Abstract
Application of C–S–Hs–PCE and sodium sulfate into Portland cement containing 20 wt% lithium slag (LS) powder was investigated, in order to strengthen early mechanical properties. Hydration properties and microstructure of cement-LS system were analyzed. Results showed that C–S–Hs–PCE was advantageous for modifying fluidity of fresh LS-cement binder, while increased dosage of sodium sulfate decreased dispersibility of fresh paste. C–S–Hs–PCE and sodium sulfate exhibited a synergistic effect on strength enhancement, hydration acceleration as well as setting behavior of LS-cement binder. Sodium sulfate increased alkalinity of interstitial solution and promoted dissolution of LS. Dissolved Al and Si from LS powder reacted with dissolved sulfate ions from sodium sulfate to produce extra hydrates, and C–S–Hs–PCE accelerated pozzolanic reaction and hydration reaction via nucleation effect collaborated with dispersing effect. The accelerated hydration generated more AFt and C–S–H gel in the matrix. Newly formed hydrates promoted exceedingly the appearance of network, leading to a refinement of pore structure as well as enhancement in mechanical strength. Application of LS into cement as a greener binder could be obtained by synergistic adoption of C–S–Hs–PCE and sodium sulfate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to the outstanding electrochemical properties, lithium has been applied widely in glass, pharmaceuticals, ceramic as well as lubrication industries, and demand quantity for lithium salts was continuously expanding recently [1, 2]. During production process of lithium salts, enormous lithium slag (LS) produced from spodumene and lithium mica was emerged as industrial waste material [3,4,5,6,7,8,9], and more than 1 million tons of LS was released worldwide annually [4, 7, 10, 11]. The discharged LS was heaped up outdoors, which occupied huge land resources, caused serious waste of natural resources and brought serious pollution [5, 12,13,14,15]. With a mass of LS piling up annually, it is urgently vital to explore environmental methods of efficaciously utilizing LS, in order to alleviate environmental pressure and achieve sustainable development of lithium industry [3,4,5,6, 8, 16, 17].
Recently, LS has been widely investigated to explore the possibility of adopting in cement and concrete to achieve the recycling of resources and benefits [3, 5, 8, 10, 11, 13, 18,19,20,21,22]. LS contained higher than 80 wt% of SiO2 and Al2O3, more than 6 wt% of SO3 and a spot of CaO and Fe2O3 [2, 17, 20, 23]. Crystalline minerals in LS included quartz (SiO2), leached spodumene (LiAlSi2O6) and gypsum (CaSO4·2H2O) [12, 14]. In this way, LS was a potentially reactive waste material, which could be utilized as SCMs to meet the huge demand of the cement industry and bring significant environmental as well as economic benefits [4, 5, 8, 10, 12,13,14,15, 24, 25]. A high amount of SiO2 and Al2O3 existed in LS would react with portlandite (CH) to produce extra non-crystalline C–S–H [12, 18], which optimized the hydration mechanism as well as microstructure of cement, and improved mechanical as well as durability properties [5, 7, 14, 18, 19]. It has been widely admitted that application of SCMs to replace cement could obviously reduce CO2 emission by higher than 40% [26, 27], along with reduction in energy consumption as well as natural raw materials consumption.
Noticeably, LS contained abundant zeolites, and possessed glassy pore structure with high internal surface area [2, 9, 28]. The application of LS would have a remarkable negative impact on workability, and lead to a slow early strength development and prolonged setting time [5, 22, 29, 30]. To achieve higher early strength, previous scholars have done a lot of improvement works. The reactivity of LS could be enhanced via curing at high temperature [10, 14], grinding to be finer particles [3, 18, 31] or utilizing chemical activators [5, 20, 22, 29]. Overall, the application of early strength agent is recognized as the most effective technology for enhancing early strength [32, 33], among which sodium silicate, sodium hydroxide, sodium sulfate and other alkali compounds were widely adopted as chemical additives [34,35,36,37]. However, sodium silicate, sodium hydroxide and other traditional alkaline activators represented obvious energy depletion and possessed a substantial CO2 footprint [38,39,40]. By far, sodium sulfate was considered as the most widely used accelerator. Compared to conventional activators, sodium sulfate was a safe, cheap, environmentally friendly, and water-soluble inorganic mineral, which can be found in the nature or in the industrial production [26, 41, 42].
It has been reported that sodium sulfate can improve alkalinity for accelerating release of active Al2O3 and SiO2 from SCMs to form extra hydrates [43,44,45]. The addition of sodium sulfate boosted hydration and early-age strength development of fly ash–cement binder, and the mechanical strength did not reversal at later ages [45,46,47,48]. Generally, the presence of sodium sulfate as an activator could raise the content of sulfate ions and correspondingly promote the production of AFt [49]. The formation of additional AFt would lead to microstructure densification as well as strength enhancement. Moreover, increased content of sodium sulfate yielded higher dissolution of minerals and correspondingly increased the activation [41, 43]. However, it has been found that setting times of binders admixed with sodium sulfate were found to be shorter, compared with those activated with sodium silicate or sodium hydroxide. It has also been found that sodium sulfate could strengthen the retarding effect of retarders [50]. Sodium sulfate delayed the hydration of C3A, decreased the production of AFt, and decreased the adsorbing capacity of retarder molecules on surfaces of cement particles [50]. It has been reported that slag cement activated by sodium sulfate had higher mechanical strength of hardened paste than that activated by sodium hydroxide [51]. Sodium sulfate added to different mixtures might vary in its function, as has been reported that sodium sulfate could enhance mechanical strength of alite paste, while results in Portland cement paste might differ. Moreover, sodium sulfate adopted into slag-cement binder might heighten [52] or decrease [53] the mechanical strength. Acceleration seemed slow because the initial pH value was low [54, 55]. Recent researches have also shown that the performance of sodium sulfate as an activator was further enhanced when it was simultaneously utilized with nano/micro silica in fly ash–cement composite binder [43, 46]. Nevertheless, there existed few researches on activation mechanism of sodium sulfate, and hence, it was vital to conduct more researches in this field.
Except chemical accelerators, nanoparticles serving as nuclei seeds, including nano SiO2 [56,57,58], nano TiO2 [56,57,58,59,60,61,62], nano ZnO [59, 61], nano Fe2O3 [58], nano CaCO3 [56, 57], carbon nanotubes (CNTs) [60] and calcium silicate hydrate seeds (C–S–Hs) [43, 63,64,65,66,67,68,69,70,71,72,73,74] recently have been adopted to improve mechanical strength of cementitious materials. Nanoparticles could serve as nucleation seeds to boost hydrates formation at the early age, and nanoparticles with lower particle size and similar chemical compositions to hydrates had greater potential to be adopted as early-strength agents in cementitious materials. Therefore, there existed an elevated interest in analyzing synthetic C–S–Hs as an accelerating agent during the past decade, due to the economic and environmental benefits [63, 68, 70, 71, 73,74,75,76]. C–S–Hs would serve as heterogeneous nucleation sites for hydration products, beneficial for seeding in facilitation of hydration kinetics through expedited nucleation and growth of hydrats, and thus efficaciously promoted cement hydration to produce hydrates [43, 70, 72, 74, 75, 77]. C–S–Hs not only reduced or even eliminated the induction period through homogenous nucleation effect, but also provided excellent physical filling effect. Particularly, the superiority of C–S–Hs in comparison with traditional accelerators is that it did not compromise long-term performance of hydrated matrix [67, 73, 78].
However, the accelerating efficiency of C–S–Hs was highly impaired due to the agglomeration. In order to regulate dispersion retention behavior of C–S–Hs, polycarboxylate (PCE) superplasticizer was injected to the co-precipitation methods [64, 68,69,70]. C–S–Hs stabilized by PCE showed low aggregation [79]. Finer sized and more stable C–S–H nanoparticles with higher surface area and low aggregation could be generated [68, 78,79,80]. Moreover, PCE remarkably delayed the transformation of C–S–H from globular to nanofoil-like, and maintained nucleation productivity of C–S–H [66, 68,69,70]. Applying well-dispersed nano-sized C–S–Hs would obviously expedite hydration kinetics and increase mechanical strength [71, 74,75,76]. C–S–Hs reduced the free activation energy of hydration crystallization to zero, and multiplied hydration formation on clinker and C–S–Hs surface, which declined the hydrates layer thickness and increased ion concentration gradient [69, 70, 74]. Furthermore, C–S–Hs could not only promote hydration of silicates in clinker [81, 82], but also accelerate pozzolanic reaction of fly ash [68], metakaolin [82], blast furnace slag [80, 83], calcined clay [80]. However, up to now researches into adoption of C–S–Hs–PCE to generate strength enhancement of LS-cement binder remained scarce.
In spite of increased analytical results with regard to hydration of composite cement in the function of different acceleration methods, researches concentrating on combined action of sodium sulfate and C–S–Hs–PCE on performances of LS blended cement merited investigation. Simple utilization of chemicals or nanoseeds was difficult to obtain high enough mechanical strength at early stage [43]. It was vitally important to excavate highly-efficient and environmentally friendly early-age strength enhancement method of LS-cement composite binder.
The primary purpose of this study was to enhance mechanical strength of cement-LS system by incorporating C–S–Hs–PCE and sodium sulfate. Mortars and pastes containing 20% LS and different dosages of C–S–Hs–PCE and sodium sulfate were prepared. Fluidity, setting time and mechanical strength were investigated, and multiple techniques were applied to reveal underlying mechanism in detail. The main results of this investigation would provide scientific guidance for the preparation of LS-based cementitious materials.
2 Experimental
2.1 Materials
PII52.5 Portland cement was supplied by Anhui Hailuo Cement Company, and LS was provided by Jiangxi Lithium Company. The chemical compositions of cement and LS are shown in Table 1. LS was ground for 10 min via a ball mill. The volume average size (d50) of cement and LS were 16.54 and 11.08 μm, respectively. LS particles were mainly in the form of crushed stone, and a small amount of rod-like crystals can be seen. Mineral compositions of LS powder were mainly lithopone, quartz and gypsum, and their contents were respectively 63.4%, 8.5 and 12.5% analyzed via XRD/Rietveld methods.
Chemical reagent sodium sulfate, calcium nitrate, and sodium hydroxide were provided by Chinese Sinopharm Chemical Reagent Co., Ltd. Methyl allyl polyethenoxy ether, acrylic acid, ammonium persulfate and thiohydracrylic acid used for the synthesis of PCE polymer were provided by Sanrui Polymer Materials Co., Ltd.
2.2 Mix proportion and sample preparation
Blended binders contained 80 wt% PII52.5 cement and 20 wt% LS. Samples for hydration analysis as well as compressive strength measurement were prepared following former reports [63, 66, 84,85,86]. Sodium sulfate and C–S–Hs–PCE suspension were dissolved in deionized water. Cement-LS binder was mixed at W/B of 0.29, and cement-LS mortars were prepared at a ratio of water to binder of 0.5, and a ratio of binder to sand of 1:3 (as shown in Table 2).
For ion dissolution analysis, pore solutions with various dosages of C–S–Hs–PCE and sodium sulfate were firstly prepared following Table 3. And then 3.0 g LS powder was mixed with these solutions. The suspensions were sealed and cured at (20 ± 1)°C, and then separated by centrifugation at 3000 r/min for 3 min. The supernatant was filtered, and 10 mL of each sample was adopted for ICP measurement [87].
2.3 Test procedure
Setting time of composite binders was measured by Vicat apparatus following Chinese Standard GB/T 1346–2011 [66, 88]. The fluidity of fresh binder was measured via a mini-slump cone (a height of 60 mm, upper diameter of 36 mm and a bottom diameter of 60 mm), according to Chinese standard GB/T 8077-2000. Compressive strength was measured following GB/T 17671–2021 [89]. Mortars of 40 mm × 40 mm × 160 mm were prepared for compressive strength measurement. The specimens were cured at 20 ± 1°c and 95 ± 5% RH. At the ages of 12 h, 1d, 3d and 28d, the compressive strengths of mortar were measured [90,91,92,93,94].
Ion dissolution was tested via inductively coupled plasma (ICP). The mix proportion of pore solution as well as the sample preparation has been expatiated in Sect. 2.3. The concentrations of Si4+, Al3+, Fe3+ and Ca2+ in the upper solution were measured [8, 87].
Hydration heat evolution was tested via TAM Air isothermal calorimeter (3114/3236 TAM 83), and hydration products of composite binder were analyzed by XRD [84, 95, 96]. Microstructure observation was observed via SEM [97, 98].
3 Results
3.1 Fluidity
Fluidity of cement-LS fresh binder were measured (shown in Fig. 1). In comparison with reference sample, increased dosage of C–S–Hs–PCE led to improved fluidity of fresh LS-cement paste. This might be ascribed to physically adsorbed PCE onto C–S–H seeds, which dissolved into pore solutions and then adsorbed onto mineral surfaces and modified the dispersity of fresh paste. This inferred that C–S–Hs–PCE was advantageous for modifying the workability of fresh binder, which was essentially different from those traditional nanoparticles, such as nano SiO2, nano CaCO3, et al. And this finding was coincident with previous reports. Plank et al. [68] and Sun et al. [82] have also affirmed that C–S–Hs–PCE could raise fluidity of fresh paste as well as the slump of fresh concrete. As a leaching residue [1, 10, 12, 23], LS contains a substantial zeolites and has a large internal surface area as well as porous structures [4, 5, 10, 17, 18, 22, 25, 29], and is obviously disadvantageous for dispersing of fresh binder. C–S–Hs–PCE was advantageous for modifying fluidity of fresh LS-cement binder. According to the fabrication process, PCE polymer and C–S–H seeds are physically combined, and plentiful amount of PCE would dissolve into pore solution during stirring fresh binder. The dissolved PCE adsorbed onto positive-charged surface through electrostatic attraction [99], and adsorb onto negative-charged surface via bridge effect of Ca2+ ions [86, 91, 92, 94, 100,101,102]. The larger adsorption amount of PCE onto cement as well as LS surfaces brings about higher dispersibility of fresh binder.
However, the addition of sodium sulfate performed a negative role in improving fluidity of LS-cement paste. Increased dosage of sodium sulfate intensified the negative effect on fluidity. When 1% C–S–Hs–PCE was added into LS-cement blended binder, the increased content of sodium sulfate (from 0 to 2%) gradually decreased the fluidity of the fresh binder. Similarly, with 2% C–S–Hs–PCE added into composite binder, increased dosage of sodium sulfate gradually led to decreased fluidity. We can also observe that the increased dosage of sodium sulfate led to the loss of consistency rapidly and the form of denser matrix.
3.2 Mechanical strength
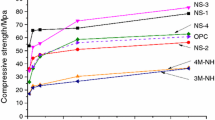
Compressive strengths of hardened LS-cement composite mortars were tested (Fig. 2). In comparison with reference (N0-0), compressive strength increased observably with increased dosage of C–S–Hs–PCE adopted. With the dosage of C–S–Hs–PCE increased from 0 to 2%, compressive strengths at 12 h, 1d, 3d and 28d increased respectively by 38.5%, 67.0%, 5.8%, and 6.2%. This indicated that C–S–Hs–PCE promoted the mechanical strength enhancement especially at early ages. C–S–Hs–PCE promoted hydration evolution ascribing to nucleation effect [63, 65, 66, 68,69,70, 80] as well as dispersing behavior of PCE superplasticizer [84, 92, 103,104,105,106], which contributed to intensified formation of hydrates and compacter microstructure [107].
The increased content of sodium sulfate also promoted the enhancement of compressive strength of LS-cement binder, especially early-age stength (at 12 h, 1d and 3d). With 2% sodium sulfate adopted, compressive strength at 12 h, 1d and 3d increased respectively by 28.8, 51.8 and 20.9% relative to the control sample (N0-0), while compressive strength at 28d did not increase. Sodium sulfate could react with CH to produce CaSO4·2H2O, and a great deal of AFt crystals were produced by rapid reaction of highly dispersed CaSO4·2H2O with C3A, leading to development of early-age strength [63]. Indeed, as reported in previous studies, sodium sulfate would reduce late hydration rates and hence decrease late age strengths [41].
Moreover, the synergistic adoption of C–S–Hs–PCE and sodium sulfate was also analyzed. With union usage of 1% C–S–Hs–PCE and 1% sodium sulfate, compressive strength exceeded that of only adopting 1% C–S–Hs–PCE as well as that only aodpting 2% C–S–Hs–PCE. And with the increased dosage of both sodium sulfate and C–S–Hs–PCE, compressive strengths increased obviously. The compressive strengths of LS-cement binder with adoption of 2% C–S–Hs–PCE and 2% sodium sulfate were enhanced by 69.2, 91.8, 48.3, and 15.2% at 12 h, 1d, 3d and 28d. The compressive strength of all mortars enhanced over time, and enhancement effect of C–S–Hs–PCE and sodium sulfate on compressive strength was obiviously overt, in comparison with simply adopitng C–S–Hs–PCE or sodium sulfate. This indicated that combined usage of C–S–Hs–PCE and sodium sulfate exhibited a synergistic effect on strength enhancement of LS-cement binder.
3.3 Hydration heat
Hydration heat evolution of LS-cement composite binder was analyzed (Fig. 3), which supplied high resolution information concerning influences of C–S–Hs–PCE and sodium sulfate on hydration progression of LS-cement binder.
Binders that differed in dosages of C–S–Hs–PCE and sodium sulfate exhibited various heat flows and hydration heat over 72 h. All the binders presented massive heat flow at the very beginning, ascribing to rapid dissolution of minerals and rapid reaction of aluminates. After induction period, the secondary exothermic peak was ascribed to the silicate reaction. C–S–Hs–PCE (see N1-0, N2-0) remarkably affected the secondary exothermic peak (see Fig. 3a), showing as elevated exothermic peak, suggesting C–S–Hs–PCE would effectively enhance hydration of silicates. This was in agreement with previous reports that C–S–Hs–PCE accelerated cement hydration [69, 80, 108].
As shown in Fig. 3a, C–S–Hs–PCE and sodium sulfate had obvious influence on the nucleation of hydrates during acceleration period. With addition of C–S–Hs–PCE and sodium sulfate, the second hydration peak was shifted to earlier time and the introduction period was cut down obviously, shown as larger and earlier hydration peaks, which was also proved by increased cumulative hydration heat (exhibited in Fig. 3b). And with increased content of C–S–Hs–PCE and sodium sulfate, the introduction period dramatically shortened, and the slop of the acceleration period increased. The slope of acceleration period was highest for N2-2 sample (2% C–S–Hs–PCE and 2% sodium sulfate), indicating that early hydration rate and hydration heat release were prodigiously enhanced. The diffusion rate of various ions from minerals was boosted by the addition of C–S–Hs–PCE and sodium sulfate. Moreover, we can also find that the slope of the acceleration period for N1-2 (1% C–S–Hs–PCE and 2% sodium sulfate) was higher than that for N2-0 (2% C–S–Hs–PCE and 0% SS). This indicated that the combined adoption of C–S–Hs–PCE and sodium sulfate would significantly promote formation of hydrates, in comparison with simple usage of C–S–Hs–PCE.
From Fig. 3b, union application of C–S–Hs–PCE and sodium sulfate boosted cumulative heat flow. Before 24 h, LS-cement binder added with 1% C–S–Hs–PCE and 2% sodium sulfate generated higher cumulative hydration heat than that only admixed with 2% C–S–Hs–PCE or synergistically adopted with 2% C–S–Hs–PCE and 1% sodium sulfate. However, after hydrating for 72, LS-cement binder admixed with 2% C–S–Hs–PCE or applied with 2% C–S–Hs–PCE and 1% sodium sulfate generated higher cumulative hydration heat than that admixed with 1% C–S–Hs–PCE and 2% sodium sulfate. Indeed, differences in cumulative hydration heat amount increased further and greater plateauing of heat production was evident. This indicated that a high amount of sodium sulfate would be beneficial for the very early-age hydration, while increased dosage of C–S–Hs–PCE would be propitious to boost later-age hydration.
3.4 Setting time
Effect of synergistic adoption of C–S–Hs–PCE and sodium sulfate on setting behavior was analyzed (Fig. 4).
From Fig. 4, C–S–Hs–PCE and sodium sulfate altered setting behavior, highly correlated with their dosages. Simply adding sodium sulfate or C–S–Hs–PCE into LS-cement composite binder shortened the setting time. And increased dosage of sodium sulfate or C–S–Hs–PCE gradually cut down initial setting time and final setting time. With 1% C–S–Hs–PCE added into LS-cement binder, increased dosage of sodium sulfate from 0 to 2% shortened initial setting time by 31.8%, and shortened final setting time by 18.6%. With 2% C–S–Hs–PCE added into LS-cement binder, increased content of sodium sulfate from 0 to 2% shortened the initial setting time by 52.1%, and shortened final setting time by 48.3%. In comparison with simply adding sodium sulfate or C–S–Hs–PCE, union adoption of C–S–Hs–PCE and sodium sulfate presented enhanced acceleration on setting behavior.
3.5 Hydration products
From XRD patterns in Fig. 5, hydrates of blended binder were analyzed, with phases such as C3S or C2S consumed and new phases formed, like CH, AFt. At early ages (1d, 3d, and 7d), compared to reference sample (N0-0), adding C–S–Hs–PCE affected intensity of characteristic peaks of CH, C3S and AFt. With increased dosage of C–S–Hs–PCE, the intensity of characteristic peaks of CH enhanced remarkably, and the intensity of characteristic peaks of C3S reduced gradually, which was possibly a consequence of a more effective reaction of cement and regarded that the hydration of cement clinker was accelerated by C–S–Hs–PCE at early age. This agreed with previous reports that C–S–Hs–PCE could facilitate hydration of C3S [66, 69].
At 28d, intensity of CH peak decreased with increased dosage of C–S–Hs–PCE (see sample of N1-0, N2-0), suggesting enhanced pozzolanic reaction was developed between CH and dissolved silicon and aluminum species from LS, producing extra C–S–H gel. This was because at later age pozzolanic reaction between LS and CH took place, and C–S–Hs–PCE boosted pozzolanic reaction of LS, resulting in a decreased amount of CH formed in the hydrated matrix.
The peak intensity of AFt was augmented significantly by adoption of sodium sulfate. When C–S–Hs–PCE content was fixed at 1%, with the increased dosage of sodium sulfate (from 0 to 2%), the appearance of AFt peak was observed to be gradually obvious. It was deducted that sodium sulfate expedited production of AFt. The same results were observed for samples containing 2% C–S–Hs–PCE. This indicated that sodium sulfate promoted formation of AFt, and this result was coincident with previous researches [5, 87, 109]. Moreover, at early ages (1d, 3d and 7d), with sodium sulfate incorporated in the binder, the intensity of CH peak increased, indicating enhanced hydration of Portland cement. And the intensity of AFt enhanced, due to the fact increased formation of CH would react with sodium sulfate to produce gypsum as an intermedium reaction product, which then reacted with aluminates from Portland cement or aluminum ions from LS to form additional AFt crystals.
3.6 Reaction of LS
After LS was soaked in various solutions, concentrations of Al and Si were tested (Fig. 6). Compared with CH solution, concentrations of Al and Si in 1% C–S–Hs–PCE solution reduced ascribing to nucleation effect of C–S–Hs–PCE, which facilitated depletion of free Al and Si. Moreover, concentrations of Al and Si in 1% C–S–Hs–PCE mixed with 2% sodium sulfate decreased further. Because adding sodium sulfate enhanced the alkalinity in solution, dissolution of Al and Si from LS minerals increased at early ages. And then, sulfate ions (SO42−) would react with Al to produce AFt and the freshly generated AFt might also act as seeding sites for the production of C–(A)–S–H, leading to reduced content of free Al and Si in interstitial solution. The lowest content of Si and S appeared in the 1% C–S–Hs–PCE-2% sodium sulfate solution, due to boosted depletion of free Al and S due to cooperative effect of sodium sulfate and C–S–Hs–PCE. Because of a high amount of sulfate ions provided by sodium sulfate, the dissolved Al3+ from LS reacted with sulfates to form AFt, which led to accelerated consumption of Al3+.
4 Conclusions
In this paper, synergistic effect C–S–Hs–PCE and sodium sulfate on LS-cement binder was analyzed. The setting behavior, dispersing performnace, mechanical strength, and hydration evolution of blended binder were evaluated. The main conclusions are shown below:
-
(1)
The addition of C–S–Hs–PCE is advantageous for modifying fluidity of fresh LS-cement binder, while increased dosage of sodium sulfate decreased fluidity of fresh paste. Compressive strength increased observably with increased dosage of C–S–Hs–PCE adopted. The union usage of C–S–Hs–PCE and sodium sulfate exhibited a synergistic effect on strength enhancement of LS-cement binder. Combined adoption of 1% C–S–Hs–PCE and 1% sodium sulfate generated higher compressive strength than simply admixing 1% C–S–Hs–PCE or simply admixing 2% C–S–Hs–PCE.
-
(2)
C–S–Hs–PCE and sodium sulfate advanced the hydration of LS-cement binder, shortened introduction period, advanced acceleration period and cut down setting time. Synergistic adoption of sodium sulfate and C–S–Hs–PCE presented enhanced shortening effect on setting time.
-
(3)
Combined adoption of C–S–Hs–PCE and sodium sulfate significantly promoted generation of C–S–H gel and CH than the application of simple usage of C–S–Hs–PCE, shown with enhanced cumulative hydration heat release. A high amount of sodium sulfate would be beneficial for the very early-age hydration, while increased dosage of C–S–Hs–PCE would be propitious to promote later-age hydration.
-
(4)
The addition of sodium sulfate increased alkalinity of interstitial solution and promoted the dissolution of LS. The free Al and Si from dissolution of LS reacted with dissolved SO42− ions from sodium sulfate to produce hydrates.
References
Yan QX et al (2012) Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int J Miner Process 110:1–5
Dong P, Ahmad MR, Chen B, Munir MJ, Kazmi SMS (2021) Preparation and study of magnesium ammonium phosphate cement from waste lithium slag. J Clean Prod 316:128371
Tan H, Li M, He X, Su Y, Yang J, Zhao H (2021) Effect of wet grinded lithium slag on compressive strength and hydration of sulphoaluminate cement system. Construct Build Mater 267:120465
He Y, Zhang Q, Chen Q, Bian J, Qi C, Kang Q, Feng Y (2021) Mechanical and environmental characteristics of cemented paste backfill containing lithium slag-blended binder. Construct Build Mater 271:121567
Zhang T, Ma B, Tan H, Liu X, Chen P, Luo Z (2020) Effect of TIPA on mechanical properties and hydration properties of cement-lithium slag system. J Environ Manag 276:111274
Li JZ, Huang SW (2020) Recycling of lithium slag as a green admixture for white reactive powder concrete. J Mater Cycles Waste Manage 22(6):1818–1827
He ZH, Du SG, Chen D (2018) Microstructure of ultra high performance concrete containing lithium slag. J Hazard Mater 353:35–43
Zhai M, Zhao J, Wang D, Wang Y, Wang Q (2021) Hydration properties and kinetic characteristics of blended cement containing lithium slag powder. J Build Eng 39:102287
Li J, Lian P, Huang S, Huang L (2020) Recycling of lithium slag extracted from lithium mica by preparing white Portland cement. J Environ Manag 265:110551
Liu Z et al (2019) A green route to sustainable alkali-activated materials by heat and chemical activation of lithium slag. J Clean Prod 225:1184–1193
He ZH, Li LY, Du SG (2017) Mechanical properties, drying shrinkage, and creep of concrete containing lithium slag. Constr Build Mater 147:296–304
Tan HB et al (2015) Utilization of lithium slag as an admixture in blended cements: physico-mechanical and hydration characteristics. J Wuhan Univ Technol-Mater Sci Ed 30(1):129–133
Tan HB et al (2020) Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J Clean Prod 250:119528
Joseph S, Snellings R, Cizer Ö (2019) Activation of Portland cement blended with high volume of fly ash using Na2SO4. Cement Concr Compos 104:103417
Lu J, Yu Z, Zhu Y, Huang S, Luo Q, Zhang S (2019) Effect of Lithium-Slag in the Performance of Slag Cement Mortar Based on Least-Squares Support Vector Machine Prediction. Materials 12(10):1652
Aljerf L, Choukaife AE (2016) Sustainable development in damascus University: a survey of internal stakeholder views. J Environ Stud 2(2):1–12
Shah SFA, Chen B, Ahmad MR, Haque MA (2021) Development of Cleaner One-part geopolymer from lithium slag. J Clean Prod 291:125241
Tan HB et al (2018) Utilization of lithium slag by wet-grinding process to improve the early strength of sulphoaluminate cement paste. J Clean Prod 205:536–551
Wu FF, Shi KB, Dong SK (2014) Properties and microstructure of HPC with lithium-slag and fly ash. Key Eng Mater 599:70–73
Wang J, Han L, Liu Z, Wang D (2020) Setting controlling of lithium slag-based geopolymer by activator and sodium tetraborate as a retarder and its effects on mortar properties. Cement Concr Compos 110:103598
Luo Q, Wen YF, Huang SW, Peng WL, Li JY, Zhou YX (2017) Effects of lithium slag from lepidolite on Portland cement concrete: Qi Luo Yufeng Wen, Shaowen Huang, Weiliang Peng, Jinyang Li & Yuxuan Zhou. In: Civil, Architecture and Environmental Engineering (pp 620-623). CRC Press
He Y, Liu S, Hooton RD, Zhang X, He S (2022) Effects of TEA on rheological property and hydration performance of lithium slag-cement composite binder. Construct Build Mater 318:125757
Wang YR et al (2019) Micro-morphology and phase composition of lithium slag from lithium carbonate production by sulphuric acid process. Constr Build Mater 203:304–313
Wang WC (2014) Effects of fly ash and lithium compounds on the water-soluble alkali and lithium content of cement specimens. Constr Build Mater 50:727–735
He Y, Chen Q, Qi C, Zhang Q, Xiao C (2019) Lithium slag and fly ash-based binder for cemented fine tailings backfill. J Environ Manag 248:109282
Zhao Y, Qiu J, Xing J, Sun X (2020) Chemical activation of binary slag cement with low carbon footprint. J Clean Prod 267:121455
Skibsted J, Snellings R (2019) Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cement Concr Res 124:105799
Zhang L, Lv SZ, Liu Y, Xiao SY, Zhou PS (2015) Influence of lithium slag on cement properties. J Wuhan Univ Technol 37(3):23–27
Liu Z, Wang JX, Li L, Wang DM (2019) Characteristics of alkali-activated lithium slag at early reaction age. J Mater Civil Eng 31(12):04019312
Xu L, Yang K, Tang C, Yang X, Wu K, Lothenbach B (2023) Lead retardation on cement hydration: Inhibition and re-acceleration of clinker dissolution. Cement Concr Compos 138:104986
Tan H, Li M, He X, Su Y, Zhang J, Pan H, Wang Y (2020) Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J Clean Prod 250:119528
Cavusoglu I, Yilmaz E, Yilmaz AO (2021) Additivity effect on properties of cemented coal fly ash backfill containing water-reducing admixtures. Construct Build Mater 267:121021
Cavusoglu I, Yilmaz E, Yilmaz AO (2021) Sodium silicate effect on setting properties, strength behavior and microstructure of cemented coal fly ash backfill. Powder Technol 384:17–28
Brykov AS et al (2002) Effect of hydrated sodium silicates on cement paste hardening. Russ J Appl Chem 75(10):1577–1579
Brykov AS, Danilov BV, Larichkov AV (2006) Specific features of portland cement hydration in the presence of sodium hydrosilicates. Russ J Appl Chem 79(4):521–524
Santana-Carrillo JL, Ortega-Zavala DE, Burciaga-Díaz O, Escalante-Garcia JI (2021) Modified blended limestone-Portland cement binders: Evaluation of 4 different sodium silicates. Cement Concr Compos 118:103935
Wu K, Hu Y, Zhang L, Xu L, Yang Z (2022) Promoting the sustainable fabrication of bricks from municipal sewage sludge through modifying calcination: microstructure and performance characterization. Construct Build Mater 324:126401
Cristelo N, Garcia-Lodeiro I, Rivera JF, Miranda T, Palomo Á, Coelho J, Fernández-Jiménez A (2021) One-part hybrid cements from fly ash and electric arc furnace slag activated by sodium sulphate or sodium chloride. J Build Eng 44:103298
Cristelo N et al (2015) Assessing the production of jet mix columns using alkali activated waste based on mechanical and financial performance and CO2 (eq) emissions. J Clean Prod 102:447–460
Habert G, de Lacaillerie JBD, Roussel N (2011) An environmental evaluation of geopolymer based concrete production: reviewing current research trends. J Clean Prod 19(11):1229–1238
Fu J, Jones AM, Bligh MW, Holt C, Keyte LM, Moghaddam F, Waite TD (2020) Mechanisms of enhancement in early hydration by sodium sulfate in a slag-cement blend–Insights from pore solution chemistry. Cement Concr Res 135:106110
Neto JDSA, Angeles G, Kirchheim AP (2021) Effects of sulfates on the hydration of Portland cement–a review. Construct Build Mater 279:122428
Zou F, Hu C, Wang F, Ruan Y, Hu S (2020) Enhancement of early-age strength of the high content fly ash blended cement paste by sodium sulfate and C-S–H seeds towards a greener binder. J Clean Prod 244:118566
Criado M, Jimenez AF, Palomo A (2010) Effect of sodium sulfate on the alkali activation of fly ash. Cement Concr Compos 32(8):589–594
Velandia DF et al (2016) Evaluation of activated high volume fly ash systems using Na2SO4, lime and quicklime in mortars with high loss on ignition fly ashes. Constr Build Mater 128:248–255
Onuaguluchi O, Ratu R, Banthia N (2022) Effect of sodium sulfate activation on the early-age matrix strength and steel fiber bond in high volume fly ash (HVFA) cement mortar. Construct Build Mater 341:127808
Li C et al (2017) Pozzolanic reaction of fly ash modified by fluidized bed reactor-vapor deposition. Cem Concr Res 92:98–109
Velandia DF et al (2018) Effect of mix design inputs, curing and compressive strength on the durability of Na2SO4-activated high volume fly ash concretes. Cement Concr Compos 91:11–20
Aydın S, Baradan B (2021) Sulfate resistance of alkali-activated slag and Portland cement based reactive powder concrete. J Build Eng 43:103205
Guo S, Zhang Y, Wang K, Bu Y, Wang C, Ma C, Liu H (2019) Delaying the hydration of Portland cement by sodium silicate: Setting time and retarding mechanism. Construct Build Mater 205:543–548
Rakhimova NR et al (2017) Mechanism of solidification of simulated borate liquid wastes with sodium silicate activated slag cements. J Clean Prod 149:60–69
Roy S et al (1998) Investigation of Portland slag cement activated by waterglass. Cem Concr Res 28(7):1049–1056
Nguyen HA, Chang TP, Thymotie A (2020) Enhancement of early engineering characteristics of modified slag cement paste with alkali silicate and sulfate. Construct Build Mater 230:117013
Myers RJ, Bernal SA, Provis JL (2014) A thermodynamic model for C-(N-) ASH gel: CNASH_ss. Derivation and validation. Cement Concr Res 66:27–47
Zhang J, Tan H, Bao M, Liu X, Luo Z, Wang P (2021) Low carbon cementitious materials: Sodium sulfate activated ultra-fine slag/fly ash blends at ambient temperature. J Clean Prod 280:124363
Luan C, Zhou Y, Liu Y, Ren Z, Wang J, Yuan L, Huang Y (2022) Effects of nano-SiO2, nano-CaCO3 and nano-TiO2 on properties and microstructure of the high content calcium silicate phase cement (HCSC). Construct Build Mater 314:125377
Ren Z, Liu Y, Yuan L, Luan C, Wang J, Cheng X, Zhou Z (2021) Optimizing the content of nano-SiO2, nano-TiO2 and nano-CaCO3 in Portland cement paste by response surface methodology. J Build Eng 35:102073
Ng DS, Paul SC, Anggraini V, Kong SY, Qureshi TS, Rodriguez CR, Šavija B (2020) Influence of SiO2, TiO2 and Fe2O3 nanoparticles on the properties of fly ash blended cement mortars. Construct Build Mater 258:119627
Amor F et al (2022) Contribution of TiO2 and ZnO nanoparticles to the hydration of Portland cement and photocatalytic properties of High Performance Concrete. Construct Build Mater 16:e00965
Liu J, Suh H, Jee H, Xu J, Nezhad EZ, Choi CS, Bae S (2021) Synergistic effect of carbon nanotube/TiO2 nanotube multi-scale reinforcement on the mechanical properties and hydration process of portland cement paste. Construct Build Mater 293:123447
Goyal R, Verma VK, Singh NB (2022) Effect of nano TiO2 & ZnO on the hydration properties of Portland cement. Mater Today Proc 65:1956–1963
Moro C, Francioso V, Velay-Lizancos M (2021) Modification of CO2 capture and pore structure of hardened cement paste made with nano-TiO2 addition: influence of water-to-cement ratio and CO2 exposure age. Construct Build Mater 275:122131
Li H, Xue Z, Liang G, Wu K, Dong B, Wang W (2021) Effect of CS-Hs-PCE and sodium sulfate on the hydration kinetics and mechanical properties of cement paste. Construct Build Mater 266:121096
Li H, Gu L, Dong B, Chen Q, Xu C, Yang X, Wang W (2020) Improvements in setting behavior and strengths of cement paste/mortar with EVA redispersible powder using CS-Hs-PCE. Construct Build Mater 262:120097
Liang G et al (2021) Synergistic effect of EVA, TEA and C–S–Hs–PCE on the hydration processand mechanical properties of Portland cement paste at early age. Construct Build Mater 272:121891
Xu C, Li H, Yang X, Dong B, Wang W (2021) Action of the combined presence of CS-Hs-PCE and triethanolamine on the performances of cement paste/mortar. Construct Build Mater 269:121345
Xu C, Liang G, Li H, Dong B, Yang X, Yang Z (2021) Insight into early-age performance of cement paste/mortar with CS-Hs-PCE and aluminum sulfate. J Mater Civil Eng 33(8):04021210
Kanchanason V, Plank J (2018) Effectiveness of a calcium silicate hydrate - Polycarboxylate ether (C-S-H-PCE) nanocomposite on early strength development of fly ash cement. Constr Build Mater 169:20–27
Kanchanason V, Plank J (2017) Role of pH on the structure, composition and morphology of C-S-H-PCE nanocomposites and their effect on early strength development of Portland cement. Cem Concr Res 102:90–98
Wang ZM, Yao YH, Tang RF, Li ST, Liu X, Sun DW (2021) The effect and mechanism of C-S–H-PCE nanocomposites on the early strength of mortar under different water-to-cement ratio. J Build Eng 44:103360
John E, Epping JD, Stephan D (2019) The influence of the chemical and physical properties of CSH seeds on their potential to accelerate cement hydration. Construct Build Mater 228:116723
John E, Stephan D, Lehmann C (2019) Accelerating cement hydration with C-S-H seeds. ZKG Int 72(4):53–59
Gu X, Tan H, He X, Zhang J, Li M, Su Y, Yang J (2022) Nano CSH seeds prepared from ground granulated blast-furnace slag-carbide slag and its application in Portland cement. Construct Build Mater 329:127204
John E, Matschei T, Stephan D (2018) Nucleation seeding with calcium silicate hydrate - A review. Cem Concr Res 113:74–85
Wang F, Kong X, Jiang L, Wang D (2020) The acceleration mechanism of nano-CSH particles on OPC hydration. Construct Build Mater 249:118734
Sun JF et al (2017) Effects of synthetic C-S-H/PCE nanocomposites on early cement hydration. Constr Build Mater 140:282–292
Pedrosa HC, Reales OM, Reis VD, das Dores Paiva, M., & Fairbairn, E. M. R. (2020) Hydration of Portland cement accelerated by CSH seeds at different temperatures. Cement Concr Res 129:105978
Zou F, Zhang M, Hu C, Wang F, Hu S (2021) Novel CASH/PCE nanocomposites: design, characterization and the effect on cement hydration. Chem Eng J 412:128569
Nicoleau L (2011) Accelerated growth of calcium silicate hydrates: experiments and simulations. Cem Concr Res 41(12):1339–1348
Kanchanason V, Plank J (2019) Effect of calcium silicate hydrate - polycarboxylate ether (C-S-H PCE) nanocomposite as accelerating admixture on early strength enhancement of slag and calcined clay blended cements. Cem Concr Res 119:44–50
Thomas JJ, Jennings HM, Chen JJ (2009) Influence of nucleation seeding on the hydration mechanisms of tricalcium silicate and cement. J Phys Chem C 113(11):4327–4334
Sun J, Dong H, Wu J, Jiang J, Li W, Shen X, Hou G (2021) Properties evolution of cement-metakaolin system with CSH/PCE nanocomposites. Construct Build Mater 282:122707
Hubler MH, Thomas JJ, Jennings HM (2011) Influence of nucleation seeding on the hydration kinetics and compressive strength of alkali activated slag paste. Cem Concr Res 41(8):842–846
He Y, Zhang X, Hooton RD (2017) Effects of organosilane-modified polycarboxylate superplasticizer on the fluidity and hydration properties of cement paste. Constr Build Mater 132:112–123
Liu M, Tan HB, He XY (2019) Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume fly ash cement system. Constr Build Mater 194:350–359
He Y, Zhang X, Liu S, Hooton RD, Ji T, Kong Y (2020) Impacts of sulphates on rheological property and hydration performance of cement paste in the function of polycarboxylate superplasticizer. Construct Build Mater 256:119428
Zou FB et al (2018) Effect of triisopropanolamine on compressive strength and hydration of steaming-cured cement-fly ash paste. Constr Build Mater 192:836–845
GB/T1346–2011, (2011) Test methods for water requirement of normal consistency, setting time and soundness of the Portland cement
GB/T17671–2021, Test methods of cement mortar strength (ISO method)
He Y et al (2020) Influence of PCE on rheological and hydration performances of cement paste. J Mater Civ Eng 32(3):04020002
He Y et al (2018) Influence of polycarboxylate superplasticizer on rheological behavior in cement paste. J Wuhan Univ Technol-Mater Sci Ed 33(4):932–937
He Y et al (2019) Effects of PCEs with various carboxylic densities and functional groups on the fluidity and hydration performances of cement paste. Constr Build Mater 202:656–668
Li H, Xu C, Dong B, Chen Q, Gu L, Yang X (2020) Enhanced performances of cement and powder silane based waterproof mortar modified by nucleation CSH seed. Construct Build Mater 246:118511
He Y et al (2019) Effect of carboxylic density on sulfate sensitivity of polycarboxylate superplasticizers. KSCE J Civ Eng 23(12):5163–5172
Zhang XW, Lu CX, Shen JY (2016) Influence of tartaric acid on early hydration and mortar performance of Portland cement-calcium aluminate cement-anhydrite binder. Constr Build Mater 112:877–884
Zhang YJ et al (2018) The synergistic effect of AFt enhancement and expansion in Portland cement-aluminate cement-FGD gypsum composite cementitious system. Constr Build Mater 190:985–994
Zhang R, Bassim N, Panesar DK (2018) Characterization of Mg components in reactive MgO Portland cement blends during hydration and carbonation. J CO2 Utilizat 27:518–527
Zhu Z, Wang Z, Xu L, Zhou Y, Chen Y, Wu K, De Schutter G (2022) Synthesis and characterization of an intermediate for CSH structure tailoring. Cement Concr Res 160:106923
Zhang Y, Kong X (2015) Correlations of the dispersing capability of NSF and PCE types of Superplasticizer and their impacts on cement hydration with the adsorption in fresh cement pastes. Cem Concr Res 69:1–9
Qi S et al (2018) Dispersion ability, adsorption and retardation effects of phosphonated small molecule superplasticizers. Adv Cement Res
Kong XM, Zhang YR, Hou SS (2013) Study on the rheological properties of Portland cement pastes with polycarboxylate superplasticizers. Rheol Acta 52(7):707–718
Ran QP et al (2009) Effect of the length of the side chains of comb-like copolymer dispersants on dispersion and rheological properties of concentrated cement suspensions. J Colloid Interf Sci 336(2):624–633
He Y, Liu S, Luo Q, Liu W, Xu M (2021) Influence of PCE-type GA on cement hydration performances. Construct Build Mater 302:124432
Kong FR et al (2016) Effects of polycarboxylate superplasticizers with different molecular structure on the hydration behavior of cement paste. Constr Build Mater 105:545–553
Shin JY et al (2008) Effects of polycarboxylate-type superplasticizer on fluidity and hydration behavior of cement paste. Korean J Chem Eng 25(6):1553–1561
Scrivener KL, Juilland P, Monteiro PJM (2015) Advances in understanding hydration of Portland cement. Cem Concr Res 78:38–56
Ma J et al (2019) Effects of limestone powder on the hydration and microstructure development of calcium sulphoaluminate cement under long-term curing. Constr Build Mater 199:688–695
Alizadeh R et al (2009) Hydration of tricalcium silicate in the presence of synthetic calcium-silicate-hydrate. J Mater Chem 19(42):7937–7946
Wang J, Ma B, Tan H, Du C, Chu Z, Luo Z, Wang P (2021) Hydration and mechanical properties of cement-marble powder system incorporating triisopropanolamine. Construct Build Mater 266:121068
Acknowledgements
This research was supported by National Natural Science Foundation of China (51808369, 51890911), CRSRI Open Research Program (CKWV20221020/KY), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22_1577, SJCX23_1722).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Y., Zhang, G., Chen, J. et al. Influence of C–S–Hs–PCE and Na2SO4 on the fluidity and mechanical performance of cement–lithium slag binder. Mater Struct 56, 158 (2023). https://doi.org/10.1617/s11527-023-02243-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-023-02243-4