Abstract
Objectives
To estimate provincial all-cause mortality rates of Saskatchewan people with rheumatoid arthritis (RA) for comparison with the general population over time and between different geographic regions.
Methods
Saskatchewan provincial administrative health databases (2001–2019) were utilized as data sources. Two RA case definitions were employed: (1) ≥ 3 physician billing diagnoses, at least 1 from a specialist (rheumatologist, general internist or orthopaedic surgeon) within 2 years; (2) ≥ 1 hospitalization diagnosis (ICD-9 code 714, and ICD-10-CA codes M05, M06). Data from these definitions were combined to create an administrative data RA cohort. All-cause mortality rates across geographic regions, between rural/urban residences and between sexes were examined.
Results
Over an 18-year span, between fiscal-year 2001–2002 and fiscal-year 2018–2019, age- and sex-adjusted mortality rates ranged from 17.10 to 21.04 (95% CI 14.77, 19.44; 18.03, 24.05)/1000 RA person-years, compared with mortality rates for the general Saskatchewan population without RA, which ranged from 9.37 to 10.88 (95% CI 9.23, 9.51; 10.72, 11.05)/1000 person-years. Fiscal-year mortality rate ratios ranged from 1.82 to 2.13 (95% CI 1.56, 2.13; 1.83, 2.46). Provincial mortality rates were higher in men than in women for both general and RA populations. Northern Saskatchewan mortality rates were significantly higher in the general population but did not achieve significance compared with other provincial regions for the RA population. Regression analysis identified age, male sex, RA and geographic region as factors contributing to increased mortality. A trend towards lower mortality rates over time was observed.
Conclusion
Higher mortality rates were observed in the RA population overall. Men had higher mortality rates, as did residents of Northern Saskatchewan compared with residents of other regions for the general population.
Résumé
Objectifs
Estimer les taux de mortalité provinciaux, toutes causes confondues, des habitants de la Saskatchewan atteints de polyarthrite rhumatoïde (PR) pour les comparer aux taux dans la population générale au fil du temps et entre différentes régions géographiques.
Méthode
Nos données sont extraites des bases de données administratives sur la santé de la Saskatchewan (2001–2019). Deux définitions de cas ont été employées pour la PR : 1) ≥ 3 factures de diagnostic médical, dont au moins une d’un(e) spécialiste (rhumatologue, interniste général[e] ou chirurgien[ne] orthopédiste) en l’espace de deux ans; 2) ≥ 1 diagnostic d’hospitalisation (code CIM-9 714 et codes CIM-10-CA M05 et M06). Les données de ces définitions ont été combinées pour créer une cohorte de personnes atteintes de PR dans les données administratives. Les taux de mortalité toutes causes confondues entre les régions géographiques, entre les lieux de résidence urbains et ruraux et entre les sexes ont été examinés.
Résultats
En l’espace de 18 ans, entre les exercices 2001-2002 et 2018-2019, les taux de mortalité rajustés selon l’âge et le sexe ont varié entre 17,10 et 21,04 (IC de 95 % : 14,77-19,44; 18,03-24,05)/1000 personnes-années pour les personnes atteintes de PR, tandis que les taux de mortalité de la population générale de la Saskatchewan non atteinte de PR se sont situés entre 9,37 et 10,88 (IC de 95 % : 9,23-9,51; 10,72-11,05)/1000 personnes-années. Les rapports de taux de mortalité par exercice ont varié entre 1,82 et 2,13 (IC de 95 % : 1,56-2,13; 1,83-2,46). Les taux de mortalité provinciaux des hommes étaient supérieurs à ceux des femmes, tant dans la population générale que chez les personnes atteintes de PR. Les taux de mortalité dans le Nord de la Saskatchewan étaient sensiblement plus élevés que dans les autres régions de la province pour la population générale, mais pas sensiblement plus élevés pour la population atteinte de PR. Selon les analyses de régression, l’âge, le sexe masculin, la PR et la région géographique étaient des facteurs contribuant à une mortalité accrue. Une tendance à la baisse des taux de mortalité au fil du temps a été observée.
Conclusion
Dans la population atteinte de PR, des taux de mortalité plus élevés ont été observés globalement. Dans la population générale, les taux de mortalité des hommes et ceux des résidents du Nord de la Saskatchewan étaient plus élevés que ceux des résidents des autres régions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is the most common of the chronic autoimmune inflammatory arthropathies having approximate prevalence of 0.7% in the Saskatchewan provincial population among people aged 18 and older (Nair et al. 2019). Saskatchewan is a geographically diverse province, with nearly half of the population located in the more southern and central urban centres, Saskatoon and Regina. A higher prevalence of RA has been observed in the northern areas of the province (Taylor-Gjevre et al. 2018). Concerningly, inequities in access to healthcare have been perceived by northern and rural Saskatchewan people with RA (Nair et al. 2016). In terms of health-related outcomes, premature and increased mortality has been associated with RA in other jurisdictions (Ogdie et al. 2017; van den Hoek et al. 2017; Jean et al. 2017; Widdifield et al. 2015). Mortality within the general population of Saskatchewan is in keeping with that seen in many Canadian provinces (Statistics Canada 2017); however, provincial mortality rates within the RA population based on administrative provincial population health data have not been previously studied. One clinical practice-based incidence cohort estimate of mortality rates was reported in 1994 and found to be higher than the general population at that time (Wolfe et al. 1994).
The objectives of this study were to estimate Saskatchewan provincial all-cause mortality rates among people with RA and determine how mortality rates compared with the general provincial population without RA and additionally whether rates varied over the study time period or among geographic regions. Consideration of mortality and any associated geographic variation may influence further healthcare initiatives towards early diagnosis and treatment of both RA and associated co-morbidities.
Methods
This study complies with the Declaration of Helsinki and was approved by the University of Saskatchewan Biomedical Research Ethics Board (BIO-REB 13-336).
This epidemiologic study of Saskatchewan RA populations is the third in a series utilizing similar methodologic and administrative data resources. Earlier studies include examination of incidence and prevalence both over time (Nair et al. 2019) and by geographic region within the province (Taylor-Gjevre et al. 2018).
Setting and design
Saskatchewan had a total population of approximately 1.18 million in 2019 (Statistics Canada 2020). A 2016 population estimate suggests half of provincial residents live outside the cities of Regina or Saskatoon (Canada: Metropolitan Areas (Population Estimates) 2020). All Saskatchewan residents are eligible for provincial insurance benefits with the exception of those who receive these benefits from the federal government (i.e., military personnel, federal penitentiary inmates and, until April 1, 2013, members of the Royal Canadian Mounted Police). All Saskatchewan health insurance beneficiaries are eligible for provincial prescription drug benefits except those who receive prescription drug benefits from the federal government (e.g., First Nations people who have a treaty relationship with the federal government, certain drugs for veterans). Despite the different source of funding, all their health information is still captured in all the administrative databases with the one caveat that prescription drug information for these groups is not available prior to 2008.
A Saskatchewan provincial population-based RA cohort study was undertaken to evaluate various epidemiologic parameters, including mortality rates between April 1, 2001 and March 31, 2019. Temporal trends for mortality in the RA cohort were examined for the population overall, as well as through stratification by sex, by urban versus rural location of residence, and by geographic region of residence.
Subjects and data sources
This retrospective, population-based cohort study was performed employing Saskatchewan provincial health administrative databases organized by fiscal year, for the periods of April 1, 2001 up until March 31, 2019. These databases can be linked through encrypted unique personal health insurance numbers. Provincial health administrative databases utilized for this study included the Discharge Abstract Database (DAD), the physician Medical Services Database (MSD) and the Person Health Registration System (PHRS).
The DAD incorporates detailed hospitalization data. Until March 31, 2001, diagnoses were recorded in compliance with the International Classification of Diseases 9th revision (ICD-9). The International Classification of Diseases, 10th revision, Canadian Version (ICD-10-CA) was then introduced. From April 1, 2001 until March 31, 2002, approximately 70% of records were coded in ICD-10-CA. After April 1, 2002, virtually all records are recorded in the ICD-10-CA format. The database contains detailed diagnostic information.
The MSD records physician services data. Physicians paid on a fee-for-service basis submit billing claims to the provincial health ministry utilizing three-digit ICD-9 codes for each diagnosis. Salaried physicians often submit ‘shadow’ billing claims for administrative purposes.
The PHRS captures characteristics of each insured individual, including their age, sex and the first 3 digits of their postal code, which is used to determine rural versus urban location of residence, as well as where in the province the person resides (i.e., geographic location of residence). The PHRS also contains dates of coverage within the provincial health insurance plan. Date of death was identified from multiple administrative databases including the PHRS and DAD.
RA cohort case definition
Rheumatoid arthritis
A previously validated algorithm for administrative data was employed in the identification of people with RA for this cohort (Widdifield et al. 2013). This study did not include further validation of this case definition for RA; however, utilization of this case definition identified a 0.7% prevalence estimate for RA in Saskatchewan, which was comparable to that of studies in other jurisdictions (Nair et al. 2019). These studies in other regions, which did encompass a validation component for this RA case definition, included administrative health database studies in Ontario and Quebec (Jean et al. 2017; Widdifield et al. 2013). Increased specificity has been associated with a RA case definition with two or more physician diagnoses and includes at least one specialist diagnosis (Kroeker et al. 2017) which is a component of the RA case definition associated with this study.
RA case definition
Individuals were identified as having RA if they had three or more physician services claims for RA (ICD-9 code 714), at least one of which was submitted by a specialist (rheumatologist, general internist or orthopaedic surgeon) within a 2-year period, or if they had one or more hospitalizations with a diagnosis of RA (ICD-9 code 714, ICD-10 codes M05, M06) in any of the up to 25 diagnosis fields. When an individual met both the physician visit and hospitalization criteria, the earliest occurrence was taken as the index date of diagnosis. For inclusion in this cohort, individuals were required to be aged 18 or older on the index date of their RA diagnosis and have uninterrupted health insurance coverage (i.e., a gap of no more than three consecutive days in coverage) from the date of their diagnosis until March 31, 2019, or their exit from the cohort in the form of their loss of health insurance or death. A 3-day gap was allowed because it is not uncommon for insured people in Saskatchewan to have a gap of a day or two in their health insurance, yet still receive medical services and have that encounter recorded in the administrative databases during the gap.
Death
The date of death was identified from multiple administrative databases, including the PHRS and DAD. An individual who died between April 1 and March 31 of the following year was described as having died in that fiscal year. For example, a person who died on or between April 1, 2001 and March 31, 2002 was described as dying in FY 0102.
Co-variates
Descriptive variables were identified for each member of the cohort. All variables were determined on the day of RA diagnosis. These included age, sex (male, female), location of residence (urban, rural, missing) and geographic region of residence (South Saskatchewan, Regina area, Central Saskatchewan, Saskatoon area, North Saskatchewan and Far North Saskatchewan). We combined North and Far North Saskatchewan regions together because of the relatively small number of residents in the Far North. Age, sex, and insurance coverage were obtained from the Person Health Registration System. Geographic region of residence was identified from the postal code. Urban versus rural location of residence was determined using Statistics Canada categorizations (Statistics Canada 2016). An individual was identified as living in an urban area if their postal code was for a census metropolitan area or census agglomeration with a population of 10,000 or more.
Statistical analysis
Crude mortality rates and 95% confidence intervals (95% CI) were calculated in 2-year groupings from FY0102 to FY1819 for both the RA cohort and the Saskatchewan general population without RA. Rates were age- and sex-adjusted to the structure of the FY0102 Saskatchewan general population without RA. Mortality rates were expressed as the number of deaths per 1000 person-years for each 2-year grouping within the study period. For variables with a small number of deaths in each strata—specifically geographic region as well as the combination of sex and urban versus rural location of residence—rates were calculated within three 5-year groupings and one 3-year group: (1) FY0102-FY0506 (FY0105), (2) FY0607-FY1112 (FY0610), (3) FY1112-FY1516 (FY1115), and (4) FY1617-FY1819 (FY1618). The average adjusted mortality rates were calculated by counting the number of deaths in each 2- or 5-year period and dividing them by the number of person-years in that same 2- or 5-year period. Mortality rate ratios were calculated by dividing age- and sex-adjusted mortality rates for the RA cohort by those of the general population without RA. A ratio value of greater than 1.0 indicates ‘excess deaths’ in the disease-specific population. Multivariable Poisson regression was used to examine the effects of these variables upon mortality rates over time. We used the negative binomial distribution to account for overdispersion.
All analyses were performed using SAS© statistical software, version 9.4 (SAS Institute Inc., Cary, NC, 2007).
Results
The RA cohort was composed of 9720 people with diagnoses of RA between April 1, 2001 and March 31, 2019.
Mortality rates in the RA and the general population
The total number of deaths and crude and adjusted mortality rates in both the RA population and the general population without RA are reported in 2-year groupings in Table 1. At this provincial level, adjusted RA mortality rates ranged over the 18-year span from a low of 17.10 (95% CI 14.77, 19.44) to a high of 21.04 per 1000 person-years (95% CI 18.03, 24.05). RA mortality rates were significantly higher than those seen in the general population without RA (Fig. 1). The higher rates in the RA population result in elevated standardized mortality rate ratios (MRRs). The standardized MRRs did not change significantly over the study period as reflected in Fig. 2. MRRs ranged from a low of 1.82 (95% CI 1.56, 2.13) to a high of 2.13 (95% CI 1.83, 2.46).
Impact of sex on mortality rates in the RA and in the general population
When stratified by sex, men consistently had a higher mortality rate than women in both the RA cohort and general population without RA (Fig. 1). Men and women with RA had higher standardized mortality rates overall compared with their counterparts in the general population without RA (Table 2).
Impact of rural versus urban residence on mortality rates for RA and for general population
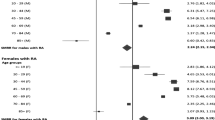
Stratification by urban versus rural location of residence revealed no significant difference in mortality rates for those living in either environment (Table 3). When urban/rural status was further substratified by sex, elevated mortality rates were found for men versus women irrespective of urban/rural status (Fig. 3).
Impact of geographic region of residence on mortality rates for RA and for general population
Stratification by geographic region of residence (Table 4) found that among people with RA, those living in South Saskatchewan had the lowest rates, ranging from 11.91 (95% CI 9.56, 14.25) to 15.92 (95% CI 13.41, 18.43) deaths per 1000 person-years in the 4 study periods, while those in the North and Far North of the province had the highest, ranging from 20.71 (95% CI 17.14, 24.27) to 23.14 (95% CI 19.68–26.60) deaths per 1000 person-years. In the general population without RA, a decreasing mortality rate over time was found in all regions (Fig. 4). Saskatoon area had the lowest adjusted mortality rates in FY1618 (9.05 deaths per 1000 person-years; 95% CI 8.84, 9.26) while the North and Far North had the highest (10.24 deaths per 1000 person-years; 95% CI 9.97, 10.52). The standardized mortality rate ratios over time for men and women combined and separately remained stable.
Regression analysis
Negative binomial regression of the number of deaths found the presence of RA, age, sex, region of residence and time had a statistically significant association (Table 5). Increased mortality was statistically significantly associated with the presence of RA (80% increase; 95% CI 1.73, 1.87), increasing age, male sex (49% increase; 95% CI 1.45, 1.53) and geographic region. Compared with the Saskatoon area, the largest urban centre in the province, mortality was higher in Central Saskatchewan (5% increase; 95% CI 1.01, 1.09), Regina area (7% increase; 95% CI 1.03, 1.11) and North and Far North Saskatchewan (18% increase; 95% CI 1.13, 1.23). After adjustment for RA, age, sex and geographic region, mortality showed a statistically significant progressive decrease over the study period (13% decrease in FY1618; 95% CI 0.83, 0.90).
When stratified by sex, the variables associated with mortality and their magnitude were similar between men and women (Table 6). Both men and women showed a statistically significant decrease in mortality over time, but the decrease was greater by FY1619 among men (17% decrease; 95% CI 0.79, 0.86) than among women (9% decrease; 95% CI 0.87, 0.95).
Discussion
In this administrative data study of the Saskatchewan population, people with RA had significantly higher mortality rates than observed in the general population. Increased mortality in RA has been reported internationally. Dadoun et al. in a meta-analysis of mortality studies determined a meta-standardized mortality ratio (SMR) of 1.47 for RA populations (Dadoun et al. 2013). In a North American (USA) Olmstead County RA inception cohort, Gonzalez et al. reported a SMR of 1.27 (Gonzalez et al. 2007).
Within the Canadian environment, standardized mortality rates in RA populations in Ontario (1996–2009) were reported at between 9.2 and 13 deaths/1000 RA cases per year (Widdifield et al. 2015). Similar standardized rates were observed in Quebec (2001–2015), ranging between 8.3 and 11.8 deaths/1000 RA cases/year (Jean et al. 2017). In British Columbia (1996–2006), all-cause mortality rates of 24.43 deaths/1000 person-years and a mortality rate ratio of 1.18 were identified (Lacaille et al. 2017). The Saskatchewan adjusted RA mortality rates identified in this 18-year study were generally comparable, although higher MRRs were observed.
There were some differences between the four provincial Canadian studies, including variations in the case definitions, in the reference populations for standardization of rates, and in the age restrictions for inclusion (Jean et al. 2017; Widdifield et al. 2015; Lacaille et al. 2017). These variations may contribute to some of the differences observed between the jurisdictions.
Within this study, regression analysis indicated 80% increase in mortality associated with RA diagnosis in the Saskatchewan population. The reasons for the increased mortality observed in RA populations have been identified in part and at various points as relating to cardiovascular disease, respiratory disorders, infection and neoplastic disease (Ogdie et al. 2017; Wolfe et al. 1994; Lacaille et al. 2017; Maradit-Kremers et al. 2005). There is some evidence, perhaps related to advances in RA therapy, that mortality rates may be improving for more recent RA inception cohorts (Lacaille et al. 2017).
Men, in both the general population and the RA population, were found to have higher mortality rates than their female counterparts. No significant difference in mortality rate based on broad categorization of urban versus rural residence was observed for either men or women. This was consistent between both the general population and the RA population and may be influenced by the population density or distribution within the provincial regions. Considering provincial geographic regions separately, it was observed that the northern regions had significantly higher general population mortality rates than the central and southern regions. Within the RA population, the adjusted mortality rates were also higher in the northern regions; however, in keeping with the smaller population size, the confidence intervals were wider and the difference between regions did not achieve significance. Regression analysis indicated, compared with the Saskatoon area, a 5% increase in mortality in central Saskatchewan, a 7% increase in the Regina area and an 18% increase in the northern regions.
Geography was noted as a determinant of health status in the 2002 Commission on the Future of Health Care in Canada. Within that report, there was recognition at the time of a widening inequality in health and healthcare access in rural areas (Romanow 2002). Barriers in access to healthcare have been previously described by rural Saskatchewan people with RA (Nair et al. 2016). Lower socio-economic affluence and higher smoking prevalence may also be implicated in the observations of higher regional mortality (Law and Morris 1998; Population Health Unit 2020). Northern Saskatchewan has been observed to have approximately half the median household income compared with the provincial median (Population Health Unit 2020). The smoking rate for men and women in Northern Saskatchewan is approximately double the rate for the province overall (Population Health Unit 2020). It should also be noted that the proportional population of Indigenous peoples is higher in the northern regions. A Northern Saskatchewan Population Health Unit Report from 2011 indicated 85% of peoples identified as Indigenous and less than 15% as non-Indigenous (Population Health Unit 2020). The health/healthcare inequities which have been recognized for Indigenous populations may be contributing to observed differences in regional mortality (Population Health Unit 2020; Tjepkema et al. 2019). The inability to stratify these study results based on Indigeneity is an unfortunate limitation in more fulsome interpretation of these findings.
The secondary use of administrative data in this study may create a limitation in the interpretation of the findings as the data were originally collected for a purpose other than this research. The completeness of the database may be impacted by missing data through incomplete shadow-billing by any salaried provincial physicians. It is also possible that some people may not have been referred to a specialist and thereby may not be identified by the case definition through either not receiving care from an orthopaedic/rheumatology/general internal medicine practitioner for RA or not requiring hospitalization where RA diagnosis was recorded during the study period.
The case definition used in this study is based on three physician diagnoses with at least one specialist diagnosis which has been shown to increase specificity (Kroeker et al. 2017). Our case definition for RA, in accommodating the physician landscape in SK during the study period, also includes non-rheumatologist specialist diagnoses. Although comparable to the case definitions used and validated in other provinces, this variation could impede comparison with similar studies (Jean et al. 2017; Widdifield et al. 2015). This case definition was not validated within the Saskatchewan population as part of this study and this may represent a limitation in the interpretation of the findings. However, it is reassuring that the RA prevalence of 0.7% in Saskatchewan, identified through this case definition, is in keeping with the prevalence reported in other jurisdictions (Taylor-Gjevre et al. 2018; Bernatsky et al. 2014; Widdifield et al. 2014; Myasoedova et al. 2010).
Recognizing such potential study limitations, these findings do nonetheless support the need for further verification and investigation into causes of death, associations with disease severity, therapeutic regimens, co-morbidities, and potentially also geographic healthcare delivery patterns. Development of programs for prevention and early diagnosis, as well as enhanced surveillance, for RA patients may be required on a province-wide scale. Such healthcare initiatives would ideally include a patient and community-centred health promotion approach towards both rheumatoid arthritis and associated co-morbidities.
Data availability
Data for this study were derived from Saskatchewan health administration databases.
References
Bernatsky, S., Dekis, A., Hudson, M., Pineau, C. A., Boire, G., Fortin, P. R., et al. (2014). Rheumatoid arthritis prevalence in Quebec. BMC Research Notes, 19(7), 937.
Canada: Metropolitan Areas (Population Estimates). https://www.citypopulation.de/php/canada-metro.php. Accessed 14 July 2020.
Dadoun, S., Zeboulon-Ktorza, N., Combescure, C., Elhai, M., Rozenberg, S., Gossec, L., & Fautrel, B. (2013). Mortality in rheumatoid arthritis over the last fifty years: Systematic review and meta-analysis. Joint, Bone, Spine, 80(1), 29–33.
Gonzalez, A., Maradit Kremers, H., Crowson, C. S., et al. (2007). The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis and Rheumatism, 56(11), 3583–3587.
Jean, S., Hudson, M., Gamache, P., Bessette, L., Fortin, P. R., Boire, G., et al. (2017). Temporal trends in prevalence, incidence and mortality for rheumatoid arthritis in Quebec, Canada: A population-based study. Clinical Rheumatology, 36, 2667–2671.
Kroeker, K., Widdifield, J., Muthukamarana, S., Jiang, D., & Lix, L. (2017). Model-based methods for case definitions from administrative health data: Application to rheumatoid arthritis. BMJ Open, 6, e016173. https://doi.org/10.1136/bmjopen-2017-016173.
Lacaille, D., Avina-Zubieta, J. A., Sayre, E. C., & Abrahamowicz, M. (2017). Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population—Closing the mortality gap. Annals of the Rheumatic Diseases, 76(6), 1057–1063.
Law, M. R., & Morris, J. K. (1998). Why is mortality higher in poorer areas and in more northern areas of England and Wales. Journal of Epidemiology and Community Health, 52(6), 344–352.
Maradit-Kremers, H., Nicola, P. J., Crowson, C. S., Ballman, K. V., & Gabriel, S. E. (2005). Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis and Rheumatism, 52(3), 722–732.
Myasoedova, E., Crowson, C. S., Kremers, H. M., Therneau, T. M., & Gabriel, S. E. (2010). Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955-2007. Arthritis and Rheumatism, 62(6), 1576–1582.
Nair, B. V., Schuler, R., Stewart, S., & Taylor-Gjevre, R. M. (2016). Self-reported barriers to healthcare access for rheumatoid arthritis patients in rural and northern Saskatchewan: A mixed methods study. Musculoskeletal Care, 14(4), 243–251.
Nair, B. V., Taylor-Gjevre, R. M., Wu, L., Jin, S., & Quail, J. M. (2019). Incidence and prevalence of rheumatoid arthritis in Saskatchewan, Canada: 2001-2014. BMC Rheumatology, 3, 28.
Ogdie, A., Maliha, S., Shin, D., Love, T. J., Baker, J., Jiang, Y., et al. (2017). Cause-specific mortality in patients with psoriatic arthritis and rheumatoid arthritis. Rheumatology (Oxford), 56(6), 907–911.
Population Health Unit. Northern Saskatchewan. Northern Saskatchewan Health Indicators Report 2011. http://www.mcrhealth.ca/media/files/Northern%20Saskatchewan%20Health%20Indicators%20Report%202011.pdf. Accessed 2 Oct 2020.
Romanow, R. J. (2002). Building on values: The future of health care in Canada. Final report. The Romanow Commission report. Ottawa: Health Canada.
Statistics Canada: Census metropolitan area (CMA) and Census agglomeration (CA). http://www.statcan.gc.ca/pub/92-195-x/2011001/geo/cma-rmr/cma-rmr-eng.htm. Accessed 12 June 2016.
Statistics Canada: Deaths and age-standardized mortality rate, by province and territory. https://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/hlth86b-eng.htm. Accessed 31 Dec 2017.
Statistics Canada: Saskatchewan annual population report calendar year 2019. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901. Accessed 20 Oct 2020.
Taylor-Gjevre, R. M., Nair, B. V., Jin, S., & Quail, J. M. (2018). Geographic variation in incidence and prevalence rates for rheumatoid arthritis in Saskatchewan, Canada 2001-2014. Canadian Journal of Public Health, 109(3), 427–435.
Tjepkema, M., Bushnik, T., Bougie, E. Life expectancy of First Nations, Metis and Inuit household populations in Canada Health Reports: Statistics Canada. https://www150.statcan.gc.ca/n1/pub/82-003-x/2019012/article/00001-eng.htm. Accessed 2 Oct 2020.
van den Hoek, J., Boshuizen, H. C., Roorda, L. D., Tijhuis, G. J., Nurmohamed, M. T., et al. (2017). Mortality in patients with rheumatoid arthritis: A 15-year prospective cohort study. Rheumatology International, 37(4), 487–493.
Widdifield, J., Bernatsky, S., Paterson, J. M., Tu, K., Ng, R., Thorne, J. C., et al. (2013). Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: A validation study using the medical records of rheumatologists. Arthritis Care and Research, 65(10), 1582–1591.
Widdifield, J., Paterson, J. M., Bernatsky, S., Tu, K., Tomlinson, G., Kuriya, B., Thorne, J. C., & Bombardier, C. (2014). The epidemiology of rheumatoid arthritis in Ontario, Canada. Arthritis and Rheumatism, 66(4), 786–793.
Widdifield, J., Bernatsky, S., Paterson, J. M., Tomlinson, G., Tu, K., Kuriya, B., et al. (2015). Trends in excess mortality among patients with rheumatoid arthritis in Ontario, Canada. Arthritis Care & Research, 67(8), 1047–1053.
Wolfe, F., Mitchell, D. M., Sibley, J. T., Fries, J. F., Bloch, D. A., Williams, C. A., et al. (1994). The mortality of rheumatoid arthritis. Arthritis and Rheumatism, 37(4), 481–494.
Funding
This work was supported by the Noreen Sutherland— Rheumatoid Arthritis—Royal University Hospital Foundation Endowment Fund.
Author information
Authors and Affiliations
Contributions
All the authors participated in the study design, interpretation of findings and preparation/review of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study complies with the Declaration of Helsinki and was approved by the University of Saskatchewan Biomedical Research Ethics Board (BIO-REB 13-336).
Consent to participate
Not applicable
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taylor-Gjevre, R.M., Nair, B.V., Jin, S. et al. Higher mortality rates associated with rheumatoid arthritis in Saskatchewan, Canada, 2001–2019. Can J Public Health 112, 722–732 (2021). https://doi.org/10.17269/s41997-021-00476-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.17269/s41997-021-00476-w