Abstract
Chitosans I and II were extracted from shrimp shells using two different methodologies that consist of three main treatments: deproteinization, demineralization, and deacetylation under microwave irradiation; Chitosan I was underwent a fourth treatment (decolorization). The methods that were proposed use fairly high concentrations of NaOH and HCl, but the time duration for such extractions is shorter compared to conventional methods. The Chitosans samples obtained by these procedures were characterized by Fourier Transform Infra-Red Spectroscopy, Scanning Electron Microscopy, X-ray diffraction, and Thermogravimetry. The experimental results show that the degree of deacetylation is higher for Chitosan II since the degree of deacetylation obtained after deproteinizing the shrimp shells with NaOH 3 N, demineralizing the product of the previous step with HCl 2 N and deacetylating the Chitin with a 45% NaOH solution with microwave-assisted synthesis for 3.5 min is 88.94, quite close to that of commercial Chitosan.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitin is a natural polymer abundant in nature [1], from which it is possible to obtain Chitosan [2]. Chitosan is a polysaccharide that belongs to the amino group, which is produced from the deacetylation of Chitin obtained from crustaceans, fungi, and insects [3]. Chitosan is characterized as a polymer that is easily bioavailable, renewable, and biodegradable [4] and has biological, bacteriostatic, and fungistatic activity [5,6,7]. This polymer has diverse applications that range from the pharmaceutical industry [8], dental surgery [9], and water treatment [10] to the cosmetics industry [11] and textiles [12].
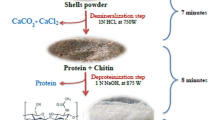
Chitosan extraction can be carried out in three steps: demineralization, deproteinization, and deacetylation; however, some authors include a fourth one (decolorization) [13]. Deproteinization is usually carried out using strong bases, while demineralization is usually carried out using strong acids. However, in most of the conventional methods that have been described by various authors for these steps, the duration time is usually quite long for the deproteinization (2–24 h) [14,15,16] and demineralization (30 m-24 h) [14,15,16] processes. Therefore, two rapid methods to obtain Chitin are proposed further on.
Deacetylation, on the other part, implies the N-deacetylation of Chitin [17] and is the most time-consuming step of all to obtain Chitosan with a considerable degree of deacetylation (DD) since it requires not only a highly concentrated sodium hydroxide solution (40–60% w/v) but also high temperature and pressure. Different methods such as high-temperature alkali treatment [18], high-temperature alkali treatment with intermittent water washing [19], use of water-miscible organic solvents [20], and enzymatic N-deacetylation [21] have been described for this process. Nevertheless, the duration time is commonly 1–8 h [18,19,20,21]. Nonetheless, the use of microwave-assisted synthesis has taken great interest and importance nowadays, since it has managed to accelerate the speed of the reactions, reducing very significantly some of the extensive time that used to be involved in the processes [22].
The methodology explained below was developed taking as a reference the methodology proposed by Dompeipen et al. [14] and Ramadhan et al. [15], in which the aim was to reduce the duration time of the demineralization and deproteinization steps, using different high concentrations of NaOH and HCl for Chitosan I and Chitosan II. On the other side, for the deacetylation step, the methodology proposed by Peniston and Johnson [22] was used as a reference, to which the microwave-assisted synthesis times were adjusted according to the microwave conditions used for this process.
Materials and methods
Material
Litopenaeus vannamei shrimp shells were collected from the port of Sisal, Yucatán, Mexico. Sodium hydroxide was purchased from Sigma Aldrich, hydrochloric acid was purchased from Fermont, and acetone was purchased from J.T Baker.
Methods
Preparation of raw material (shrimp shells)
40 g of shrimp shells was washed carefully with tap water to remove as much protein as possible and then dried in a convection oven to a constant weight. Finally, 20 g of the shrimp shells was weighed, ground to a fine powder, and placed in a container (for Chitosan I); the other 20 g was also weighed, ground to a fine powder, and placed in another container (for Chitosan II).
Deproteinization
Procedure for Chitosan I
The 20 g of shrimp shells powder was transferred to a 500-mL beaker and a 1:10 (w/v) ratio of 4 N NaOH solution was added to it. The mixture was stirred and heated at 60 °C for one hour, then filtered, washed with distilled water to neutral pH, and dried at 60 °C in a drying oven to constant weight [14,15,16].
Procedure for Chitosan II
The other 20 g of shrimp shells powder was transferred to a 500-mL beaker and added to a 1:10 (w/v) ratio of 3 N NaOH solution. The mixture was stirred and heated at 60 °C for one hour, then filtered, washed with distilled water to neutral pH, and dried at 60 °C in a drying oven to constant weight [14,15,16].
Demineralization
Procedure for Chitosan I
The product obtained in the previous step (deproteinization with NaOH 4 N) was transferred to a 500-mL volumetric flask, and a 3 N HCl solution was added in a 1:15 (w/v) ratio. The mixture was stirred and heated at 65 °C for half an hour, then filtered, neutralized with distilled water, and dried at 60 °C in a drying oven to constant weight [14,15,16].
Procedure for Chitosan II
The product obtained in the previous step (deproteinization with NaOH 3 N) was transferred to a 500-mL volumetric flask, and a 2 N HCL solution was added in a 1:15 (w/v) ratio. The mixture was stirred and heated at 65 °C for half an hour, then filtered, neutralized with distilled water, and dried at 60 °C in a drying oven to constant weight [14,15,16].
Decolorization
For this step, the product obtained (Chitin) in the previous step (demineralization with 3 N HCl) was transferred to a 500-mL beaker, and acetone was added in a 1:10 ratio (w/v) [23]. The mixture was stirred for 1 day, then filtered, neutralized with distilled water, and dried at 60 °C in a drying oven to constant weight. However, for the product obtained (Chitin) after deproteinization with 2 N HCl, the decolorization step was omitted.
Deacetylation
In this step, both the decolorized and non-decolorized Chitin were transferred to 250-mL conical flasks, respectively. Then, 62 mL of 45% w/v NaOH solution was added to the flask containing the non-decolorized Chitin and mixed; 62 mL of 45% w/v NaOH solution was added to the flask containing the decolorized Chitin and mixed. Subsequently, the two conical flasks were placed in the center of the turntable of the microwave oven and irradiated for 3.5 min at 1800 watts [22]. The obtained products (Chitosan) were filtered, washed with distilled water to neutral pH, and dried in a drying oven at 60 °C to constant dry weight.
Fourier Transform Infrared (FT-IR) analysis
For each sample analysis, 0.200 g of potassium bromide (KBr) and 0.005 g of Chitosan were ground, and then both powders were mixed into a fine powder using an agate mortar and pestle. Afterward, the powder was carefully poured into the sample holder of the Smart Collector accessory and scanned with a resolution of 1 cm−1 in a frequency region of 4000–400 cm−1 using a Thermo Nicolet 6700 FT-IR spectrophotometer, and the characteristic peaks of the IR transmission spectra were recorded. In addition, the degree of deacetylation (DD) was calculated from FT-IR spectra using the following equation [24]:
where A1655 represents the amide I vibration; A3450 is the OH vibration; and 1.33 is the constant of the ratio of A1655/A3450 (N-acetylated Chitosan).
Scanning Electron Microscopy analysis
In each sample analysis, 0.005 g of Chitosan powder was mounted on the sample holder of the equipment, and a gold–palladium coating was applied for 35 s using the sputtering metallizer and Q150R evaporator. Consequently, scanning electron microscopy analysis was performed using the JEOL JSM-7600F field-emission SEM equipment to obtain an elemental analysis of surfaces [using energy-dispersive spectroscopy (EDS)], and images (using the LABE and SE-I detector) of the Chitosan samples to observe their shape, size, composition, and surface morphology. The aforementioned was performed with an accelerating voltage of 15 kV, a magnification of 5000x, and a resolution of 0.615 nm.
X-Ray Diffraction (XRD) analysis
The samples of Chitosan powder (0.02 g) were ground, spread on the sample stage, and underwent to XRD using a Bruker D8 Advance X-ray diffractometer under the following operating conditions: 40 kV and 30 mA with Cu-Kα1 at λ 1.5418 Å radiation; primary grating of 0.5 mm and a secondary grating of 5 mm, step time of 0.5 s, and step size of 0.02 degrees. Relative intensity was recorded over the scattering range (2θ) of 3–60° in steps of 0.04°.
Thermogravimetry analysis
Samples of Chitosan powder (~ 0.02 g) were weighed using a tared balance and were sealed in sandwich form. Subsequently, thermogravimetric analysis was carried out using TGA 5500 equipment under the following operating conditions: heating from 30 to 600 °C at a heating rate of 10 °C min−1 with a dry nitrogen atmosphere.
Results and discussion
Currently, the extraction of Chitosan has become increasingly important, and several methods have been proposed for this purpose since Chitosan is a biopolymer that offers a wide variety of applications and can be extracted from various sources such as crustaceans and insects. However, some of the methods that have been suggested are too time-consuming to be performed, which is why an increasing number of attempts have been made in recent years to reduce the time taken to extract Chitosan from shrimp shells.
The use of chemical reagents with high normal values greatly reduces the duration times of each of the steps necessary to obtain Chitosan. Even so, great care must be taken, since some of them, being too high, react very violently with the raw material. One of the great advantages of working with chemical reagents that have high normal values is obtaining concise results in less time. In contrast to chemical reactions, microwave-assisted synthesis is a process that offers considerably shorter reaction times, resulting in higher yields.
Chitosan extraction can be finally summarized in three steps: deproteinization, demineralization, and deacetylation. The decolorization step was performed exclusively for the purpose of obtaining a whiter Chitin and Chitosan sample.
On the other part, when the deproteinization process occurs, the rupture of the chemical bonds present in the proteins of the shrimp shells is originated. Sodium hydroxide (NaOH) tends to depolymerize and degrade Chitin [25]. Demineralization, on the other side, is a process in which an acid is utilized to remove minerals such as calcium carbonate and calcium phosphate [26].
The formation of Chitosan I and Chitosan II was verified utilizing FT-IR spectroscopy (Fig. 1) and a sample of commercial Chitosan of the Sigma Aldrich brand was taken as a reference, in which the characteristic bands of this product were observed. The first bands at ~ 1659 and ~ 1660 cm−1 can be attributed to the vibration of NH bend and largely indicate that there was an elimination of the acetyl group and the bands at ~ 3441 and ~ 3443 cm−1 can be attributed to the vibration of the OH.
The Chitosan formation was also confirmed by XRD analysis (Fig. 2). Where the prominent characteristic diffraction peaks of the Chitosan powder crystals are observed at 9.4° and 19.32–20.05°.
The obtained Chitosans (I and II) and the commercial Chitosan were analyzed with the SEI, EDS, and LABE detectors of the SEM (Fig. 3) to compare their surface morphology and composition.
Finally, the TGA curves of the Chitosan samples were plotted at two main stages of decomposition. The first was at 100 °C with a mass loss of 5.1% for Chitosan I, 4.8% for Chitosan II, and 11.6% for commercial Chitosan. Such loss is due to the evaporation of H2O molecules [27]. The second was variable, at 300 °C with a loss of 46.1% for Chitosan I, at 350 °C with a loss of 47% for Chitosan II, and at 300 °C with a loss of 47.2% for commercial Chitosan. This loss was caused by the depolymerization of the Chitosan chains [28]. Additionally, the details of the TGA are shown in the table below (Table 1), which also includes the degree of deacetylation and elemental analysis of the samples by EDS.
Conclusion
The study confirms that the proposed extraction method for Chitosan II involves less time duration (1 h and 33.5 min) than conventional methods (4–11 h) without considering the washing, drying, and neutralizing time, only considering the time required for the reaction of the raw material with the chemical reagents. Based on the information obtained from FTIR, XRD, SEM, TGA, and DD characterization, it is concluded that the Chitosan II has shown similar performance to commercial Chitosan.
References
T. Uragami, S. Tokura, Material science of chitin and chitosan (Springer, Berliin, 2006), pp.1–2
H. Hernández-Cocoletzi, E. Almanza, et al., Obtaining and characterization of chitosan from shrimp exoskeletons. (Scielo, Surfaces and Vacuum, 2009), https://www.scielo.org.mx/pdf/sv/v22n3/v22n3a12.pdf. Accessed 16 October 2022
D. Sánchez, J. López et al., NVNM. (2019). https://doi.org/10.1016/b978-0-12-812491-8.00064-3
A. Sahu, P. Goswami, U. Bora, Mater Med. (2009). https://doi.org/10.1007/s10856-008-3549-4
T. Uragami, S. Tokura, Material science of chitin and chitosan (Springer, Berlin, 2006), pp.195–207
S. Roller, N. Covill, Int. J. Food Microbiol. (1999). https://doi.org/10.1016/s0168-1605(99)00006-9
H. No, N. Park et al., Int. J. Food Microbiol. (2002). https://doi.org/10.1016/s0168-1605(01)00717-6
L. Donaruma, R. Ottenbrite, O. Vogl, Anionic polymeric drugs (Wiley, Hoboken, 1980)
T. Uragami, S. Tokura, Material science of chitin and chitosan (Springer, Berlin, 2006), pp.238–241
C. Elson, D. Davies, E. Hayes, Water Res. (1980). https://doi.org/10.1016/0043-1354(80)90190-6
T. Uragami, S. Tokura, Material science of chitin and chitosan (Springer, Berlin, 2006), pp.258–261
E. Pascual, M.R. Julia, J. Biotechnol. (2001). https://doi.org/10.1016/s0168-1656(01)00311-x
A. Ibram, A. Lonescu, E. Cadar, Comparison of extraction methods of chitin and chitosan from different sources. Eur. J. Med. Sci. (2019). https://doi.org/10.26417/688wvv48e
E.J. Dompeipen, M. Kaimudin et al., Isolasi Kitin dan Kitosan dari Limbah Kulit Udang. Majalah BIAM 092(1), 32–39 (2016)
L.O.A.N. Ramadhan, C.L. Radiman et al., Deasetilasi Kitin secara Bertahap dan Pengaruhnya terhadap Derajat Deasetilasi serta Massa molekul Kitosan. Jurnal Kimia Indonesia 5, 17–21 (2010)
A. Patria, Production and characterization of Chitosan from shrimp shells waste. AACL Bioflux 6(4), 339–344 (2013)
T. Uragami, S. Tokura, Material science of chitin and chitosan (Springer, Berlin, 2006), pp.33–44
A. Castelli, L. Bergamasco et al., Some insights into the kinetics of non-conventional alkaline deacetylation of chitin. Adv. Chitin Sci. 1, 198–203 (1996)
S. Mima, M. Miya et al., J. Appl. Polym. Sci. (1983). https://doi.org/10.1002/app.1983.070280607
I. Batista, G. Roberts, Makromol Chem. (1990). https://doi.org/10.1002/macp.1990.021910217
A. Martinou, D. Kafetzopoulos, V. Bouriotis, Carbohydr. Res. (1995). https://doi.org/10.3390/md13031133
Q.T. Peniston and E.L. Johnson. (1979) Process for activating chitin by microwave treatment and improved activated chitin product. Patent USPTO 4159932
D.D. Salman, W.S. Ulaiwi, A. Qais, Preparation of chitosan from Iraqi shrimp shell by autoclave, studying some physicochemical properties and antioxidant activity. J. Pharm. Sci. Res. 10, 3120–3123 (2018)
J. Domszy, G. Robert, Evaluation of infrared spectroscopic techniques for analyzing chitosan. Macromol. Chem. Phys. 186, 1671–1677 (1985)
J. Wong, R. Tyagi, A. Pandey, Current developments in biotechnology and bioengineering (Elsevier, Amsterdam, 2016), pp.305–371
A. Khanafari, R. Marandi, S. Sanati, Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran J. Environ. Sci. Eng. 5, 19–24 (2008)
S. Erdogan, M. Kaya, Int. J. Biol. Macromol. (2016). https://doi.org/10.1016/j.ijbiomac.2016.04.059
M. Ziegler-Borowska, D. Chełminiak, H. Kaczmarek, J. Therm. Anal. Calorim. (2015). https://doi.org/10.1007/s10973-014-4122-7
Acknowledgments
Thanks to Dr. Daniel Hernández P. for the advice and knowledge provided; Dr. Patricia Quintana for access to LANNBIO; M.C. Daniel Treviño for obtaining the diffractograms; and William González for the acquisition of TGA data. The XRD analyses were performed at the National Laboratory of Nano and Biomaterials, Cinvestav-IPN; funded by projects FOMIX-Yucatán 2008-108160, CONACYT LAB-2009-01-123913, 292692, 294643, 188345, and 204822.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work and the authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toledo Ruíz, O.V., Azamar Barrios, J.A. Comparison between two Chitosans extracted from shrimp shells using two different methods versus the commercial Chitosan. MRS Advances 8, 519–524 (2023). https://doi.org/10.1557/s43580-023-00512-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-023-00512-6