Abstract
Nanostructures are playing important roles in fundamental research and practical application. The ease of their processing to secondary structures through assembly and the use as catalysts in chemical reactions have inspired much research interest in developing nanoparticles synthesis and characterization techniques. In this snapshot review, the advances in colloidal synthesis, chemical processing, and emerging characterization of novel nanostructures will be reviewed. The working mechanism and rational design of nanostructures with different chemical components, sizes, and shapes will be firstly introduced, with a focus on understanding the complex structure–property correlation of these nanostructured materials. In the second part, advanced in situ electron microscopy characterization technologies will be introduced to illustrate new understandings on nanostructure synthesis, nucleation, and phase transformation. Some operando characterization techniques will be further elaborated to study the complicated catalytic reactions occurring on nanoparticle surfaces, followed by a perspective on the further advances of nanostructures to the next generation.
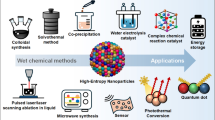
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoparticles are nanometer-sized particles with well-defined shape, composition, surface property, size, and size distribution [1]. Due to the small sizes, nanoparticles exhibit many unique physiochemical properties that are not observed in their bulk counterparts. For example, metallic nanoparticles, particularly those made of Au, Ag, and Cu, have localized surface plasmon resonance (LSPR) due to the resonant oscillation of free electrons with the light, leading to extinction of light at resonant wavelengths [2,3,4,5]. The LSPR is sensitive to the chemical compositions and nanoparticles made of different materials have their characteristic extinction peak positions in optical spectrum, making them attractive materials for light-related applications [6,7,8,9]. Semiconductor nanoparticles have unique electronic bandgaps and have therefore been widely used in energy conversion and light-emitting diodes (LEDs) [10, 11]. This remarkable property is induced by quantum confinement effect of quantum dots (QDs) and is of great importance in fundamental science and practical applications.
The physical and chemical properties of nanoparticles are determined not only by chemical compositions but also by particle shapes, sizes, and aggregation states [12,13,14,15,16]. Under properly prepared conditions, they can be either chemically processed to other materials or physically assembled into superstructures with defined symmetry, lattice parameter, and crystal habit [17,18,19,20,21,22]. Therefore, synthesizing nanoparticle with precise shape control, narrow size distribution, and defined compositions and their further process to secondary structures are central in nanomaterials science and engineering. To this end, researchers have developed many synthetic methods to prepare nanoparticles with diverse sizes and shapes and to study their size- and shape-dependent physical properties [23, 24]. Colloidal synthesis has been introduced to prepare nanoparticles using wet chemistry in colloidal solutions. This type of methods uses precursors in well-prepared solutions, followed by nucleation and growth of inorganic nanoparticles under the presence of capping ligands for kinetic and thermodynamic control [25]. One typical example in this regard is the synthesis of QDs in hot-injection process. At initial nucleation, the quick injection of metal precursors into high-temperature solutions leads to fast burst of small nuclei, followed by growth of the small nuclei into large nanocrystals with tunable sizes. Such nucleation-growth mechanism was later modified to synthesize nanocrystals of different chemical compositions, including metal, magnetic, and perovskite nanocrystals using colloidal synthesis [26,27,28]. Solid-state chemistry has also been used to synthesize nanoparticles in dry states or to chemically transform nanoparticles from one type to another, with either shape changes or composition changes [29]. A well-known reaction is calcination, a high-temperature treatment process for nanoparticle synthesis or modification in solid state [30]. The combination of precise control over nanoparticle formation and its further processing to more complex nanostructures makes nanoparticles highly accessible for different applications, from energy conversion to biomedical engineering and quantum engineering [31, 32].
In addition to chemical synthesis, characterization of nanoparticles is equally important but remains challenging in materials science. To synthesize nanoparticle with desirable properties, it is essential to understand nanoscale mass transportation, chemical transformation, and wave propagation in nanostructured materials. For example, studying the correlation between LSPR and nanoparticle structures requires thorough characterization of nanoparticle size, size distribution, and shapes [33,34,35]. Based on systematic study, many empirical and theoretical formula have been put forward to design nanostructures of desirable optical properties and to predict physical properties of complex nanostructures [36]. However, the small sizes and the dynamic nature of nanoparticles make it challenging to characterize the structures and capture chemical processes occurring involving nanoparticles. While engineers are pushing the imaging limits of electron microscopy down to atomic level, this characterization is widely performed in vacuum at ambient temperature, which is in totally different conditions compared with practical situations or real-time chemical reactions. The lack of in situ and operando characterization techniques makes it challenging to understand the formation mechanism of nanoparticles in many complex reactions and to evaluate many important chemical reactions involving nanoparticles as catalysts.
This snapshot review addresses recent advances in nanoparticle synthesis, in situ nanoparticle characterization, and operando techniques for nanoparticle catalytic reactions. Progress in nanoparticle preparation, including colloidal synthesis and solid-state methods, will be firstly discussed in this review. A few important synthetic approaches will be introduced for preparing metal, oxide, and metal-halide perovskite nanoparticles and heterostructures. Key reaction parameters will be elucidated on nanoparticle formation with controlled sizes, size distribution, shapes, compositions, and heterogeneity. The second part will be focused on advanced new in situ transmission electron microscopy (TEM) techniques for visualizing interfacial processes and surface instability of nanoparticles as well as nanocrystal nucleation events using high-speed or artificial intelligence-assisted TEM. New understanding on these important processes in nanoparticle synthesis will be discussed to elucidate how the emerging characterization techniques resolve existing puzzles during nanoparticle formation. As one of the most important applications, catalysis will be used as an example in the last part to overview combined operando techniques such as X-ray diffraction, TEM, and scanning probe techniques for understanding the roles and evolution of nanoparticle systems in catalyzing important chemical reactions. This review will be concluded with a summary of challenges in nanoparticle engineering and a perspective will be provided for its further advance to the next generation.
Recent advances in nanoparticle synthesis
Colloidal synthesis in solutions has been extensively used for preparing and transforming nanoparticles through chemical reactions. After a few decades’ development, a large number of different types of nanoparticles have been successfully made in colloidal solutions, including inorganic and organic nanoparticles with diverse shapes, sizes, compositions, and structures. Among these established nanomaterials, nanoframes or nanocages are challenging to make because of their highly porous and complex structures. The preparation requires multiple chemical steps for structural and compositional control, typically including synthesis of solid nanoparticles followed by chemical etching. In addition, biological materials such as DNA, RNA, peptides, and proteins can be assembled into superstructures, including nanocages, using the specific interactions between programmable biological materials [37,38,39,40,41,42,43]. In an interesting system, the peptide high-order structures could guide nanocrystal formation and anisotropic assembly into different hierarchical structures depending on molecular concentration [42]. At low concentration, Pt{100}-specific peptide has ST-turn configuration, leading to formation of cubic Pt nanocrystals. At high concentration, the peptide spontaneously transforms to a β-sheet, which drives the assembly of the Pt nanocubes into linear superstructures. Assembling nanoframes or nanocages is possible using DNA strands with pre-designed DNA sequences and stepwise assembly strategies [37]. The strategy to construct polyhedral nanocages is to firstly assemble DNA single strands into sticky-ended, three-point-arm motifs, and then construct polyhedral nanocages through sticky-end association. Synthesizing inorganic cages requires alternative structural control to form the nanocages or nanoframes. One typical approach is to use chemical etching or structural reconstruction of nanocrystals [44,45,46]. For amorphous inorganic nanoframes, like silica cages, a template-directed self-assembly method was introduced to realize structural control over small nanoparticles (Fig. 1) [47]. The templates are surfactant micelles, and silica precursor is introduced into the solution, forming 2-nm silica cluster through hydrolysis reaction. The negatively charged silica clusters are assembled on the positively charged micelles through electrostatic interactions into template-directed nanostructures. Nanocages with similar cage-like structures are also prepared by replacing silica with other inorganic materials, including Au, Ag, and vanadium oxide, making it a generalizable approach to nanocages of different chemical compositions.
Schematic illustration of projections from the reconstruction and cryo-EM images of a 3D dodecahedral cage. The scale bar is 10 nm. Reproduced with permission from [47]
While nanocages manifest structural complexity, heterostructures are complex nanomaterials that contain two or more separated composition domains. The simplest heterostructure consists of two domains separated by one defined interface, known as heterojunctions, across which the chemical compositions of the heterostructures change. To this end, semiconductor heterostructures are of particular interest due to the heterojunction-associated properties, in which the semiconductor domain (like perovskite nanocrystals) is bound with domains of another materials [48, 49]. A few typical examples of the secondary materials include metals [50], metal chalcogenide [51], metal dichalcogenide [52], metal oxides [53], and perovskite of different compositions [54]. Many of these heterostructures are synthesized through hetero-nucleation-growth method or epitaxy growth, with the use of primary component as seeds, followed by growth of the secondary components on the primary nanoparticles. For example, halide perovskite-lead chalcohalide nanocrystal heterostructures were reported in an epitaxial growth using CsPbBr3 nanoclusters as “seeds” to initiate growth of the lead chalcohalide (Fig. 2) [55]. The halide perovskite nanoparticles share an epitaxial interface and lead lattices of certain facets with the formed lead chalcohalide nanoparticles through an epitaxial growth pathway. The chemical compositions of the perovskite nanocrystals could be changed through ion exchange reactions, leading to a wealth of perovskite heterostructures of tunable components. Associated with the heterostructures are the component-dependent electronic structure changes of individual components, which represents a reliable way to engineer wave functions of nanocrystals and is not accessible in single-component materials. The resulting energy level alignment at the heterojunction enhances the splitting and trapping of photogenerated excitons: the photoexcited holes localized in the chalcohalide domain and the excited electrons partially delocalized in the entire nanostructures, leading to significant suppression of photoluminescence of the heterostructures.
Schematic illustration of the synthesis of the CsPbBr3-Pb4S3Br2 heterostructures. STEM images of the CsPbBr3-Pb4S3Br2 nanoparticles at two different magnifications. Reproduced with permission from [55]
Compared with colloidal synthesis, some types of gas-state synthesis like flame synthesis are capable of large-scale production of nanoparticles with potential control over particle structures. They can also produce heterostructures with large specific surface areas and abundant active sites, with supported metal catalysts being a typical example. Due to these advantages, gas-phase methods have been investigated and used for synthesizing nanostructured materials at elevated temperatures, including carbon blacks by pyrolysis of plant oils, fumed oxides, and metals [56, 57]. Therefore, they are broadly used in production and functionalization of consumer products, such as tires, paints, commercial photocatalysts, pharmaceutics, and microelectronics. Even though gas-phase synthesis as one aerosol technique is gas-to-particle or droplet-to-particle formation process, its working mechanism and reaction pathway are complicated because of the presence of simultaneous chemical and physical processes depending on reaction temperature and relative position to flames [58]. As shown in Fig. 3, a few important processes are outlined in the flame synthesis, including nucleation, surface growth, aggregation, coagulation, and agglomeration in the downstream of the synthesis [56]. Such reaction sequences produce fractal-like agglomerates of nanostructures of similar chemical compositions. During gas-to-particle transformation, a precursor gas or vapor is used as reactants and nucleation occurs in the gas phase, forming nanoparticles during surface growth. In the droplet-to-particle transformation, the initial nucleation occurs in a droplet containing all reactants, followed by formation of nanostructures through precipitation within the droplet into compact or porous nanostructures [59]. After nucleation as reaction proceeds, the reactants are moving away from the flame, leading to temperature decreases in the content. Inter-particle interactions are becoming obvious, leading to aggregation of dispersed nanoparticles and coagulation with coalescence. This sequence of events creates either solid or sinter-bonded nanostructures depending on coalescence.
Schematic illustration of the synthesis of catalytic materials in flames. Reproduced with permission from [56]
In situ TEM characterization
Compared with controlled synthesis of nanoparticles, characterization could be even more challenging due to the small size and dynamic nature of the nanostructured materials. Electron microscopy (EM), advanced optical microscopy, and some scanning probe techniques can image the structures and compositions of nanoparticles in atomic resolution. The combination of advanced imaging systems with data processing further extends the possibilities of these characterization methods, facilitating the understanding and development of nanoparticle formation and applications. Electron tomography and cryo-EM are exciting progresses in this regard for characterizing nanoparticles with complex and transformable structures [60,61,62]. However, characterizing transient and dynamic chemical processes faces additional challenges due to the vacuum conditions of electron microscopes. In situ TEM techniques are then developed to overcome these challenges and provide insightful understanding on phase transitions and chemical transformations of nanoparticles. Nucleation of nanocrystals, for example, is a typical transient process and generally involves mechanism of a spontaneous transition from disordered to crystalline states [63, 64]. However, understanding such a dynamic event is mainly theoretical. In classical nucleation theory, many solid crystalline materials nucleate through direct assembly of monomers by density fluctuations over the surface and volume free-energy barriers [65, 66]. In many molecular and nanoparticle crystallization, nonclassical nucleation is dominant and contains two-step nucleation mechanism, in which metastable intermediate clusters form and transform to more stable crystal phases to avoid high energy barrier of direct crystallization [67, 68]. This mechanism is typical and frequently observed in the crystallization of nanoparticles, small molecules, and biological molecules like proteins [69,70,71,72]. In experiments, the presence of disordered intermediates has been observed in solution-phase atomic crystallization, either on surfaces or in nanoparticles [73,74,75,76]. Considering the presence of multiple intermediate status with similar local energy minimum in single nucleation event, dynamic reconstruction is highly expected during deposition and rearrangement of reduced atoms in the early stages of crystallization. To solve the nucleation issues in crystallization, advanced TEM equipped with a scintillator-coupled camera featuring an electrostatic subframing system was developed to improve temporal resolution in imaging dynamic processes occurring on nanoparticles. A heterogeneous nucleation and growth of Au nanoparticles was used as a model system to study time-dependent nucleation process in nanoscale [67]. Interestingly, reversible disorder-order transitions were reported on the hetero-nucleation of Au nanoparticles on graphene, rather than a single phase transition (Fig. 4). Such structural fluctuation is a result of size-dependent thermodynamic stability of the ordered and disordered states. This high-speed atomic-resolution TEM imaging enables in situ observation of transient and dynamic events of nanoparticles and provides a powerful tool to understand chemical reactions and physical transitions occurring in nanoparticle systems.
In situ characterization of crystal nucleation on graphene via high-speed atomic-resolution TEM. Atomic models of the nanocrystals are shown beneath each frame on the left side. The areas highlighted in red and light-green represent Au lattices and gold(I) cyanide domains, respectively. Blue circles outline nanoclusters in a disordered state, and purple circles indicate undefinable states, in which the classification occurs. Reproduced with permission from [67]
In addition to the quick nucleation in nanoscale, in situ TEM is also capable of characterizing atomic-resolution structural evolution and chemical reactions occurring on nanoparticle surfaces [77]. These transformations are important because many particles’ formation and catalytic reactions involve complex ions, atoms, and molecules diffusion and migration at the surfaces of nanoparticles. This sequence of events determines the nanoparticle shapes through kinetic control or catalytic reactivity of nanoparticles, making it critically important to characterize these chemical transformations for nanomaterials engineering [78, 79]. One of the great challenges in this regard is to balance between spatial and temporal resolution in electron microscopes to capture transient processes in atomic resolution. That is, to understand structural evolution with improved time resolution, short exposure time is necessary to capture images in a relatively fast fashion but unfortunately reduces the quality of the images with low single-to-noise ratio. Therefore, developing techniques to mitigate the noises with improved image quality and high temporal resolution is becoming important. In addition to the development of hardware to solve the problem [80], artificial intelligence (AI) is introduced as a software toolset to improve image quality [81]. AI sets a potential pathway forward because of its power in image recognition and processing, which can be applied on electron microscopy images for denoising purposes. Supervised convolutional neural networks have been introduced to denoise TEM images for nanoparticles, which improves image resolution of initial TEM data [82, 83]. Taking catalytic nanoparticles as example, Pt nanoparticles decorated on CeO2 structures were introduced and imaged under an environmental TEM at varying temperatures and pressures [81, 84]. Figure 5a shows two initial TEM images of a small Pt nanoparticle acquired at a time interval of 0.2 s in a CO atmosphere at room temperature. In this raw data set, the presence of noises and low contrast make it difficult to analyze the structures and determine the location of nanoparticles in the dynamic process. After the images are regenerated by an unsupervised deep video denoising algorithm, the atomic structures of the nanoparticles and the location can be easily determined in Fig. 5b, which significantly improves the quality of the TEM images and provide precise information to understand the dynamic processes occurring on nanoparticle surfaces in a single-atom fashion.
Visualizing interfacial processes and surface instabilities in nanoparticle systems through in situ TEM and artificial intelligence. TEM images a before and b after denoising using an unsupervised deep video algorithm. Reproduced with permission from [81]
Operando techniques: a new era of materials science
Combining environmental TEM with other in situ characterization provide more powerful tools for understanding chemical processes, such as catalytic reactions, under operating conditions. These operando techniques have been developed recently for revealing dynamic structural and compositional changes of nanoparticles during catalysis, which advances our understanding of complex structural and phase transitions of nanoparticles under chemical and electrochemical conditions. Single nanoparticle X-ray imaging is developed to map dynamics of structural and strain changes of catalysts and represents as a promising way to decode active sites of nanoparticles in catalyzing important chemical reactions [85]. Among these X-ray techniques, coherent X-ray diffraction imaging has been developed as a reliable method to characterize structures of nanoparticles in nanometer resolution. More importantly, it allows construction of three-dimensional crystalline electron density and strain field of single nanoparticle in various reaction conditions, including some operating conditions [85,86,87]. In the case of PtRh alloy-based catalysts, the coherent X-ray diffraction was used to reconstruct the three-dimensional nanoparticle shape through reconstruction of electron density, and strain field of single alloy nanoparticle under different gas conditions (Fig. 6a). This operando X-ray technique produces precise facet types and orientations of the nanoparticles and the change of facets upon chemical reactions. Moreover, the surface strain evolution and nanoparticle composition changes during the catalytic reaction can also be in situ investigated simultaneously, making it possible to correlate these structural and compositional evolution to process of chemical reactions. As shown in Fig. 6b, it was found that under Ar flow conditions, only < 111 > facets exhibited an outward relaxation. Switching reaction conditions from reducing to CO oxidation leads to a reduction of the surface strain state. These facet-dependent structural and compositional heterogeneities are expected to elucidate catalytic reaction pathways, reactivity, and selectivity in many important catalytic reactions [85]. Combining a few operando characterization may provide multi-dimensional information on nanoparticle formation and transformation. In terms of electroreduction of CO2 into multi-carbon products, this combined technique is expected to provide insightful understanding of reaction mechanism of Cu nanoparticles in catalyzing the chemical transformation [88]. An operando electrochemical scanning transmission electron microscopy (STEM) was developed in this work to track dynamic shape and structural evolution of Cu nanocrystals and to analyze active sites of Cu nanoparticles. This technique consists of STEM cells containing a liquid pocket with a three-electrode configuration. Cu nanoparticles are deposited on carbon working electrode, coupled with Pt counter and Pt pseudoreference electrode, which allows measuring nanoscale morphology changes and crystallinity evolution of the Cu nanoparticles under catalytic conditions. At working potential, particle movement was initially observed, followed by nanoparticle aggregation and coalescence. After airflow, Cu nanoparticles were rapidly oxidized to form Cu2O nanoparticles with cubic shapes (Fig. 7). This correlated operando STEM study reveals that Cu nanoparticles are rich in grain boundary in catalytic conditions and support high-density active undercoordinated sites for enhancing selectivity of chemical reactions. This work further demonstrates that combining operando structural analysis with electron, X-ray probes and single-molecule spectroscopy will be promising to investigate dynamic and transient chemical reactions and evolutions of nanoparticles in reaction conditions.
Operando single alloy nanoparticle X-ray imaging during a catalytic reaction. a Top (left panels) and side view (right panels) of the reconstructed nanoparticle and b strain field mapping at the nanoparticle surface under different gas conditions. Reproduced with permission from [85]
Operando studies reveal active Cu nanograins for electrocatalysis. Conventional ex situ characterization methods provide limited information to the study of nanocatalysts during the catalytic reactions. The emerging operando methods reveal that Cu nanoparticles undergo structural transformation during which the surface oxide is reduced, ligands are disordered, and a progressive coalescence/aggregation leads to the formation of active metallic Cu nanograins. Reproduced with permission from [88]
Conclusion
In summary, this snapshot review summarizes recent progress in nanoparticle synthesis, in situ characterization, and emerging operando techniques for studying nanoscale mass transportation and chemical transformation. The development of new wet-chemistry and solid-state methods provides many exciting approaches to nanoparticles with controlled structures, precision compositions, and designer properties. The working principles in nanoparticle formation and their shape and composition control are elucidated in this review to explain how to prepare nanoparticles with designated structures and properties. In characterizing chemical and physical process of nanoparticles, some advanced TEM techniques have been introduced in this snapshot review to represent recent progresses in nanoparticle characterization including high-speed, atomic-resolution TEM and AI-assisted TEM imaging, making it possible to probe nucleation and dynamic interfacial evolution of nanoparticles in working conditions. The improved EM with assistance of AI significantly improve the spatial and temporal resolution of the nanoparticles, providing new understanding of nanoparticle nucleation and transformation that are not attainable based on traditional characterization techniques. In the last part, combined operando techniques have been developed to systematically study the catalytic reaction mechanism, dynamics, and pathways using nanoparticles as catalysts. Such correlated operando methods open the door to studying catalytic intermediates and structural evolution of nanoparticles in their working conditions. Compared with traditional methods, these emerging operando techniques provide multi-dimensional, correlated data simultaneously for elucidating chemical transformation and structural evolution of nanoparticles in real-time fashion. Based on the promise of these cutting-edge techniques and methods, it is reasonable to expect more operando methods to unravel the unknown fields of nanomaterials and nanoscience.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
C. Burda, X. Chen, R. Narayanan, M.A. El-Sayed, Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105, 1025 (2005)
Z. Li, W. Wang, Y. Yin, Colloidal assembly and active tuning of coupled plasmonic nanospheres. Trends Chem. 2, 593 (2020)
J. Zhao, S. Xue, R. Ji, B. Li, J. Li, Localized surface plasmon resonance for enhanced electrocatalysis. Chem. Soc. Rev. 50, 12070 (2021)
V. Amendola, R. Pilot, M. Frasconi, O.M. Maragò, M.A. Iatì, Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 29, 203002 (2017)
Z. Li, J. Jin, F. Yang, N. Song, Y. Yin, Coupling magnetic and plasmonic anisotropy in hybrid nanorods for mechanochromic responses. Nat. Commun. 11, 2883 (2020)
K.M. Mayer, J.H. Hafner, Localized surface plasmon resonance sensors. Chem. Rev. 111, 3828 (2011)
X. Zhang, Y.L. Chen, R.-S. Liu, D.P. Tsai, Plasmonic photocatalysis. Rep. Prog. Phys. 76, 046401 (2013)
Z. Li, N.V. Myung, Y. Yin, Light-powered soft steam engines for self-adaptive oscillation and biomimetic swimming. Sci. Robot. 6, eabi4523 (2021)
Z. Li, Z. Meng, F. Tian, Z. Ye, X. Zhou, X. Zhong, Q. Chen, M. Yang, Z. Liu, Y. Yin, Fast Fourier transform-weighted photoacoustic imaging by in vivo magnetic alignment of hybrid nanorods. Nano Lett. 22, 5158 (2022)
F.P. García de Arquer, D.V. Talapin, V.I. Klimov, Y. Arakawa, M. Bayer, E.H. Sargent, Semiconductor quantum dots: Technological progress and future challenges. Science 373, eaaz8541 (2021)
C.R. Kagan, L.C. Bassett, C.B. Murray, S.M. Thompson, Colloidal quantum dots as platforms for quantum information science. Chem. Rev. 121, 3186 (2020)
Z. Li, Q. Fan, Y. Yin, Colloidal self-assembly approaches to smart nanostructured materials. Chem. Rev. 122, 4976 (2021)
J. Zhang, Z. Li, Y. Bai, Y. Yin, Gold nanocups with multimodal plasmon resonance for quantum-dot random lasing. Appl. Mater. Today 26, 101358 (2022)
Y. Xia, Y. Xiong, B. Lim, S.E. Skrabalak, Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60 (2009)
S. Wang, S. Lee, J.S. Du, B.E. Partridge, H.F. Cheng, W. Zhou, V.P. Dravid, B. Lee, S.C. Glotzer, C.A. Mirkin, The emergence of valency in colloidal crystals through electron equivalents. Nat. Mater. 21, 580 (2022)
J.S. Du, W. Zhou, S.M. Rupich, C.A. Mirkin, Twin pathways: Discerning the origins of multiply twinned colloidal nanoparticles. Angew. Chem. 133, 6934 (2021)
Z. Li, J. Zhang, J. Jin, F. Yang, R. Aleisa, Y. Yin, Creation and reconstruction of thermochromic Au nanorods with surface concavity. J. Am. Chem. Soc. 143, 15791 (2021)
Z. Cai, Z. Li, S. Ravaine, M. He, Y. Song, Y. Yin, H. Zheng, J. Teng, A. Zhang, From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 50, 5898 (2021)
L. Xu, H.-W. Liang, Y. Yang, S.-H. Yu, Stability and reactivity: Positive and negative aspects for nanoparticle processing. Chem. Rev. 118, 3209 (2018)
B. Li, D. Zhou, Y. Han, Assembly and phase transitions of colloidal crystals. Nat. Rev. Mater. 1, 1 (2016)
M.A. Boles, M. Engel, D.V. Talapin, Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 116, 11220 (2016)
Z. Li, C. Qian, W. Xu, C. Zhu, Y. Yin, Coupling morphological and magnetic anisotropy for assembling tetragonal colloidal crystals. Sci. Adv. 7, eabh1289 (2021)
J. Feng, F. Yang, X. Wang, F. Lyu, Z. Li, Y. Yin, Self-aligned anisotropic plasmonic nanostructures. Adv. Mater. 31, 1900789 (2019)
F. Qi, L. Li, Z. Li, L. Qiu, Z. Meng, Y. Yin, Magnetic/plasmonic hybrid nanodisks with dynamically tunable mechano-chiroptical responses. ACS Nano 17, 1427 (2023)
Y. Yin, A.P. Alivisatos, Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437, 664 (2005)
V. Pareek, A. Bhargava, R. Gupta, N. Jain, J. Panwar, Synthesis and applications of noble metal nanoparticles: A review. Adv. Sci. Eng. Med. 9, 527 (2017)
N.A. Frey, S. Peng, K. Cheng, S. Sun, Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 38, 2532 (2009)
S.A. Kulkarni, S.G. Mhaisalkar, N. Mathews, P.P. Boix, Perovskite nanoparticles: Synthesis, properties, and novel applications in photovoltaics and LEDs. Small Methods 3, 1800231 (2019)
W.H. Eisa, A.M. Abdelgawad, O.J. Rojas, Solid-state synthesis of metal nanoparticles supported on cellulose nanocrystals and their catalytic activity. ACS Sustain. Chem. Eng. 6, 3974 (2018)
M.G. Kim, J.M. Kang, J.E. Lee, K.S. Kim, K.H. Kim, M. Cho, S.G. Lee, Effects of calcination temperature on the phase composition, photocatalytic degradation, and virucidal activities of TiO2 nanoparticles. ACS Omega 6, 10668 (2021)
F. Lyu, Y. Bai, Z. Li, W. Xu, Q. Wang, J. Mao, L. Wang, X. Zhang, Y. Yin, Self-templated fabrication of CoO–MoO2 nanocages for enhanced oxygen evolution. Adv. Func. Mater. 27, 1702324 (2017)
M.A. Green, A. Ho-Baillie, H.J. Snaith, The emergence of perovskite solar cells. Nat. Photonics 8, 506 (2014)
J. Olson, S. Dominguez-Medina, A. Hoggard, L.-Y. Wang, W.-S. Chang, S. Link, Optical characterization of single plasmonic nanoparticles. Chem. Soc. Rev. 44, 40 (2015)
Z. Li, Q. Fan, C. Wu, Y. Li, C. Cheng, Y. Yin, Magnetically tunable plasmon coupling of Au nanoshells enabled by space-free confined growth. Nano Lett. 20, 8242 (2020)
Z. Li, Q. Fan, Z. Ye, C. Wu, Z. Wang, Y. Yin, A magnetic assembly approach to chiral superstructures. Science 380, 1384 (2023)
N. Liu, M. Hentschel, T. Weiss, A.P. Alivisatos, H. Giessen, Three-dimensional plasmon rulers. Science 332, 1407 (2011)
Y. He, T. Ye, M. Su, C. Zhang, A.E. Ribbe, W. Jiang, C. Mao, Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 452, 198 (2008)
S.M. Douglas, H. Dietz, T. Liedl, B. Högberg, F. Graf, W.M. Shih, Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414 (2009)
K.A. Afonin, E. Bindewald, A.J. Yaghoubian, N. Voss, E. Jacovetty, B.A. Shapiro, L. Jaeger, In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat. Nanotechnol. 5, 676 (2010)
N.P. King, W. Sheffler, M.R. Sawaya, B.S. Vollmar, J.P. Sumida, I. André, T. Gonen, T.O. Yeates, D. Baker, Computational design of self-assembling protein nanomaterials with atomic level accuracy. Science 336, 1171 (2012)
Y. Hsia, J.B. Bale, S. Gonen, D. Shi, W. Sheffler, K.K. Fong, U. Nattermann, C. Xu, P.-S. Huang, R. Ravichandran, Design of a hyperstable 60-subunit protein icosahedron. Nature 535, 136 (2016)
E. Zhu, S. Wang, X. Yan, M. Sobani, L. Ruan, C. Wang, Y. Liu, X. Duan, H. Heinz, Y. Huang, Long-range hierarchical nanocrystal assembly driven by molecular structural transformation. J. Am. Chem. Soc. 141, 1498 (2018)
E. Zhu, X. Yan, S. Wang, M. Xu, C. Wang, H. Liu, J. Huang, W. Xue, J. Cai, H. Heinz, Peptide-assisted 2-D assembly toward free-floating ultrathin platinum nanoplates as effective electrocatalysts. Nano Lett. 19, 3730 (2019)
S.E. Skrabalak, J. Chen, Y. Sun, X. Lu, L. Au, C.M. Cobley, Y. Xia, Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 41, 1587 (2008)
C. Chen, Y. Kang, Z. Huo, Z. Zhu, W. Huang, H.L. Xin, J.D. Snyder, D. Li, J.A. Herron, M. Mavrikakis, Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339 (2014)
Z. Niu, N. Becknell, Y. Yu, D. Kim, C. Chen, N. Kornienko, G.A. Somorjai, P. Yang, Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts. Nat. Mater. 15, 1188 (2016)
K. Ma, Y. Gong, T. Aubert, M.Z. Turker, T. Kao, P.C. Doerschuk, U. Wiesner, Self-assembly of highly symmetrical, ultrasmall inorganic cages directed by surfactant micelles. Nature 558, 577 (2018)
S. Bera, N. Pradhan, Perovskite nanocrystal heterostructures: Synthesis, optical properties, and applications. ACS Energy Lett. 5, 2858 (2020)
P.V. Kamat, N. Pradhan, K. Schanze, P.S. Weiss, J. Buriak, P. Stang, T.W. Odom and G. Hartland: Challenges and Opportunities in Designing Perovskite Nanocrystal Heterostructures (ACS Publications, 2020).
S.K. Balakrishnan, P.V. Kamat, Au–CsPbBr 3 hybrid architecture: Anchoring gold nanoparticles on cubic perovskite nanocrystals. ACS Energy Lett. 2, 88 (2017)
X. Zhang, X. Wu, X. Liu, G. Chen, Y. Wang, J. Bao, X. Xu, X. Liu, Q. Zhang, K. Yu, Heterostructural CsPbX3-PbS (X= Cl, Br, I) quantum dots with tunable Vis–NIR dual emission. J. Am. Chem. Soc. 142, 4464 (2020)
X.-D. Wang, Y.-H. Huang, J.-F. Liao, Y. Jiang, L. Zhou, X.-Y. Zhang, H.-Y. Chen, D.-B. Kuang, In situ construction of a Cs2SnI6 perovskite nanocrystal/SnS2 nanosheet heterojunction with boosted interfacial charge transfer. J. Am. Chem. Soc. 141, 13434 (2019)
Z.J. Li, E. Hofman, J. Li, A.H. Davis, C.H. Tung, L.Z. Wu, W. Zheng, Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 28, 1704288 (2018)
D. Baranov, G. Caputo, L. Goldoni, Z. Dang, R. Scarfiello, L. De Trizio, A. Portone, F. Fabbri, A. Camposeo, D. Pisignano, Transforming colloidal Cs4PbBr6 nanocrystals with poly (maleic anhydride-alt-1-octadecene) into stable CsPbBr3 perovskite emitters through intermediate heterostructures. Chem. Sci. 11, 3986 (2020)
M. Imran, L. Peng, A. Pianetti, V. Pinchetti, J. Ramade, J. Zito, F. Di Stasio, J. Buha, S. Toso, J. Song, Halide perovskite–lead chalcohalide nanocrystal heterostructures. J. Am. Chem. Soc. 143, 1435 (2021)
G.A. Kelesidis, S.E. Pratsinis, A perspective on gas-phase synthesis of nanomaterials: Process design, impact and outlook. Chem. Eng. J. 421, 129884 (2021)
R. Koirala, S.E. Pratsinis, A. Baiker, Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 45, 3053 (2016)
S.E. Pratsinis, Vapour synthesis of ceramic powders. Ceram. Trans. 12, 227 (1990)
G.L. Messing, S.C. Zhang, G.V. Jayanthi, Ceramic powder synthesis by spray pyrolysis. J. Am. Ceram. Soc. 76, 2707 (1993)
T. Ni, T. Frosio, L. Mendonça, Y. Sheng, D. Clare, B.A. Himes, P. Zhang, High-resolution in situ structure determination by cryo-electron tomography and subtomogram averaging using emClarity. Nat. Protoc. 17, 421 (2022)
F. Eisenstein, H. Yanagisawa, H. Kashihara, M. Kikkawa, S. Tsukita, R. Danev, Parallel cryo electron tomography on in situ lamellae. Nat. Methods 20, 131 (2023)
K.M. Yip, N. Fischer, E. Paknia, A. Chari, H. Stark, Atomic-resolution protein structure determination by cryo-EM. Nature 587, 157 (2020)
J.J. De Yoreo, P.U. Gilbert, N.A. Sommerdijk, R.L. Penn, S. Whitelam, D. Joester, H. Zhang, J.D. Rimer, A. Navrotsky, J.F. Banfield, Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760 (2015)
D. Erdemir, A.Y. Lee, A.S. Myerson, Nucleation of crystals from solution: Classical and two-step models. Acc. Chem. Res. 42, 621 (2009)
J. Chen, E. Zhu, J. Liu, S. Zhang, Z. Lin, X. Duan, H. Heinz, Y. Huang, J.J. De Yoreo, Building two-dimensional materials one row at a time: Avoiding the nucleation barrier. Science 362, 1135 (2018)
M. Sleutel, J. Lutsko, A.E. Van Driessche, M.A. Durán-Olivencia, D. Maes, Observing classical nucleation theory at work by monitoring phase transitions with molecular precision. Nat. Commun. 5, 5598 (2014)
S. Jeon, T. Heo, S.-Y. Hwang, J. Ciston, K.C. Bustillo, B.W. Reed, J. Ham, S. Kang, S. Kim, J. Lim, Reversible disorder-order transitions in atomic crystal nucleation. Science 371, 498 (2021)
J.S. Du, Y. Bae, J.J. De Yoreo: Non-classical crystallization in soft and organic materials. Nat. Rev. Mater., 1 (2024)
T.H. Zhang, X.Y. Liu, Nucleation: What happens at the initial stage? Angew. Chem. Int. Ed. 48, 1308 (2009)
Z. Ou, Z. Wang, B. Luo, E. Luijten, Q. Chen, Kinetic pathways of crystallization at the nanoscale. Nat. Mater. 19, 450 (2020)
P.R.T. Wolde, D. Frenkel, Enhancement of protein crystal nucleation by critical density fluctuations. Science 277, 1975 (1997)
M.H. Nielsen, S. Aloni, J.J. De Yoreo, In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 345, 1158 (2014)
N.D. Loh, S. Sen, M. Bosman, S.F. Tan, J. Zhong, C.A. Nijhuis, P. Král, P. Matsudaira, U. Mirsaidov, Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 9, 77 (2017)
J. Yang, J. Koo, S. Kim, S. Jeon, B.K. Choi, S. Kwon, J. Kim, B.H. Kim, W.C. Lee, W.B. Lee, Amorphous-phase-mediated crystallization of Ni nanocrystals revealed by high-resolution liquid-phase electron microscopy. J. Am. Chem. Soc. 141, 763 (2019)
L. Fei, X. Gan, S.M. Ng, H. Wang, M. Xu, W. Lu, Y. Zhou, C.W. Leung, C.-L. Mak, Y. Wang, Observable two-step nucleation mechanism in solid-state formation of tungsten carbide. ACS Nano 13, 681 (2018)
J. Zhou, Y. Yang, Y. Yang, D.S. Kim, A. Yuan, X. Tian, C. Ophus, F. Sun, A.K. Schmid, M. Nathanson, Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 570, 500 (2019)
S. Rasouli, D. Myers, K. Higashida, N. Nakashima, P. Crozier, P. Ferreira, Electrochemical evolution of fuel cell platinum nanocatalysts on carbon nanotubes at the atomic scale. ACS Appl. Energy Mater. 6, 11861 (2023)
P. Haluai, M.R. McCartney, P.A. Crozier, Studying Charge Redistribution in Photocatalytic Nanoparticles Using In Situ Light Illumination Coupled with Electron Holography (Oxford University Press, Oxford, 2023)
M. Tan, M. Leibovich, C. Fernandez-Granda, P.A. Crozier, In Situ Study of Surface Oxygen Exchange and Transport on Ceria at Different Temperatures (Oxford University Press, Oxford, 2023)
K. Koo, Z. Li, Y. Liu, S.M. Ribet, X. Fu, Y. Jia, X. Chen, G. Shekhawat, P.J. Smeets, R. Dos Reis, Ultrathin silicon nitride microchip for in situ/operando microscopy with high spatial resolution and spectral visibility. Sci. Adv. 10, eadj6417 (2024)
P.A. Crozier, A.M. Morales, M. Leibovich, S. Mohan, P. Haluai, M. Tan, J. Vincent, A. Gilankar, Y. Wang, C. Fernandez-Granda, The Impact of Artificial Intelligence on In Situ Electron Microscopy (Oxford University Press, Oxford, 2023)
J.L. Vincent, R. Manzorro, S. Mohan, B. Tang, D.Y. Sheth, E.P. Simoncelli, D.S. Matteson, C. Fernandez-Granda, P.A. Crozier, Developing and evaluating deep neural network-based denoising for nanoparticle TEM images with ultra-low signal-to-noise. Microsc. Microanal. 27, 1431 (2021)
S. Mohan, J.L. Vincent, R. Manzorro, P. Crozier, C. Fernandez-Granda, E. Simoncelli, Adaptive denoising via gaintuning. Adv. Neural. Inf. Process. Syst. 34, 23727 (2021)
J.L. Vincent, P.A. Crozier, Atomic level fluxional behavior and activity of CeO2-supported Pt catalysts for CO oxidation. Nat. Commun. 12, 5789 (2021)
Y.Y. Kim, T.F. Keller, T.J. Goncalves, M. Abuin, H. Runge, L. Gelisio, J. Carnis, V. Vonk, P.N. Plessow, I.A. Vartaniants, Single alloy nanoparticle x-ray imaging during a catalytic reaction. Sci. Adv. 7, eabh0757 (2021)
D. Kim, M. Chung, S. Kim, K. Yun, W. Cha, R. Harder, H. Kim, Defect dynamics at a single Pt nanoparticle during catalytic oxidation. Nano Lett. 19, 5044 (2019)
D. Kim, M. Chung, J. Carnis, S. Kim, K. Yun, J. Kang, W. Cha, M.J. Cherukara, E. Maxey, R. Harder, Active site localization of methane oxidation on Pt nanocrystals. Nat. Commun. 9, 3422 (2018)
Y. Yang, S. Louisia, S. Yu, J. Jin, I. Roh, C. Chen, M.V. Fonseca Guzman, J. Feijóo, P.-C. Chen, H. Wang, Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262 (2023)
Acknowledgments
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z. A snapshot review of nanostructure synthesis and operando characterization. MRS Advances 9, 1067–1076 (2024). https://doi.org/10.1557/s43580-024-00861-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-024-00861-w