Abstract
Variation of the conditions in the synthesis of thin films of CdS is presented. Using a chemical bath deposition (CBD) methodology, parameters such as the ratio of the molar concentration of cadmium and sulfur ions, pH, and temperature were modified in deposition times of 120 min and on glass substrates. Low Cd/S molar ratios favor thin film thickness. This is verified by optical characterization by a comparison of the transmission spectra. It was also found that the possible tuning of the gap energy is in the range of 2.52 to 2.63 eV, controlling the Cd/S molar ratio.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the high demand for products of the electronics industry that exists today, components based on semiconductor materials have become a priority technological need [1]. Semiconductor materials are a type of advanced ceramics, which are of high interest for the design of electronic components such as resistors, transistors, and photovoltaic structures. Conventional manufacturing technologies in the electronics industry include highly sophisticated techniques, such as sputtering, chemical vapor deposition, and plasma deposition, among others [2, 3]. These synthesis and processing techniques require high temperatures and pressures, resulting in a significant environmental impact.
Chemical bath deposition (CBD) technique can be a sustainable option for manufacturing of semiconductor thin films, through CBD methods, thin films of several materials can be obtained at a very low cost, with very good characteristics for applications in semiconductor devices such as photodiodes, LDR photoresistors, and the window layer of the polycrystalline solar cell [4, 5].
The CBD technique is based on the controlled precipitation of a material that is deposited homogeneously on the substrate of various materials. The substrates are immersed in a chemical solution, with specific conditions of pH, temperature, concentration, and deposition time that controls the kinetic growth and the thickness of films [6].
There are multiple investigations on CdS thin films synthesized by DBQ that report their main properties like this: the forbidden bandgap at room temperature between typical values of 2.57 and 2.40 eV, high absorption coefficient, absorbs up to 63% of incident radiation with photon energy greater than bandgap energy, and hexagonal and cubic structures [7]. It has been proposed that the properties of the CdS films can also be associated with the conditions of the growth reaction. Factors such as temperature and pH have been studied extensively, but little has been discussed about the influence on the proportion of the precursors of the material to be synthesized.
In this work, a methodology was developed that allowed to observe the influence of the main DBQ parameters on the growth kinetics and on the optical properties of the CdS films. The composition of the baths was studied, analyzing how it affects the molar proportion of cadmium and sulfur ions; this aspect of composition is something that has been extraordinarily little studied by other authors. Likewise, to identify additional improvements in the proposed methodology, some other variable conditions of pH and reaction temperature were analyzed.
Experimental detail
Tables 1 and 2 report the compositions of the CBD reactors used in this work.
The deposit times for the films were 15, 30, 60, 90 and 120. For temperature variation, the 1:9 molar ratio, deposition time previously mentioned, and pH were fixed. In the case of pH, it is a consequence of molar ratio as it is directly affecting the chemical composition.
The optical characterization was carried out obtaining the absorption and transmission spectra in a range of 300 to 1100 nm, with a Hach DR6000 molecular absorption spectrophotometer. Energy bandgap (Eg) can be calculated with the Tauc equation, which is expressed as follows:
where hv is the photon energy, and Eg is the semiconductor optical bandgap, A is a constant, and n = ½ for direct bandgaps of semiconductors such as CdS [8].
Results and discussion
The molar ratios with the highest concentration of thiourea (1:9, 1:6, and 1:3) were tested in the chemical bath, with an initial pH of 10 and final pH of 8, respectively. In addition, in the residue of the solution of the synthesis of the films, these present a yellow-whitish powder. The yellow-whitish powder at the bottom of the solution indicates that the growth mechanism was cluster by cluster, while ion-by-ion mechanism was related to reactors with semi-transparent and little dusty solutions [9]. For the 3:1 and 6:1 ratio, its deposit was very similar, and, in both solutions, whitish crystals were formed which were dissolving over time, and a final pH of 10 was obtained. The reactors of the films that grew with a molar ratio of 3:1, 6:1, and 9:1, which presented a semi-transparent solution.
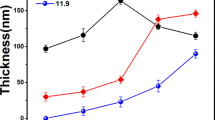
For the temperature variation, it was decided to fix the composition of the Cd/S molar ratio 1:9, since visually, its characteristics were the best compared to the other molar ratios. Figure 1a shows the transmission spectra of the 2-h deposition films with the different Cd/S compositions analyzed. All the films show a high transmittance above the rim located around 450 nm. From these spectra, it can be inferred that the higher the molar ratio of sulfur, the growth kinetics is favored, achieving higher thicknesses for the 1:9 ratio Cd/S composition. From the observation of the spectra of Fig. 1b, it is possible to show that, at temperatures higher than 70 °C, it is possible to deposit the CdS on the substrate, but at temperature of 50 °C or room temperature, not good results are obtained in growth, which is corroborated with the transmittance spectra with an average transmittance close to 100%, as observed in Fig. 1b.
Table 3 shows the average transmittance percentage at 480 nm, for each of the molar ratios. It is observed numerically that those with a higher sulfur molar ratio and higher temperature at the time of synthesis tend to have lower transmittance percentages. The average transmittance data are shown at 480 nm, because it is the most noticeable inflection point in the transmission spectra; in it, it is seen that for the 80 °C variant, it is the one with the lowest transmittance, followed by the ratio of 9 : 1 at 90 °C.
Figure 2 shows the Tauc graphs for calculating the energy of space by extrapolating the straight part of the graph line (αhν) 2 against the photon energy. It is observed that by controlling the proportion of cadmium and sulfur ions in the solution, it is possible to adjust the Eg values of the films, in a range from 2.52 to 2.63 eV. There is a trend in the increase of Eg values with increasing sulfur concentration; the 1: 6 molar ratio was the only one that did not follow this trend.
Values greater than 2.57 eV are not commonly reported in the literature [7, 10], and they represent structural changes in the polycrystalline lattice of the material that may be associated with changes in the electrical properties of the semiconductor due to quantum confinement effects, which can be very interesting due to the simplicity of this methodological proposal and to identify possible applications of this formulation in the design of electronic components such as LDR photoresistors.
Conclusions
pH values close to 10better favor the growth of thin films of CdS by CBD. The Cd/S molar ratio affects the growth of the films, being higher as the sulfur concentration increases up to the 1:9 ratio. The 3:1, 6:1, and 9:1 ratios were ineffective for homogeneous, and well-adhered CdS thin films to the substrate. Based on the experimental observations on the characteristics of the residues, the reaction mechanism proposed for thicker films is group by group. Temperature is directly related to the growth of thin films by CBD; as it decreases, the average transmission increases due to the decrease in thicknesses. 70 °C is an optimal temperature for the growth of CdS. A tuning effect is observed in the Eg values when the Cd/S composition ratio is modified, finding Eg values between 2.52 and 2.63 eV. These are higher values than those typically reported, which may be related to the induction of quantum confinement effects, which is interesting considering that only minimal changes were applied in the composition of the chemical baths.
References
G. Zavala Aznar, Industria Electrónica (ProMéxico, Ciudad de México, 2014). https://www.gob.mx/cms/uploads/attachment/file/76339/141216_DS_Electronico_ES.pdf
P.J. Kelly, R.D. Arnell, Magnetron sputtering: a review of recent developments and applications, Vacuum, 56(3), 159–172 (2000). ISSN 0042-207X. https://doi.org/10.1016/S0042-207X(99)00189-X
S. Kumar, N. McEvoy, H.-Y. Kim, K. Lee, N. Peltekis, E. Rezvani, H. Nolan, A. Weidlich, R. Daly, G.S. Duesberg, Crecimiento de CVD y procesamiento de grafeno para aplicaciones electrónicas (2011). https://doi.org/10.1002/pssb.201100179
S. Alhammadi, H. Jung, S. Kwon, H. Park, J.-J. Shim, M.H. Cho, M. Lee, J.S. Kim, W.K. Kim, Effect of Gallium doping on CdS thin film properties and corresponding Cu(InGa)Se2/CdS:Ga solar cell performance, Thin Solid Films, 660, 207–212 (2018). ISSN 0040-6090. https://doi.org/10.1016/j.tsf.2018.06.014
F.J. Willars-Rodríguez, I.R. Chávez-Urbiola, R. Ramírez-Bon, P. Vorobiev, Y.V. Vorobiev, Effects of aluminum doping in CdS thin films prepared by CBD and the performance on Schottky diodes TCO/CdS:Al/C, J. Alloys Compd. 817, 152740 (2020). ISSN 0925-8388. https://doi.org/10.1016/j.jallcom.2019.152740
P.K Nair, M.T.S Nair, V.M Garcı́a, O.L Arenas, Y Peña, A. Castillo, I.T Ayala, O. Gomezdaza, A. Sánchez, J. Campos, H. Hu, R. Suárez, M.E. Rincón, Semiconductor thin films by chemical bath deposition for solar energy related applications, Solar Energy Mater. Solar Cells, 52(3–4), 313-344 (1998). ISSN 0927-0248. https://doi.org/10.1016/S0927-0248(97)00237-7
F. Liu, Y. Lai, J. Liu, B. Wang, S. Kuang, Z. Zhang, J. Li, Y. Liu, Characterization of chemical bath deposited CdS thin films at different deposition temperature. J. Alloy. Compd. 493(1–2), 305–308 (2010). https://doi.org/10.1016/j.jallcom.2009.12.088
F. Ouachtari, A. Rmili, B. Elidrissi, A. Bouaoud, H. Erguig, P. Elies, Influence of bath temperature, deposition time and S/Cd ratio on the structure, surface morphology, chemical composition and optical properties of CdS thin films elaborated by chemical bath deposition. J. Mod. Phys. 2(9), 1073–1082 (2011). https://doi.org/10.4236/jmp.2011.29131
A. Slonopas, H. Ryan, B. Foley, Z. Sun, K. Sun, T. Globus, P. Norris, Growth mechanisms and their effects on the opto-electrical properties of CdS thin films prepared by chemical bath deposition, Mater. Sci. Semicond. Process., 52, 24–31 (2016). ISSN 1369-8001. https://doi.org/10.1016/j.mssp.2016.05.011.
J.P. Enríquez, X. Mathew, Influence of the thickness on structural, optical and electrical properties of chemical bath deposited CdS thin films. Sol. Energy Mater. Sol. Cells 76(3), 313–322 (2003). https://doi.org/10.1016/S0927-0248(02)00283-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiménez-Flores, Y., Lefranc-Cabrera, J., Gómez-Barrales, P.D. et al. Optimal conditions of pH, composition, and temperature in synthesis of CdS thin films by chemical bath deposition technique. MRS Advances 6, 1001–1004 (2021). https://doi.org/10.1557/s43580-021-00181-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-021-00181-3