyma rays which interrupted well developed xylem. Rhizomes show eccentric growth. The leaf blade is amphistomatous, with dorsiventral structure. Crystals druses were found in leaf epidermal and mesophyll cells. The peduncle cross section is characterized by more or less polygonal shape with medullary collateral vascular bundles arranged in a circle, and a few “cortical” vascular bundles. Secretory canals were not found. Multilayer sclerenchyma is present in the mesophyll of involucral bract and palea. Inflorescence anatomy show similar structures with those described for Asteraceae members. Densely distributed vermiform (lanate) trichomes as well as biseriate glandular trichomes are present on the peduncle and on both leaf sides, but much more so on the abaxial. Anatomical uniformity indicates very close relationships between examined taxa regarding conserve nature of the genome of the genus. However, taxonomic treatments of these taxa remain unclear (Blečić and Mayer 1967; Caković et al. 2015; Cvetković et al. 2018). Our results reveal qualitative characters which contribute to the general anatomy of the genus Amphoricarpos. Some of the described anatomical traits could be a guideline for future investigations of other taxa of the Xerantheminae.
References
Adedeji O, Jewoola OA (2008) Importance of leaf epidermal characters in the Asteraceae family. Not Bot Hort Agrobot 36:7–16
Anderberg AA, Baldwin BG, Bayer RJ, Breitwieser I, Jeffrey C, Dillon MO, Eldenäs P, Funk VA, Garcia-Jacas N, Hind DJN, Karis PO, Lack HW, Nesom G, Nordenstam B, Oberprieler C, Panero JL, Puttock C, Robinson H, Stuessy TF, Susanna A, Urtubey E, Vogt R, Ward J, Watson LE (2007) Compositae. In: Kadereit JW, Jeffrey C (eds) Flowering plants. Eudicots. Asterales, vol VIII K. Kubitzki [ed] The families and genera of vascular plants. Springer Verlag, Berlin, pp 123–146
Anderson LC, Creech JB (1975) Comparative leaf anatomy of Solidago and related Asteraceae. Am J Bot 62:486–493
Andreucci AC, Ciccarelli D, Desideri I, Pagni AM (2008) Glandular hairs and secretory ducts of Matricaria chamomilla (Asteraceae): morphology and histochemistry. Ann Bot Fenn 45:11–18
Appezzato-da-Glória B, Hayashi A, Cury G, Soares M, Rocha R (2008) Occurrence of secretory structures in underground systems of seven Asteraceae species. Bot J Linn Soc 157:789–796
Ascensão L, Pais MSS (1987) Glandular trichomes of Artemisia campestris (ssp. maritima): ontogeny and histochemistry of the secretory product. Bot Gaz 148:221–227
Ascensão L, Pais MS (1988) Ultrastructure and histochemistry of secretory ducts in Artemisia campestris ssp. maritima (Compositae). Nord J Bot 8:283–292
Atrrog BAA, Natic M, Tosti T, Opsenica Milojković D, Djordjević I, Tešević V, Jadranin M, Milosavljević S, Lazić M, Radulović S, Tešić Z (2008) Lipophilicity of some guaianolides isolated from two endemic subspecies of Amphoricarpos neumayeri (Asteraceae) from Montenegro. Biomed Chromatogr 23:250–256
Barres L, Sanmartín I, Anderson CL, Susanna A, Buerki S, Galbany-Casals M, Vilatersana R (2013) Reconstructing the evolution and biogeographic history of tribe Cardueae (Compositae). Am J Bot 100:867–882
Barthlott W (1981) Epidermal and seed surface characters of plants: systematic applicability and some evolutionary aspects. Nord J Bot 1:345–355
Bartoli A, Galati BG, Tortosa RD (2011) Anatomical studies of the secretory structures: glandular trichomes and ducts, in Grindelia pulchella Dunal (Astereae, Asteraceae). Flora 206:1063–1068
Batista MF, De Souza LA (2017) Flower structure in ten Asteraceae species: considerations about the importance of morpho-anatomical features at species and tribal level. Braz J Bot 40:265–279
Blečić V, Mayer E (1967) Die europäischen Sippen der Gattung Amphoricarpos Visiani. Phyton (Austria) 12:150–158
Borchert R (1984) Functional anatomy of the calcium-excreting system of Gleditsia triacanthos L. Bot Gaz 145:474–482
Bošnjak K (1936) Iz hercegovačke flore. Glasnik Hrvatskog Prirodoslovnoga Društva 41–48:62–63
Bremer K (1994) Asteraceae: cladistics & classification. Timber Press, Portland
Brubaker CL, Horner HT (1989) Development of epidermal crystals in leaflets of Stylosanthes guianensis (Leguminosae; Papilionoideae). Can J Bot 67:1664–1670
Caković D, Stešević D, Schönswetter P, Frajman B (2015) How many taxa? Spatiotemporal evolution and taxonomy of Amphoricarpos (Asteraceae, Carduoideae) on the Balkan Peninsula. Org Divers Evol 15:429–445
Ciccarelli D, Garbari F, Pagni AM (2007) Glandular hairs of the ovary: a helpful character for Asteroideae (Asteraceae) taxonomy? Ann Bot Fenn 44:1–7
Cury G, Appezzato-da-Glória B (2009) Internal secretory spaces in thickened underground systems of Asteraceae species. Aust J Bot 57:229–239
Cvetković M, Ðorđević I, Jadranin M, Vajs V, Vučković I, Menković N, Milosavljevic S, Tešević V (2014) Further Amphoricarpolides from the surface extracts of Amphoricarpos complex from Montenegro. Chem Biodivers 11:1428–1437
Cvetković M, Anđelković B, Stevanović V, Jadranin M, Đorđević I, Tešević V, Milosavljević S, Gođevac D (2018) NMR-based metabolomics study of Amphoricarpos species from Montenegro. Phytochem Lett 25:1–5
da Silva EMS, Hayashi AH, Appezzato-da-Glória B (2014) Anatomy of vegetative organs in Aldama tenuifolia and A. kunthiana (Asteraceae: Heliantheae). Braz J Bot 37:505–517
Dadpour MR, Naghiloo S, Neycharan SF (2012) The development of pistillate and perfect florets in Xeranthemum squarrosum (Asteraceae). Plant Biol 14:234–243
Davis GL (1966) Systematic embryology of the angiosperms. Wiley, New York, London, Sydney
Dittrich M (1996) Die Bedeutung morphologischer und anatomischer Achänen-Merkmale für die Systematik der Tribus Echinopeae Cass. und Carlineae Cass. Boissiera 51:9–102
Djordjević I, Vajs V, Bulatović V, Menković N, Tešević V, Macura S, Janaćković P, Milosavljević S (2004) Guaianolides from two subspecies of Amphoricarpos neumayeri from Montenegro. Phytochemistry 65:2337–2345
Djordjević I, Jadranin M, Vajs V, Menković N, Tešević V, Macura S, Milosavljević S (2006) Further Guaianolides from Amphoricarpos neumayeri ssp. murbeckii from Montenegro. Z Naturforsch 61b:1437–1442
Duarte MR, Budel JM, Matzenbacher NI, Menarim DO (2011) Microscopic diagnosis of the leaf and stem of Lucilia nitens Less., Asteraceae. Lat Am J Pharm 30:2070–2075
Duke SO, Paul RN (1993) Development and fine structure of glandular trichomes of Artemisia annua L. Int J Plant Sci 154:107–118
Duke MV, Paul RN, Elsohly HN, Sturtz G, Duke SO (1994) Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int J Plant Sci 155:365–372
Evans LS, Citta A, Sanderson SC (2012) Flowering branches cause injuries to second-year main stems of Artemisia tridentata Nutt. subspecies tridentata. West N Am Nat 72:447–457
Fahn A (1979) Secretory tissues in plants. Academic Press, London
Franca RDO, De-Paula OC, Carmo-Oliveira R, Marzinek J (2015) Embryology of Ageratum conyzoides L. and A. fastigiatum R.M. King & H. Rob. (Asteraceae). Acta Bot Bras 29:08–15
Fritz E, Saukel J (2011a) Anatomy of subterranean organs of medicinally used Cardueae and related species and its value for discrimination. Sci Pharm 79:157–174
Fritz E, Saukel J (2011b) Secretory structures of subterranean organs of some species of the Cardueae and their value for discrimination. Acta Biol Cracov Ser Bot 53:63–73
Fritz E, Saukel J (2011c) Interxylary cork of Saussurea discolor and S. pygmaea (Asteraceae). Biologia 66:454–457
Funk VA, Robinson HE (2005) Daisies and sunflowers: Family Asteraceae. In: Krupnick GA, Kress WJ (eds) Plant conservation. A natural history approach. University of Chicago Press, Chicago and London, pp 115–137
Funk VA, Bayer RJ, Keeley S, Chan R, Watson L, Gemeinholzer B, Schilling E, Panero JL, Baldwin BG, Garcia-Jacas N, Susanna A, Jansen RK (2005) Everywhere but Antarctica: using a supertree to understand the diversity and distribution of the Compositae. Biol Skr 55:343–374
Garnatje T, Vallès J, Garcia S, Hidalgo O, Sanz M, Canela MA, Siljak-Yakovlev S (2004) Genome size in Echinops L. and related genera (Asteraceae, Cardueae): karyological, ecological and phylogenetic implications. Biol Cell 96:117–124
Gavrilović M, Soković MD, Stanković M, Marin PD, Stevanović ZD, Janaćković P (2016) Antimicrobial and antioxidative activity of various leaf extracts of Amphoricarpos Vis. (Asteraceae) taxa. Arch Biol Sci 68:803–810
Gavrilović M, Tešević V, Đorđević I, Rajčević N, Bakhia A, Garcia-Jacas N, Susanna A, Marin PD, Janaćković P (2018) Leaf micromorphology, antioxidative activity and a new record of 3-deoxyamphoricarpolide of relict and limestone endemic Amphoricarpos elegans Albov (Compositae) from Georgia. Arch Biol Sci 70:613–620
Gavrilović M, Rančić D, Škundrić T, Dajić-Stevanović Z, Marin PD, Garcia-Jacas N, Susanna A, Janaćković P (2019a) Anatomical characteristics of Xeranthemum L. (Compositae) species: taxonomical insights and evolution of life form. Pak J Bot 51:1007–1019
Gavrilović M, Garcia-Jacas N, Susanna A, Marin PD, Janaćković P (2019b) How does micromorphology reflect taxonomy within the Xeranthemum group (Cardueae-Asteraceae)? Flora 252:51–61
Ginko E, Dobeš C, Saukel J (2016) Suitability of root and rhizome anatomy for taxonomic classification and reconstruction of phylogenetic relationships in the tribes Cardueae and Cichorieae (Asteraceae). Sci Pharm 84:585–602
Greuter W (2003) The euro+med treatment of Cardueae (Compositae): generic concepts and required new names. Willdenowia 33:49–61
Häffner E (2000) On the phylogeny of the subtribe Carduinae (tribe Cardueae, Compositae). Englera 21:1–208
Harris EM (1995) Inflorescence and floral ontogeny in Asteraceae: a synthesis of historical and current concepts. Bot Rev 61:93–278
Hayat MQ, Ashraf M, Khan MA, Yasmin G, Shaheen N, Jabeen S (2009) Diversity of foliar trichomes and their systematic implications in the genus Artemisia (Asteraceae). Int J Agric Biol 11:542–546
Herrando-Moraira S, the Cardueae radiations Group (in alphabetical order) Calleja JA, Galbany-Casals M, Garcia-Jacas N, Liu J-Q, López-Alvarado J, López-Pujol J, Mandel JR, Massó S, Montes-Moreno N, Roquet C, Sáez L, Sennikov A, Susanna A, Vilatersana R (2019) Nuclear and plastid DNA phylogeny of the tribe Cardueae (Compositae) with Hyb-Seq data: a new subtribal classification and a temporal diversification framework. Mol Phylogenet Evol. https://doi.org/10.1016/j.ympev.2019.05.001
Hind DJN (2007) Asteraceae. In: Heywood VH, Brummitt RK, Culham AC, Seberg O (eds) Flowering plant families of the world. Kew Publications, Richmond; Firefly Books, Ontario and Buffalo, New York, pp 46–52
Jadranin M, Djordjević I, Tešević V, Vajs V, Menković N, Soković M, Glamočlija J, Milosavljević S (2013) Sesquiterpene lactones of Amphoricarpos autariatus ssp. autariatus from Montenegro - antifungal leaf - surface constituents. Rec Nat Prod 7:234–238
Janaćković P, Gavrilović M, Rančić D, Dajić-Stevanović Z, Giweli AA, Marin PD (2019) Comparative anatomical investigation of five Artemisia L. (Anthemideae, Asteraceae) species in view of taxonomy. Braz J Bot 42:135–147
Jensen WA (1962) Botanical Histochemistry: principles and practices. WH Freeman and Co., San Francisco
Johansen DA (1940) Plant Microtechnique. McGraw-Hill Book Company, New York, London
Johri BM, Ambegaokar KB, Srivastava PS (1992) Comparative embryology of angiosperms, vol 2. Springer-Verlag, New York
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2002) Plant systematics – a phylogenetic approach, 2nd edn. Sinauer, Sunderland
Karanović D, Luković J, Zorić L, Anačkov G, Boža P (2015) Taxonomic status of Aster, Galatella and Tripolium (Asteraceae) in view of anatomical and micro-morphological evidence. Nord J Bot 33:484–497
Kuo-Huang LL, Ku MSB, Franceschi VR (2007) Correlations between calcium oxalate crystals and photosynthetic activities in palisade cells of shade-adapted Peperomia glabella. Bot Stud 48:155–164
Lavialle MP (1912) Recherches sur le développement de l'ovaire en fruit chez les Composées. Ann Sci Nat Bot 15:39–141
Lersten NR, Horner HT (2000) Calcium oxalate crystals types and trends in their distribution patterns in leaves of Prunus (Rosaceae: Prunoideae). Plant Syst Evol 224:83–96
Luković J, Malenčić D, Zorić L, Kodranov M, Karanović D, Kiprovski B, Boža P (2013) Anatomical characteristics and antioxidant ability of Centaurea sadleriana reveals an adaptation towards drought tolerance. Cent Eur J Biol 8:788–798
Lusa MG, Da Costa FB, Appezzato-da-Glória B (2016) Histolocalization of chemotaxonomic markers in Brazilian Vernonieae (Asteraceae). Bot J Linn Soc 182:581–593
Makbul S, Coskuncelebi K, Gültepe M, Okur S, Güzel ME (2012) Scorzonera ahmet-duranii sp. nov. (Asteraceae) from Southwest Anatolia, and its phylogenetic position. Nord J Bot 30:2–11
Makbul S, Coşkunçelebi K, Okur S, Gültepe M (2016) Contribution to the taxonomy of Turkish Scorzonera (Asteraceae) taxa based on vegetative anatomy. Nord J Bot 34:670–684
Melo-de-Pinna GF (2000) Anatomia dos órgãos vegetativos dos gêneros Richterago Kuntze e Ianthopappus Roque & D.J.N. Hind (Mutisieae-Asteraceae). Tese de doutorado, Universidade de São Paulo, São Paulo
Melo-de-Pinna GF, Menezes NL (2002) Vegetative organ anatomy of Ianthopappus corymbosus Roque & Hind (Asteraceae-Mutisieae). Braz J Bot 25:505–514
Meric C (2008) Calcium oxalate crystals in Conyza canadensis (L.) Cronq. and Conyza bonariensis (L.) Cronq. (Asteraceae: Astereae). Acta Biol Szeged 52:295–299
Meric C (2009) Calcium oxalate crystals in Aster squamatus and Bellis perennis (Asteraceae: Astereae). Phytol Balcan 15:255–259
Metcalfe CR, Chalk L (1950) Anatomy of Dicotyledones I. Clarendon Press, Oxford
Metcalfe CR, Chalk L (1957) Anatomy of the Dicotyledons, vol 2. Clarendon Press, Oxford
Murbeck S (1891) Beiträge zur Kenntnis der Flora von Südbosnien und der Hercegovina. Acta Univ Lund 27:1–182
Nakata PA (2003) Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci 164:901–909
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Oliveira AMA, Santos VLP, Franco CRC, Farago PV, Duarte MR, Budel JM (2011) Comparative morpho-anatomical study of Baccharis curitybensis Heering ex Malme and Baccharis spicata (lam.) Baill. Lat Am J Pharm 30:1560–1566
Omer LS, Moseley MF Jr (1981) The vegetative anatomy of Jaumea carnosa (Less.) Gray (Asteraceae), a salt marsh species. Am J Bot 68:312–319
Pagni AM, Orlando R, Masini A, Ciccarelli D (2003) Secretory structures of Santolina ligustica Arrigoni (Asteraceae), an Italian endemic species. Isr J Plant Sci 51:185–192
Palser B (1975) The bases of angiosperm phylogeny: embryology. Ann Mo Bot Gard 62:621–646
Pandey AK, Singh RP (1982) Development and structure of seeds and fruits in the Compositae, tribe Senecioneae. Bot Jahrb Syst 103:413–422
Petit DP (1997) Generic interrelationships of the Cardueae (Compositae): a cladistic analysis of morphological data. Plant Syst Evol 207:173–203
Prychid CJ, Rudall PJ (1999) Calcium oxalate crystals in monocotyledons: a review of their structure and systematics. Ann Bot 84:725–739
Robinson H (2009) An introduction to micro-characters of Compositae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds) Systematics. Evolution and Biogeography of Compositae. IAPT, Vienna, pp 89–100
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, Oxford
Stuessy TF (2009) Plant taxonomy: the systematic evaluation of comparative data. Columbia Univ Press, New York
Susanna A, Garcia-Jacas N (2007) Tribe Cardueae Cass. In: Kadereit JK, Jeffrey C (eds) The families and genera of vascular plants, Springer-Verlag, vol 8. Berlin, Heidelberg, pp 123–146
Susanna A, Galbany-Casals M, Romashchenko K, Barres L, Martín J, Garcia-Jacas N (2011) Lessons from Plectocephalus (Compositae, Cardueae-Centaureinae): ITS disorientation in annuals and Beringian dispersal as revealed by molecular analyses. Ann Bot 108:263–277
Turril WB (1929) The plant life of the Balkan Peninsula: a Phytogeographical study. Clarendon Press, Oxford
Visiani R (1844) De un nuovo genero della tribù delle Xerantemee. Giorn Bot Ital 1:194–199
Wang YJ, Raab-Straube E, Susanna A, Liu JQ (2013) Shangwua (Compositae), a new genus from the Qinghai-Tibetan plateau and Himalayas. Taxon 62:984–996
Williams BC (1947) The structure of the meristematic root tip and origin of the primary tissues in the roots of vascular plants. Am J Bot 34:455–462
Wu CC, Kuo-Huang LL (1997) Calcium crystals in the leaves of some species of Moraceae. Bot Bull Acad Sinica 38:97–104
Acknowledgments
The authors thank to the Ministry of Education, Science and Technological Development of the Republic of Serbia for financial support (Grant Nos. 173029 and TR31005). Also, many thanks to Mr. Miloš Bokorov from University Center for Electron Microscopy, Novi Sad, for his contribution in SEM analysis, and to Radenko Radošević, technical associate, from Faculty of Agriculture, University of Belgrade, for technical assistance in anatomical laboratory. We also wish to express our gratitude to Milutin Praščević, Alpinetum of Prokletije, Plav (Montenegro) for the immense help in collecting the plant material.
Author information
Authors and Affiliations
Contributions
PJ and MG conducted the field work. PJ, MG and DR conducted anatomical analysis. PJ, MG and DR wrote the manuscript. PJ, ZDS, NGJ, AS and PDM supervised the research and gave comments to the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gavrilović, M., Rančić, D., Garcia-Jacas, N. et al. Anatomy of Balkan Amphoricarpos Vis. (Cardueae, Asteraceae) taxa. Biologia 75, 209–222 (2020). https://doi.org/10.2478/s11756-019-00406-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00406-9