Abstract
Septins are a family of eukaryotic guanosine phosphate-binding proteins that form linear heterooligomeric complexes, which, in turn, polymerize end-on-end into filaments. These filaments further assemble into higher-order structures at distinct subcellular locations. Dynamic changes in the organization of septin cortex structures appear during cell cycle progression. A variety of regulatory proteins and posttranslational modifications are involved in changes to the structure of septin assemblies during the entire cell cycle. In particular, septin-associated protein kinases mediate changes to septin higher order structures or interconnect cellular morphogenesis with the cell cycle. Yeast cyclin-dependent kinase, a master cell cycle regulator, is required for the initiation of a new septin ring. Here, using epifluoresence and electron microscopy, we show that upon phosphorylation by the Cdc28 kinase, septin filaments disassemble into hetero-octameric building blocks, and that filament depolymerization is specifically G1 cyclin-dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septins are conserved guanosine phosphate-binding proteins ubiquitous in eukaryotic species. These filament-forming proteins were first discovered in screens for temperature-sensitive mutations affecting the budding yeast cell cycle (Hartwell and Smith 1985). Five Saccharomyces cerevisiae septin genes are expressed in mitotically-dividing cells, while two septins, Spr3 and Spr28, are present only in cells undergoing meiosis and sporulation (Garcia III et al. 2016). The mitotic septins assemble into two types of heterooctameric linear complexes that differ only in their terminal subunit: Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11 and Shs1-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Shs1 (Garcia III et al. 2011). Complexes with Cdc11 terminal subunits polymerize end-to-end into paired filaments in vitro, whereas the formation of rings, curved bundles and a bird’s nest-like network was observed in the presence of both complex types (Garcia III et al. 2011).
Various regulatory proteins and posttranslational modifications control septin structure organisation at the bud neck. Several protein kinases, including several cyclin-dependent kinases (CDKs), are associated with the septin cortex at particular points in the cell division cycle. CDKs are serine/threonine protein kinases, which require a separate cyclin subunit for their activity. CDKs play crucial roles in cell cycle control, and they modulate transcription in response to several extra- and intracellular impulses. In S. cerevisiae, six CDKs have been identified: Cdc28 (Cdk1), Pho85 (ortholog of mammalian Cdk5), Kin28 (ortholog of mammalian Cdk7), Srb10 (ortholog of mammalian Cdk8), Bur1 (ortholog of mammalian Cdk9) and Ctk1 (ortholog of mammalian Cdk12) (Huang et al. 2007; Lee and Greenleaf 1991; Liao et al. 1995; Lorincz and Reed 1984; Simon et al. 1986; Yao et al. 2000). A single CDK, Cdc28, is sufficient for cell cycle control in budding yeast, but many of its functions are also supported by the non-essential Pho85. The other CDKs have regulatory functions in transcription processes. Cdc28 alternately associates with nine G1, S, G2/M phase cyclins (Cln1–3 and Clb1–6), whereas Pho85 associates with ten cyclins. Cdc28 was first identified in a genetic screen for cell cycle control genes (Hartwell and Smith 1985). This proline-directed kinase prefers the consensus sequence S/T-P-X-K/R (where X is any amino acid), although it also phosphorylates the minimal consensus sequence S/T-P (Nigg 1993). The first evidence for a functional link between septins and a CDK came from Cvrčková et al. (1995), who described the synthetic lethality of a particular Cdc12 allele with cln1Δ cln2Δ. Tang and Reed (2002) demonstrated that two serines at the C-terminus of Cdc3 are phosphorylated by Cdc28. In wild-type cells, the septin ring inherited from the previous cell cycle must disassemble shortly before or simultaneously with assembly of a new ring during G1 phase. A nonphosphorylable mutant of Cdc3 was defective in the disassembly of the old septin ring at the end of the cell cycle. In addition, G1 cyclin was also shown to be required for efficient ring disassembly. In more recent work, the Shs1 septin showed strong genetic interactions with G1 cyclins, and is a direct target of the CDKs associated with G1 cyclins (Egelhofer et al. 2008). The Cdc3 and Shs1 septins were identified among the 308 targets of Cdc28 in a screen combining CDK chemical inhibition and quantitative mass spectrometry (Holt et al. 2009). Recently, in experiments using polarized fluorescence microscopy none of the previously described shs1 phosphorylation-deficient mutants (Egelhofer et al. 2008) had a rate of disassembly different from the wild-type (McQuilken et al. 2017). All of these mutants were comparably highly disorganized and had a wide distribution of septin orientations, analogous to the shs1 null mutant. Phosphorylation of Shs1 by CDK and Pho58 seems to be important for modulating septin higher-order organization, but not for the splitting process (McQuilken et al. 2017). Shs1 is also multiphosphorylated at the C-terminus in the filamentous fungus Ashbya gossypii, however, phosphorylation of this protein is not required for the septin to organize into rings (Meseroll et al. 2012).
Protein-protein interactions and posttranslational modifications induce changes in septin filament polymerization and higher-order structure formation, but these processes are still only partially understood at the molecular level. Here we show that phosphorylation by a cyclin-dependent kinase dramatically alters the assembly of septin filaments in vitro.
Materials and methods
Growth conditions and media
E. coli cells were grown in 1% Tryptone, 0.5% yeast extract, and 0.5% NaCl (LB) or in 1.2% Tryptone, 2.4% yeast extract, 72 mM K2HPO4, 17 mM KH2PO4 and 0.4% glycerol (TB) supplemented with the appropriate antibiotics. General genetic manipulations were conducted using standard procedures (Ausubel et al. 1995). Strains used in this work are listed in Table 1.
Construction of plasmids
In order to construct expression vectors, fragments of different size were amplified using yeast chromosomal DNA as a template, the Q5 or Phusion High-Fidelity DNA polymerase (New England BioLabs) and the appropriate primers (Table 2). The PCR products were digested with restriction endonucleases (Table 2) and the resulting fragments were introduced into expression vectors. The yEGFP PCR product was digested with EagI and SalI and the fragment introduced between the EagI and SalI sites of pFM455 (Farkasovsky et al. 2005), resulting in plasmid pFM873. All plasmid constructs were confirmed by sequencing.
Heterologous expression and isolation of Cdc28, Cdc37, Cak1, Cln2, Clb2, Cks1 and septin complex
E. coli strain BL21 (DE3) Rosetta (Novagen) was transformed with the bicistronic plasmids pFM453 (CDC10 and CDC11) and pFM873 (MBP-CDC12 and CDC3-yEGFP). This strain was used to express a yeast septin complex containing a Cdc3-yEGFP fusion protein, which was purified as described earlier Farkasovsky et al. 2005). For bacterial expression of Cdc28, the plasmids pFM1165 (CDC28), pFM1101 (CDC37) and pFM1102 (CAK1) were transformed into the E. coli strain BL21 (DE3) Rosetta (Novagen). The cells were grown in TB medium, supplemented with ampicillin, kanamycin, streptomycin and chloramphenicol, at 37 °C and induced by addition of 0.2 mM IPTG at an optical density of OD600 = 0.6. After 6 h at 28 °C, cells were harvested by centrifugation, resuspended in isolation buffer IB1 (25 mM NaHPO4 pH 7.8, 5% glycerol, 0.3 M NaCl, 1 mM MgCl2, 5 mM β-mercaptoethanol, Complete protease inhibitors (Roche), 2 mM PMSF), supplemented with 10 mM imidazole, and disrupted by sonication. After high-speed centrifugation at 100000×g, the supernatant was incubated with Ni-NTA-Sepharose (Qiagen), loaded onto a column, washed with buffer IB2 (10 mM Tris-Cl pH 7.8, 5% glycerol, 0.5 M NaCl, 1 mM MgCl2, 5 mM β-mercaptoethanol, 5 mM ATP, Complete protease inhibitors, 0.2 mM PMSF), supplemented with 20 mM imidazole, washed with buffer IB3 (10 mM Tris-Cl pH 7.8, 5% glycerol, 0.3 M NaCl, 1 mM MgCl2, 5 mM β-mercaptoethanol) and the protein was eluted with 250 mM imidazole in buffer IB3. The GST tag, used to improve the protein solubility, was cleaved with thrombin (Serva), separated by GSH-Sepharose (GE Healthcare) and the Cdc28 complex was further concentrated.
Cln2 and Clb2, with maltose-binding protein tags, were expressed in E. coli strain BL21 (DE3) Rosetta under similar conditions as Cdc28. Cells were resuspended in column buffer (50 mM Tris-HCl pH 7.9, 150 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol, Complete protease inhibitors (Roche), 2 mM PMSF), sonicated and centrifuged at 30000×g at 4 °C for 20 min. The supernatant was diluted with column buffer and loaded on amylase resin (New England Biolabs) equilibrated with column buffer. The column was washed with 10 volumes of column buffer, the proteins were eluted with column buffer supplemented with 10 mM maltose, concentrated, and stored at −80 °C.
GST-Cks1 was expressed in E. coli strain BL21 (DE3) Rosetta. The cells were grown in TB medium, supplemented with ampicillin and chloramphenicol, at 37 °C and induced by the addition of 0.2 mM IPTG at an optical density of OD600 = 0.6. After 4 h at 28 °C, cells were harvested by centrifugation, resuspended in isolation buffer IB4 (25 mM Tris-Cl pH 7.9, 150 mM NaCl, 1 mM MgCl2, 5 mM DTE, 5% glycerol, 1 mM ATP, Complete protease inhibitors, 2 mM PMSF), sonicated and centrifuged at 28000×g and 4 °C for 20 min. The supernatant was applied to a GSH-Sepharose column, equilibrated in IB4. The column was washed with 10 volumes of IB4, again with 10 volumes of IB4 without protease inhibitors, and the fusion protein was cleaved with thrombin on the column overnight at 4 °C. The protein was eluted with column buffer, concentrated and stored at −80 °C.
Kinase reaction
For the kinase reaction, 0.3 μM septins and 0.01 μM Cdc28 were incubated with Cks1 and cyclin Cln2 or Clb2 (each 0.01 μM) at 30 °C for 2 h in kinase buffer (50 mM HEPES pH 7.6, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, Complete protease inhibitors (Roche)) with 0.5 mM ATP. As a control, septins alone or with Cdc28 were incubated in the kinase reaction buffer with ATP. Dephosphorylation with lambda phosphatase was carried out according to the manufacturer’s instructions (New England BioLabs). Reactions were stopped after 2 h with 5 × SDS-loading buffer.
Immunobloting
Kinase reaction samples were separated by SDS-PAGE, blotted on Polyvinylidene difluoride membrane and the blot was immunoprobed with Q5 PhosphoSerine antibodies (Qiagen). Horseradish peroxidase-conjugated secondary anti-mouse antibodies and a commercial (Perkin Elmer) or home-made enhanced chemiluminescent (Haan and Behrmann 2007) system was used to detect phosphorylated serines.
Fluorescence microscopy
A Zeiss Axiovert microscope equipped with Zyla 4.2 PLUS (Andor) was employed for the epifluorescence microscopy of septin filaments in vitro. A Cdc3-yEGFP-Cdc10-Cdc11-Cdc12 complex in high salt buffer was diluted to 1–5 mg/mL and dialyzed into 20 mM HEPES pH 7.9, 50 mM NaCl, 1 mM MgCl2, 1 mM dithioerythritol for up to 12 h at 4 °C. Dialyzed samples were processed through a kinase reaction, applied to slides and observed by fluorescence microscopy. JFilament, an ImageJ plugin, was used to measure length of the filaments (Rueden et al. 2017; Smith et al. 2010).
Electron microscopy
Negative staining of samples was performed as previously described (Bröcker et al. 2012). In brief, samples (diluted into 20 mM Tris pH 7.9, 50 mM NaCl, 1 mM MgCl2, 1 mM DTE; septins at 0.03 μM) were applied onto freshly glow-discharged, carbon-coated copper grids, left for 2 min on the grid, and then blotted and stained with 0.5% (w/v) uranyl formate. Specimens were examined with a JEOL JEM 1400 electron microscope equipped with a LaB6 filament at an acceleration voltage of 120 kV. Images were taken in minimal dose mode at a magnification of 50,000× and a defocus of 1–2 μm, and recorded with a 4k × 4 k CMOS F-416 camera (TVIPS).
Results
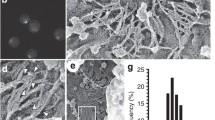
To study the interaction of Cdc28/cyclin with septin filaments, we reconstituted the system in vitro. We chose two cyclins, Cln2 and Clb2, to mimic the situations at the G1 to S phase transition (Cln2) and early M phase (Clb2). We expressed all yeast proteins heterologously in E. coli and purified them biochemically (Materials and Methods, Online Resource 1). To ensure the expression of a fully functional Cdc28, we co-expressed it with the Cdc37 chaperone and the cyclin-dependent kinase-activating kinase Cak1. Cks1, the regulatory subunit and adaptor of CDK, was expressed in E. coli separately. Cln2 was used in the form of a maltose-binding fusion protein to enhance its stability. In order to analyse septin filament formation in vitro, we expressed a functional yeast septin complex, containing a Cdc3-yEGFP fusion protein, in E. coli, isolated it in a similar way as the wild-type septin complex, and monitored filament formation by fluorescence microscopy (Sadian et al. 2013). Filaments were formed by dialyzing septins overnight against a low-salt buffer (50 mM NaCl); these filaments were then used in kinase reactions. The yEGFP-labeled septin complex (Cdc3-yEGFP-Cdc10-Cdc11-Cdc12) forms straight filaments in vitro (average length 56 μm, SD = 22 μm, Fig. 1a). After the addition of Cdc28 and 0.5 mM ATP and subsequent incubation at 30 °C, no difference was visible when compared to the isolated septins (average length 54 μm, SD = 29 μm, Fig. 1b). A dramatic change occurs in septin filament structure when Cln2, the G1 cyclin is incubated together with Cdc28, septins and ATP. Under these conditions, the septin filaments apparently depolymerise: no filaments were visible (Fig. 1c). After adding lambda phosphatase to the depolymerized septin filaments and incubating further at 30 °C, septin filaments reappeared (average length 18 μm, SD = 8 μm, Fig. 1d). A kinase reaction using the mitotic cyclin Clb2, in the presence of Cdc28 and ATP, produced no significant structural changes when examined by fluorescence microscopy, though the filaments did show slightly more branching than the control (average length 58 μm, SD = 29 μm, Fig. 1e).
G1 cyclin-dependent septin depolymerisation in vitro. Yeast septin octamers (0.3 μM) containing Cdc3-yEGFP, polymerized by dialysis and incubated in kinase buffer, a alone, b in the presence of 0.01 μM Cdc28, c in the presence of 0.01 μM Cdc28/Cln2, d in the presence of Cdc28/Cln2 treated with lambda phosphatase, e in the presence of Cdc28/Clb2. Samples were viewed using epifluorescence microscopy. All panels are shown at the same magnification. Scale bar, 10 μm
To confirm our fluorescence microscopy observations and to obtain more structural detail, we examined these kinase reaction products using electron microscopy. The majority of the septin filaments formed bundles showing a characteristic striation (Fig. 2a) as well as pairs of filaments as described in previous reports (Farkasovsky et al. 2005; Patasi et al. 2015; Versele and Thorner 2004). The addition of Cdc28 to the kinase reaction did not produce noticeably different results than the septin alone (Fig. 2b), however septin samples treated with Cdc28/Cln2, in contrast to the septin polymers alone (Fig. 2a), contained only septin octamers (Fig. 2c). Phosphatase treatment of these octamers once again initiated septin filament assembly (Fig. 2d). Finally, the sample treated with Cdc28/Clb2 contained both straight and branched filaments (Fig. 2e).
Electron microscopy of the kinase reaction products of septin filaments. Representative EM images of negatively stained septin filaments diluted to 0.03 μM are shown. Yeast septin octamers polymerized by dialysis and incubated in kinase buffer, a alone, b in the presence of Cdc28, c in the presence of Cdc28/Cln2, d in the presence of Cdc28/Cln2 treated with lambda phosphatase, e in the presence of Cdc28/Clb2. The protein concentration in all reactions is the same as in Fig. 1. Ten-fold dilution was used for EM. All scale bars in (a–e) are 200 nm
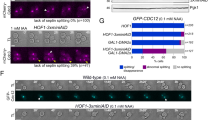
As described previously (Tang and Reed 2002), the Cdc3 septin is phosphorylated on its C-terminus (S503 and S509) by Cdc28 during early G1 phase. Filament disassembly seems to depend on septin phosphorylation (Figs. 1 and 2), because treatment with phosphatase reverses this process. To support this interpretation, we examined the phosphorylation of Cdc3 in vitro by western blotting. We first performed kinase reactions and then used antibodies to detect phosphoserine on the blot. Cdc28 with Cln2 cyclin displayed a positive Cdc3 signal, whereas Cdc28 with Clb2 or Cdc28 alone showed no signal. After phosphatase treatment of a kinase reaction mixture containing Cdc28/Cln2, the Cdc3 western blot signal disappeared, as expected (Fig. 3a). No other septin appeared to be phosphorylated using this antibody-based detection system. To increase the very mild effect of Clb2 on septin filaments, we performed the kinase reaction using a 10-fold excess of this cyclin. Surprisingly, this did produce a Cdc3 phosphorylation signal on the western blot (Fig. 3a, last column). The filaments formed under these conditions are moderately shorter than the control (average length 35 μm, SD = 21 μm, p ≤ 0.0001) and show more frequent branching (Fig. 3b).
Cdc3 is specifically phosphorylated by Cdc28/Cln2. a Kinase reactions were separated by SDS-PAGE, blotted on PVDF membrane and incubated with anti-phosphoserine antibodies. Secondary antibodies conjugated to horse-radish peroxidase in an enhanced chemiluminescent system were used to detect phosphoserine (upper panel). The blot was then stained with Coomassie Brilliant Blue (lower panel). Kinase reaction mixtures from left to right: septin alone, septin with Cdc28, septin with Cdc28/Cln2, septin with Cdc28/Clb2, septin with Cdc28/Cln2 treated with phosphatase, septin with Cdc28/Clb2. * 10-fold excess of Clb2. b Kinase reaction with Cdc28 and 10-fold excess of Clb2 viewed using epifluorescence microscopy. Scale bar, 10 μm
Taken together, these data suggest that the Cdc28 kinase specifically phosphorylates septin Cdc3 in a G1 cyclin-dependent manner in vitro, thereby causing the septin filaments to depolymerise.
Discussion
The structure of the septin polymer network at the cell cortex is highly regulated during the S. cerevisiae cell cycle and is controlled by multiple classes of regulatory proteins. Protein kinase phosphorylation plays a very important role in the formation of the septin ring, in the transformation of higher-order structures throughout the entire cell cycle, and in the assembly of the septin collar for morphogenesis checkpoint monitoring (Perez et al. 2016). As shown previously (Tang and Reed 2002), mutations preventing the phosphorylation of putative CDK recognition motifs in Cdc3 decrease the disassembly of the old septin ring following cytokinesis. In S. cerevisiae, therefore, the activity of Cdc28 is presumably required for disassembly of the old septin ring and the consequent initiation of the new one, although the molecular mechanisms at the root of this process remain unknown.
To examine the structural changes to septin filaments which might arise following phosphorylation, we first heterologously expressed the cyclin-dependent protein kinase Cdc28 and cyclins Cln2 and Clb2. We then used different yeast CDK/cyclin combinations in kinase reactions with yEGFP-labelled septin complex (Cdc3-yEGFP-Cdc10-Cdc11-Cdc12) to visualize filaments using fluorescence microscopy. Phosphorylation of this complex by Cdc28/Cln2 caused the filaments to become completely depolymerised. This was confirmed by electron microscopy, which showed only septin octamers (Figs. 1c and 2c). This septin filament disassembly was reversible if treated with lambda phosphatase (Fig. 1d and 2d). Together, these results show that phosphorylation likely causes structural changes. Cdc3 phosphorylation was also confirmed by western blotting (Fig. 3).These results agree with previously published data (Tang and Reed 2002), which suggested that septin phosphorylation may regulate the equilibrium between the assembled and free protomers. Septin phosphorylation seems not only to be the signal for septin ring disassembly, but also directly affects filament structural stability. We propose that phosphorylation of the Cdc3 C-terminus disturbs the coiled-coil interaction proposed to stabilize septin filaments (Bertin et al. 2008). It is still unclear if it is phosphorylation of Cdc3 alone which is responsible for filament disassembly of if phosphorylation of some other septin, not identified in our blot, is also necessary. It may be that the antibody used does not recognise all possible phosphoserine epitopes. Recently, it was reported that the Candida albicans homolog of Cln1 targets the Cdc28 kinase activity to the Cdc11 septin (Sinha et al. 2007); it is therefore possible that S. cerevisiae Cdc11 is also a CDK target. In accordance with this hypothesis, S. cerevisiae Cdc11 contains two potential phosphorylation sites, T90 and S100, matching minimal consensus motif S/T-P. A more detailed investigation into septin phosphorylation will be needed to address this.
We also observed that the Cdc28/Clb2 combination phosphorylates Cdc3 in vitro, but only when an excess of Clb2 is used. One possible reason is that this cyclin interacts with Cdc3 in a different, less efficient way, thereby requiring a higher concentration for phosphorylation. Non-specific phosphorylation also cannot be excluded.
Mutation to the Cdc3 phosphorylation sites only delays initiation of septin ring assembly and bud emergence (Tang and Reed 2002), so it is unlikely that septin phosphorylation is the only pathway regulating septin disassembly. It remains a possibility, however, that there are other proteins which maintain the septin structure at the bud neck that are inhibited by phosphorylation, thereby causing septin ring disassembly independently of septin phosphorylation. Another possibility is that the phosphorylation of regulatory proteins might be required to activate septin disassembly. A good candidate for such a regulatory protein is Syp1, which has been implicated in both the disassembly of the septin ring in the late stages of cell division and in the formation of a septin ring at the incipient bud site (Qiu et al. 2008). Interestingly, Syp1 is a highly phosphorylated protein (Albuquerque et al. 2008; Swaney et al. 2013), and eight of the phospho-sites detected in this protein match the T/S-P consensus for the kinase CDK/Cdc28. The sumoylation of septins also seems to be involved in septin ring disassembly given that a yeast strain containing a septin with mutations to its sumoylation site accumulates extra septin rings (Johnson and Blobel 1999). Finally, it should be noted that septins are associated with the cytoplasmic membrane, and phosphorylation conditions in and around the membrane may be quite different from those in free solution. Further investigation will be needed to determine whether phosphorylated septins bind to the membrane, or if membrane-bound septins are phosphorylated at the same sites as in free solution.
In summary, we have shown that the G1 cyclin-dependent phosphorylation of septin filaments by yeast CDK in vitro induces dramatic changes in filament structure. Septin phosphorylation might be one of the mechanisms by which disassembly of septin filaments is induced after completion of cytokinesis.
Abbreviations
- CDK:
-
Cyclin-dependent kinase
- DTT:
-
Dithiothreitol
- EGFP:
-
Enhanced green fluorescent protein
- IPTG:
-
Isopropyl-β-D-thiogalactopyranosid
- NTA:
-
Nitrilotriacetic acid
- PMSF:
-
Phenylmethylsulfonylfluorid
- SD:
-
Standard deviation
References
Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteomics 7:1389–1396. https://doi.org/10.1074/mcp.M700468-MCP200
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1995) Current protocols in molecular biology. John Wiley and Sons, New York
Bertin A, McMurray MA, Grob P, Park SS, Garcia G 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E (2008) Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci U S A 105:8274–8279. https://doi.org/10.1073/pnas.0803330105
Bröcker C, Kuhlee A, Gatsogiannis C, Balderhaar HJ, Hönscher C, Engelbrecht-Vandré S, Ungermann C, Raunser S (2012) Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci U S A 109:1991–1996. https://doi.org/10.1073/pnas.1117797109
Cvrčková F, De Virgilio C, Manser E, Pringle JR, Nasmyth K (1995) Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev 9:1817–1830
Egelhofer TA, Villén J, McCusker D, Gygi SP, Kellogg DR, Hardwick KG (2008) The Septins Function in G1 Pathways that Influence the Pattern of Cell Growth in Budding Yeast. PLoS ONE 3:e2022. https://doi.org/10.1371/journal.pone.0002022
Farkasovsky M, Herter P, Voss B, Wittinghofer A (2005) Nucleotide binding and filament assembly of recombinant yeast septin complexes. Biol Chem 386:643–656. https://doi.org/10.1515/BC.2005.075
Garcia G III, Bertin A, Li Z, Song Y, McMurray MA, Thorner J, Nogales E (2011) Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation. J Cell Biol 195:993–1004. https://doi.org/10.1083/jcb.201107123
Garcia G III, Finnigan GC, Heasley LR, Sterling SM, Aggarwal A, Pearson CG, Nogales E, McMurray MA, Thorner J (2016) Assembly, molecular organization, and membrane-binding properties of development specific septins. J Cell Biol 212:515–529. https://doi.org/10.1083/jcb.201511029
Haan C, Behrmann I (2007) A cost effective non-commercial ECL-solution for western blot detections yielding strong signals and low background. J Immunol Methods 318:11–19. https://doi.org/10.1016/j.jim.2006.07.027
Hartwell LH, Smith D (1985) Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics 110:381–395
Holt LJ, Tuch BB, Villén J, Johnson AD, Gygi SP, Morgan DO (2009) Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325:1682–1686. https://doi.org/10.1126/science.1172867
Huang D, Friesen H, Andrews B (2007) Pho85, a multifunctional cyclin dependent protein kinase in budding yeast. Mol Microbiol 66:303–314. https://doi.org/10.1111/j.1365-2958.2007.05914.x
Johnson ES, Blobel G (1999) Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol 147(5):981–994
Lee JM, Greenleaf AL (1991) CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expr 1:149–167
Liao SM, Zhang J, Jeffery DA, Koleske AJ, Thompson CM, Chao DM, Viljoen M, van Vuuren HJ, Young RA (1995) A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 374:193–196
Lorincz AT, Reed SI (1984) Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature 307:183–185
McQuilken M, Jentzsch MS, Verma A, Mehta SB, Oldenbourg R, Gladfelter AS (2017) Analysis of Septin reorganization at cytokinesis using polarized fluorescence microscopy. Front Cell Dev Biol 5:42. https://doi.org/10.3389/fcell.2017.00042
Meseroll RA, Howard L, Gladfelter AS (2012) Septin ring size scaling and dynamics require the coiled-coil region of Shs1p. Mol Biol Cell 23:3391–3406. https://doi.org/10.1091/mbc.E12-03-0207
Nigg EA (1993) Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol 3:296–301
Patasi C, Godočíková J, Michlíková S, Nie Y, Káčeriková R, Kválová K, Raunser S, Farkašovský M (2015) The role of Bni5 in the regulation of septin higher-order structure formation. Biol Chem 396:1325–1337. https://doi.org/10.1515/hsz-2015-0165
Perez AM, Finnigan GC, Roelants FM, Thorner J (2016) Septin-associated protein kinases in the yeast Saccharomyces cerevisiae. Front Cell Dev Biol 4:119. https://doi.org/10.3389/fcell.2016.00119
Qiu W, Neo SP, Yu X, Cai M (2008) A novel septin-associated protein, Syp1p,is required for normal cell cycle-dependent septin cytoskeleton dynamics in yeast. Genetics 180:1445–1457. https://doi.org/10.1534/genetics.108.091900
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf 18:529. https://doi.org/10.1186/s12859-017-1934-z
Sadian Y, Gatsogiannis C, Patasi C, Hofnagel O, Goody RS, Farkasovsky M, Raunser S (2013) The role of Cdc42 and Gic1 in the regulation of septin filament formation and dissociation. eLife 2:e01085. https://doi.org/10.7554/eLife.01085
Simon M, Seraphin B, Faye G (1986) KIN28, yeast split gene coding for a putative protein kinase homologous to CDC28. EMBO J 5:2697–2701
Sinha I, Wang YM, Philp R, Li CR, Yap WH, Wang Y (2007) Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev Cell 13:421–432. https://doi.org/10.1016/j.devcel.2007.06.011
Smith MB, Li H, Shen T, Huang X, Yusuf E, Vavylonis D (2010) Segmentation and tracking of cytoskeletal filaments using open active contours. Cytoskeleton 67:693–705. https://doi.org/10.1002/cm.20481
Swaney DL, Beltrao P, Starita L, Guo A, Rush J, Fields S, Krogan NJ, Villén J (2013) Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat Methods 10:676–682. https://doi.org/10.1038/nmeth.2519
Tang CS, Reed SI (2002) Phosphorylation of the septin cdc3 in G1 by the cdc28 kinase is essential for efficient septin ring disassembly. Cell Cycle 1:42–49
Versele M, Thorner J (2004) Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol 164:701–715. https://doi.org/10.1083/jcb.200312070
Yao S, Neiman A, Prelich G (2000) BUR1 and BUR2 encode a divergent cyclin-dependent kinase-cyclin complex important for transcription in vivo. Mol Cell Biol 20:7080–7087
Acknowledgments
We gratefully acknowledge financial support from the Humboldt Foundation 3.4 - 1006989 - SVK - IP (MF and SR) and VEGA (Vedecká Grantová Agentúra, Ministerstvo Školstva Slovenskej Republiky) Grant 2/0002/15 (MF). We thank Jacob Bauer for helpful discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All of the experiments complied with the current Slovak law.
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1282 kb)
Rights and permissions
About this article
Cite this article
Káčeriková, R., Godočíková, J., Wang, Z. et al. Modulation of septin higher-order structure by the Cdc28 protein kinase. Biologia 73, 1025–1033 (2018). https://doi.org/10.2478/s11756-018-0116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0116-4