Abstract
All-solid-state batteries can greatly improve safety and energy/power density, bringing superionic conductors to the research frontier of this field. Among all studied solid electrolytes, sulfides have become the most promising technical system due to their highest ionic conductivity, desirable mechanical ductility, and good interfacial contact with electrodes. Theoretical calculations predicted that lithium thioborate materials may have extremely high lithium-ion conductivity, good electrochemical stability, and thus great application promise. In this paper, the research history of lithium thioborate (Li–B–S) solid electrolytes is summarized and reviewed, with elaborations on milestone discoveries. Furthermore, both glassy and crystalline Li–B–S solid electrolytes are discussed in details, followed by introductions of Li–B–S system from experimental and theoretical perspectives. Moreover, the existing challenges and potential solutions are also proposed for the benign development of this field in the future.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All-solid-state batteries (ASSBs) hold promise as the next-generation advanced energy storage technology for safer, longer-lasting, and more energy dense alternatives to the commercialized Lithium-ion batteries (LIBs) with liquid electrolytes [1,2,3,4,5]. Solid electrolytes (SEs) as the key of ASSBs have attracted immense research interests, covering polymers [6, 7], oxides [8, 9], hydrides [10], halides [11, 12], and sulfides [13,14,15], etc. Among them, sulfide SEs is one of the most promising technical routes and material systems for realizing ASSBs due to their highest lithium-ion conductivity (10–3–10–2 S cm−1) and excellent mechanical properties. However, the mass production of sulfide ASSBs still faces many difficulties including moisture sensitivity, narrow electrochemical windows, and detrimental interfacial reactions at cathode/anode side [16,17,18,19,20,21]. Designing novel superionic conductors with better overall performance is thus essential for the benign development of this field [22,23,24,25].
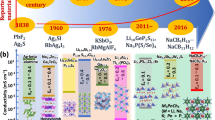
Lithium thioborate SEs have gradually become research hotspots due to their excellent theoretically predicted properties [26,27,28]. Li–B–S SEs were first studied in the early 1980s, with a focus on glassy materials (e.g., Li2S–B2S3, Li2S–B2S3–LiI) [29, 30]. Sequentially, crystalline Li–B–S SEs were found, including Li6+2x [B10S18]Sx (x ≈ 2), Li2B2S5, Li3BS3, Li5B7S13, and Li9B19S33 [31,32,33,34]. Although these crystals have been successfully synthesized, they were not designed for solid electrolytes. It was not until 2019 that Sendek et al. [27] predicted four phases in Li–B–S crystal system (Li2B2S5, Li3BS3, Li5B7S13, and Li9B19S33) with outstanding single-crystal Li+ conductivity at room temperature by density-functional theory molecular dynamics (DFT-MD) calculation. Besides, pseudo-quaternary lithium oxythioborate halide glasses (Li2S–B2S3–SiO2–LiI, or “LIBOSS”) and new lithium thioborate halides crystalline materials, Li7.5 [B10S18]X1.5(X = Cl, Br, I) and Li6B7S13I, were discovered by Linda et al. [35,36,37]. These studies indicate that Li–B–S SEs may offer comparable or significantly improved performance such that an emerging field of promising superionic conductor is on the rise. Figure 1 shows the comparison of the number of published papers about Li–B–S, Li–Sn–S, Li–P–S, Li–Si–P–S, Li–Sn–P–S, and Li–Ge–P–S sulfide SEs included on Web of Science from 2000 to 2021, demonstrating a much less research enthusiasm in Li–B–S system. This may be attributable to the following reasons. (1) Most commercial boron contains impurities such that the amorphous boron used as starting materials in previous works was not pure. (2) Boron chalcogenides used in the literature would attack containers/glass tubes at temperatures > 400 °C. (3) Compared with the simple synthesis of thiophosphate, thioborate requires higher temperature (> 700 °C), longer time (> 24 h), and more complicated process. (4) The synthesized product is often a multiphase mixture and the preparation of pure compounds is difficult. Although these syntheses have been reported long time ago, the products synthesized still contain impurities and it is difficult to obtain a single target product [38,39,40]. So far, only a relatively small number of lithium thioborates have been reported and structurally determined such that enough space is left for exploration and more impactful research.
In this work, the major content of this paper, including key technologies, development history, compositional categories, and crystalline or glassy materials of Li–B–S SEs, is summarized and shown in Fig. 2. The research results of previous works are elaborated in details from the perspectives of crystalline and glassy materials. Moreover, based on the comprehensive survey of the previous studies, the experimentally and theoretically predicted results of the existing Li–B–S SEs are summarized. The research and development history of Li–B–S SEs from the very first material in 1981 to date are summarized. Finally, the problems that still need to be solved in the Li–B–S SEs system are discussed to provide a basis for the follow-up in-depth study, and potential future directions are proposed. This review thus serves as a comprehensive summary of the reported results in Li–B–S SEs system and important reference for the future development of new solid electrolytes.
Glass systems
Glass-phase sulfide SEs were highly expected in the 1980s due to their open structure with isotropic ion conduction path and the effectively eliminated grain boundary resistance [41, 42]. The ionic conductivity of glass-phase sulfide SEs was considered to be 1–2 orders of magnitude higher than that of crystalline sulfide SEs with the same composition at early times [43]. Table 1 provides a detailed summary of the ionic conductivities and activation energies of Li–B–S glass materials. Results show that the ionic conductivity of (1 − x)B2S3 − xLi2S (0.5 \(\le \) x \(\le \) 0.75) glass electrolytes is not prominent (\(\sim \) 10–4 S cm−1), but can reach 2 mS cm−1 after doping (Li2S–B2S3–SiO2–LiI), meeting the target for utilization in a bulk-type solid-state cell. Similar to other sulfide glass electrolytes, it proves again that the ion concentration greatly affects the ionic conductivity of glass electrolyte. In addition, Li–B–S glass transition temperature changes significantly with the increase of Li2S and there may even be two glass transition temperatures due to phase separation (e.g., 67 Li2S–33B2S3 and 67 Li2S–26 B2S3–7P2S5).
Figure 3 shows a timeline of the development history of Li–B–S SEs from 1981 to date with the key milestones. The earliest research started with B2S3–Li2S glass (0.09 mS cm−1) in 1981. The glass–ceramic phase began to appear depending on the glass composition and heat treatment conditions in the 1990s. Crystallization of glass was believed to have reduced ionic conductivity, which is attributable to the formation of individual crystals with lower ionic conductivity [48]. Therefore, B2S3–Li2S glass–ceramic phase was only briefly mentioned in the glass phase. Moreover, the first crystal synthesized (Li6+2x [B10S18]Sx) in 1990 was reported without description of ionic conductivity. Due to the complexity of the synthesis steps and the inconspicuous ionic conductivity, the study of Li–B–S SEs were interrupted around 2000. Only a few works were reported on Li–B–S during this period, until Sendek et al. put forward theoretical calculations in 2020, showing that lithium thioborate might have high ionic conductivities.
In 1981, the first work reported by Levasseur [29] on (1 − x)B2S3–xLi2S (0 \(\le \) x \(\le \) 0.5) glassy systems showed that the ionic conductivity (10–4 S cm−1) was 1000 times higher than oxide counterparts. This work used graphite crucible to synthesize B2S3-based glasses, but most of subsequent studies used sealed quartz tubes. In 1990, Zhang et al. [38] found that B2S3 would react with quartz tube above 400 °C. To overcome this challenge, a new process was developed to coat the quartz tube with carbon and to synthesize (1 − x) B2S3–xLi2S (0.5 \(\le \) x \(\le \) 0.75) glasses in carbon-coated containers, leading to a higher Li2S content than previous studies. Differential scanning calorimetry (DSC) results show that glass transition temperature (Tg) decreased with increasing content of Li2S. A single Tg was observed in the composition range of 0.7 \(\le \) x \(\le \) 0.75, while two Tgs were observed at x = 0.67, indicating the existence of two glass phases from the fast quench of liquids. When x \(\le \) 0.6, an exothermal peak appeared, possibly due to a weak crystallization process. Using 11B NMR, three-coordinated boron was found with newly created non-bridging sulfur atoms, as four-coordinated boron decreased with increasing Li2S [49, 50]. The difference in the ratio of negatively charged four-coordinated units to non-bridging trigonal units for sulfur glasses could be responsible for conductivity changes. In 1991, Menetrier et al. [51] further studied the ionic conduction of (1 − x)B2S3–xLi2S glasses (0.5 \(\le \) x \(\le \) 0.75). According to Menetrier’s study, three-coordinated boron atoms have stronger bonding with Li+, resulting in the unusual increase of the activation energy and the pre-exponential factor, which has a great influence on ionic conductivity. Besides, the faster quenching speed can induce more disorder in the relative positions of the structural entities, which may create more potential pathways for Li diffusion and result to a higher mobility.
It has been reported that for glass systems, increasing concentration and mobility of charge carrier ions is the key to improve the ionic conductivity [41]. It is generally assumed that adding lithium halides is an effective way to increasing Li+ concentration and thus improve ionic conductivity [52,53,54]. In 1983, Wada et al. [30] studied the B2S3–Li2S–LiI glass system. With the addition of LiI, 30Li2S·26B2S3·44LiI glass showed the highest ionic conductivity of 1.7 \(\times \) 10–3 S cm−1 at room temperature and an activation energy of 0.30 eV. Furthermore, the conductivities of pellets obtained under different pressures were also measured [Fig. 4(a)], which increased along with the pressure. Raman spectra show that iodide anions do not react with and remain outside of the vitreous network [49]. The iodide anions act as plasticizers, facilitating the cooperative movements and thus the significant decrease in glass transition temperatures [55, 56]. Burckhard et al. [46] performed a systematic investigation of the composition dependence of the conductivity in the B2S3–Li2S–LiI system. They found that the B2S3–Li2S–LiI glass system can be divided into two different parts, as shown in Fig. 4(b). Part I refers to Li2S-rich B2S3–Li2S–LiI glass with transparent yellow/brown color, while part II is opaque because of a phase separation. The whole glass forming region is separated by a line, which can be drawn approximately between the composition points 66.7Li2S–33.3B2S3 and 66.7Li2I–33.3Li2S. The glass in the intermediate region can be either transparent or opaque depending on the cooling speed. The main parameter influencing the conductivity is the lithium iodide content, but its major effect is on the activation energy. Larger I ion replaces smaller S ion at low I− concentrations, reducing the activation energy [45]. At higher concentrations, LiI aggregates begin to form, resulting in a high barrier region and an overall increase in activation energy. This phenomenon has been confirmed by Vinatier et al. using 7Li NMR [57]. It is also worth to note that the presence of lithium iodide in glasses does not modify the ratio between four-coordinated boron and three-coordinated boron [58], but impairs the stability against electrochemical oxidation, since LiI results to microdomains with I− ions not covalently bonded to the glass network. The isolated I− ions tend to be oxidized when in contact with cathodes (I− ions are oxidized at 2.9 V vs. Li/Li+), resulting in a narrow electrochemical stability window. Kbala et al. [59] synthesized amorphous B2S3–Li2S–LiI glass thin films by vacuum evaporation technique, showing similar conductivities of 10–3 S cm−1 at 25 °C with B2S3–Li2S glass. A thermal treatment at 90 °C of the thin films containing lithium iodide would enhance the conductivity and decrease activation energy (0.18 eV), which was identified as Phipps effect and can be attributed to a quick ion diffusion along thin film–substrate interface. In 1984, Menetrier et al. [60] tested 26B2S3–32Li2S–42LiI and Li as SE and anode, respectively, to achieve secondary battery at room temperature. The Li/glass interface was successfully tested in symmetrical cells and it was therefore used as negative electrode in batteries to obtain high energy density cells. The best results were obtained with a composite TiS2-liquid electrolyte cathode [60, 61]. 50 mg of TiS2 could be discharged up to Li0.8TiS2 and charged again with a continuous current of 0.1 mA cm−2 (without any relaxation time) with a small polarization. In order to reduce the test time, the amount of TiS2 is limited to 50 mg, but it can undoubtedly be greatly increased to obtain high-capacity batteries.
(a) Plots of log \(\upsigma \) vs. 103 T−1 (left) and log \(\upsigma \) vs. pressure (right) for pellets at 25 °C in the B2S3–Li2S–LiI system [30]; (b) Glass forming region (left) and glass transition temperature (right) of the ternary system B2S3–Li2S–LiI [46]; (c) Schematic illustration of the quaternary-phase diagram (Li2S–B2S3–SiO2–LiI) (left) with horizontal slices indicating ternary-phase diagram (Li2S–B2S3–LiI) of exact SiO2 content and the conversion from ternary-phase diagram to quaternary-phase diagram (right). [36]; (d) Room-temperature conductivity, activation energy, and pre-exponential factor of LIBOSS [36].
Since Zhang et al. [44] found that the introduction of P2S5 into SiO2-based glass can improve ionic conductivity and electrochemical stability toward lithium metal anode, they also doped P2S5 into B2S3–Li2S glass system (33 [(1 − x)B2S3–xP2S5]–67 Li2S (0 \(\le \) x \(\le \) 0.3 and 0.9 \(\le \) x \(\le \) 1)) with carbon-coated quartz tube. The room-temperature conductivity of 33 [(1 − x)B2S3–xP2S5]–67 Li2S glass reached the highest value of 0.141 mS cm−1 when y = 0.3. The addition of P2S5 into a B-rich glass partially converted trigonal boron to tetrahedral boron. In another study of the Li2S–B2S3–As2S3 glass system, the expected increase of ionic conductivity was not observed [62]. IR and Raman spectrum results show [62] that ternary glass Li2S–B2S3–As2S3 may include both Li2S–B2S3 and Li2S–As2S3 binary glass phases. Similar to the previous report [58], As2S3 can lead to the modification of three-coordinated boron and four-coordinated boron content, but the evolution of the value of the ionic conductivity is not significant. Seino et al. [47] synthesized Li4SiO4-doped Li2S–B2S3 glasses by rapid quenching and transformed them into glass ceramics by heat treatment in 2006. When 5 mol% of Li4SiO4 was doped into 70Li2S–30B2S3, the glass ceramic showed an ionic conductivity of 1 × 10−3 S cm−1 and wide electrochemical window of about 10 V. Recently, Linda et al. [36] reported a new class of vitreous quaternary-phase diagram glass (Li1.05B0.5SixO2xS1.05I0.45, 0 \(\le \) x \(\le \) 1) that were prepared directly from a melt. These new glasses exhibit high ionic conductivities up to 2 mS cm−1, rendering them one of the best-known lithium-ion-conductive glasses [Fig. 4(c), (d)]. A small amount of silica can severely modify the thioborate network to create additional free volume to accommodate LiI and enable a high fraction of LiI dissolution. As more LiI dissolves into the glass matrix, the density of lithium-ion carriers increases to improve the ionic conductivity. Moreover, the glass with oxygen exhibits low H2S evolution upon exposure to moisture under ambient conditions. An all-solid-state battery was assembled using the LIBOSS glass SE. Figure 5(a) shows that upon the first cycle charge at 2.7 V (vs. Li), a slight irreversible capacity is evident due to initial electrolyte oxidation at the cathode interface. As a proof of concept, an ASSB with a TiS2 cathode and LIBOSS electrolyte [36] shows a close-to-theoretical capacity with extremely stable cycling stability for over 130 cycles [Fig. 5(b)]. Rate capabilities of an identical cell cycled within the same voltage range and at 25 °C are shown in Fig. 5(c). This is further coupled with improved moisture stability in comparison with \(\upbeta \)-Li3PS4 and Li7P3S11 by increasing SiO2 content as shown in Fig. 5(d). Differential scanning calorimetry (DSC) studies of the compositions in Fig. 5(e) show that there is only a small increase in Tg and Tx when SiO2 increases. This promising material class with its enhanced material properties by both doping salt and mixed former effect make it a promising candidate SE, which can be extended to a broader scope to include alternative halides and glass forming oxides and a new avenue of SE materials.
(a) Charge–discharge curves of Li-In/LIBOSS/TiS2 all-solid-state batteries cycled at 0.1 °C and 25 °C [36]; (b) Cycling performance of the cell at 0.1C and 25 °C [36]; (c) Cycling data at 60 °C and 1C rate [36]; (d) H2S evolution from pelletized β-Li3PS4, Li7P3S11, LIBOSS (x = 0.25), and LIBOSS (x = 0.5) upon exposure to ambient air [36]; (e) Differential scanning calorimetry curves of x = 0, 0.25, 0.375 ,and 0.5 compositions under a N2 flow of 50 mL min−1 [36]; Tg glass transition temperature, Tx onset of LiI crystallization, Tl melting of LiI.
Crystal systems
The crystalline phases of Li–B–S system and their solid solutions were discovered in the 1990s, but no ionic conductivity or electrochemical stability has been reported in these works [63,64,65,66,67]. Many crystalline lithium thioborates, like Li6+2x [B10S18]Sx (x \(\approx \) 2), have been found to exhibit a thioborate network composed of supertetrahedral clusters (also referred to as superadamantanoid) or B10S20 structural units [34]. Similar supertetrahedral structures were observed in lithium/sodium phosphidosilicates and sodium/silver thioborates, such as Na19Si13P25, Ag6B10S18, and Na6B10S18 [Fig. 6(a)] [63, 68, 69]. Supertetrahedral networks are of great interests because the structure forces cations and anions to distribute into the void spaces between the clusters. For frameworks with a large void space, the cations are weakly bonded to the surrounding anions, which facilitates cation mobility within the structure [70, 71]. The summary of crystal structure and room-temperature ionic conductivity of Li–B–S system materials is shown in Table 2. The lithium thioborate with supertetrahedral structure shows high ionic conductivity (the ionic conductivity of Li5B7S13 is 1 mS cm−1), and the doping of halogen can stabilize the supertetrahedral structure and improve the ionic conductivity (the ionic conductivity of Li7.5B10S18I1.5 is 1.4 mS cm−1).
Li6+2x [B10S18]Sx (x \(\approx \) 2) is the first reported structurally characterized lithium thioborate prepared at 750 °C in a graphitized sealed tube [34]. The connection of the corner-sharing B10S20 macrotetrahedra results in a three-dimensional polymeric network [72]. Two different types of lithium ions which are non-exchangeable exist in the network from NMR. A small fraction (20%) of the lithium ions form skeleton and most of the rest distributed among a large number of potential positions between the disordered sulfide ions in the extended channels of the structure. Sufficient unoccupied sites distribute in the surrounding of each Li+ ion so that the mobility is high. Linda et al. [37] discovered crystalline Li7.5B10S18X1.5 (X = Cl, Br, I) based on Li6+2x [B10S18]Sx (x \(\approx \) 2) structure. Replacing sulfide ions in the channel by iodide ions with greater polarization rate will weaken the interactions among mobile Li+ ions to affect Li+ ion conduction.
In 1993, two crystalline thioborates (Li5B7S13 and Li9B19S33, whose structures are shown in Fig. 6) were synthesized by Krebs et al. [31, 73]. Similar to Li6+2x [B10S18]Sx (x \(\approx \) 2), a high mobility was expected for Li5B7S13 and Li9B19S33 because of extended Li diffusion channels in the structure [Fig. 6(b)]. An alternation of B4S6S4/2 tetrahedra and B10S16S4/2 supertetrahedra forms networks in Li5B7S13, while Li9B19S33 only contains B10S16S4/2 tetrahedra. Two B10S16S4/2 entities share a BS45− subunit to form infinite three-dimensional networks of corner-sharing B19S30S6/2 units [Fig. 6(c)], whose anionic structure is isotypic with Na23Si19P33 [69]. In essence, B4S10, B10S20, and B19S33 supertetrahedra are all composed of corner-sharing BS4 tetrahedra.
In 1995, Krebs et al. [32] then prepared Li2B2S5 with stoichiometric amounts of boron and excessive sulfur at 650 °C by employing solid-state reactions. In the Li2B2S5 structure [Fig. 6(d)], two different orientations of the B2S5 ring exist with an alternation between two adjacent layers. They are connected by lithium ions in a structure similar to PbO [72]. Lithium ions are located in highly twisted tetrahedrons surrounded by sulfur between layers.
In 1996, Li3BS3 was also successfully synthesized with isolated (BS3)3− species [33, 74]. Like the structure of Na3BS3, K3BS3 and Rb3BS3 of the novel orthothioborates, Li3BS3 contains isolated planar (BS3)3− anions and coordinating alkali metal cations [Fig. 6(e)] [65]. A theoretical work [26] on Li3BS3 predicted a rather low activation energy barrier (0.25 eV) for ion migration, but no experimental evidence has been reported up to date.
Syntheses of Li–B–S SEs
Most thioborates were synthesized by solid-state reactions of mixed reactants at a high temperature, following the similar heating procedure. The starting reactants of these reactions are usually B powder, S powder, and metal sulfides. From the aspect that high reactivity is beneficial to the progress of the reaction, amorphous boron is more preferred compared with the less reactive high-purity crystalline boron. Since many reactants are sensitive to water and air, the entire reaction process needs to be carried out in a glove box. The silica tubes loaded with reactants are usually sealed off by a flame torch in vacuum and then heated in a tube furnace. Due to the high affinity of boron to oxygen, it attacks the typical container materials (e.g., silica tubes) at temperatures above 400 °C [76], following the reaction of
To overcome this issue, two common solutions have been proposed. The first involves the use of quartz glass with the inner surface coated with a tight layer of glassy carbon, which is also the best solution. Another simpler method is to use crucibles made of glassy carbon, pressed graphite, or boron nitride, which are furnished with a tight screw cap. The comparison of these sample containers is presented in Table 3.
The reagents are dry mixed in an agate mortar and pressed into a small pellet, which is subsequently placed in a glassy carbon crucible and further sealed into a quartz tube. The quartz tubes are usually sealed in vacuum and placed vertically in a furnace. Excessive S powder is usually added to promote crystallization and yield sufficiently pure products, which is usually achieved by dissolving part or all of the products as a flux at higher temperatures. In addition, in solid-state reactions, high-pressure reactions are often used to facilitate the formation of BS4− tetrahedrons [77]. Because of the difficulties associated with the synthesis methods, only a relatively small number of thioborates have been synthesized and structurally determined up to date.
Theoretical studies
Theoretical studies have always been an important help for discovering new types of SEs with high ionic conductivity and wide electrochemical window [78, 79]. In 2020, Sendek et al. [27] reinvestigated crystalline Li–B–S system, using the machine learning-based models. It is surprising to note that four phases in the crystalline Li–B–S system were theoretically predicted to have high bulk ionic conductivities, including Li2B2S5, Li3BS3, Li9B19S33, and Li5B7S13. Specifically, the ionic conductivities of Li2B2S5, Li3BS3, Li9B19S33, and Li5B7S13 were predicted to reach 0.16 mS cm−1, 9.7 mS cm−1, 80 mS cm−1, and 62 mS cm−1, respectively, from extrapolations and calculations [Fig. 7(a)]. These predicted values, especially Li9B19S33 and Li5B7S13, are among the highest Li-ion conductivities for single-crystal SEs. The electronic band gaps of Li5B7S13, Li2B2S5, Li3BS3, and Li9B19S33 were also calculated to be 4.7, 3.5, 4.2, and 4.0 eV, respectively [Fig. 7(b), (c)]. Since a wide band gap is considered essential for small electronic conductance and excellent electrochemical stability, the band gaps of Li–B–S phases are large enough for SE and applications in ASSBs [80]. Different from any other well-known superionic lithium conductors, the phase diagram of Li–B–S crystal system suggests that one lithium thioborate can transform into another superionic phase without great changes in conductivity upon oxidation and reduction [Fig. 7(d)]. This provides a good strategy to realize both wide electrochemical window and high ionic conductivity if oxidation/reduction products are the same system of the fast ion-conducting phases. Figure 7(e) shows the stability window width and ionic conductivity of various SEs. Considering the two aspects of ion conductivity and electrochemical window, Sendek et al. [27] predict that Li–B–S possesses higher ionic conductivity than most known ionic conductors. The ionic conductivity and electrochemical stability window are important parameters for achieving high power and high energy density, and predictions suggest that Li–B–S may be better than LGPS. In general, predicted value of Li–B–S shows the best overall performance. Although there is a lack of experimental proof, the excellent performance shown by the Li–B–S system is still worthy of attention and future experimental efforts.
(a) Theoretically predicted temperature dependence of Li ionic conductivity for four crystalline Li–B–S systems [80]; (b) Theoretical analysis of electrochemical stability of Li–B–S system [80]; (c) Superionic Molar fraction of stable superionic phases vs. composition and potential. The molar fraction of thermodynamically stable superionic phases, plotted as a function of the B/(B + S) ratio and electrode potential [80]; (d) Ternary-phase diagram of the Li–B–S system [80]; (e) Stability window width and ionic conductivity of various SEs. The thermodynamic electrochemical stability window widths computed with PBE DFT and the experimentally reported ionic conductivities of several known solid Li-ion conductors, including the predicted values for the best Li–B–S electrolyte compositions [80].
Although Li–B–S system was theoretically predicted by Sendek et al. [27] to have significantly improved ionic conductivity and electrochemical stability over the best-performing SE, no experimental evidence has been reported to corroborate the simulation results. As mentioned throughout this article, synthesis routes of Li5B7S13, Li2B2S5, and Li3BS3 have been reported, and their ion conductivities were measured to be 1 mS cm−1, 3.4 \(\times \) 10–4 mS cm−1, and 1.19 \(\times \) 10–4 mS cm−1, respectively. These measured ionic conductivities are far below the theoretically predicted values, which might be caused by defects, grain boundary resistance, and microstructure, etc. Unfortunately, the influences of such factors on the overall conductivity are generally not predictable. Moreover, the synthetic conditions of Li–B–S system are quite demanding. Although there is good agreement in X-ray diffraction (XRD) peak positions between synthesized products and standard samples, impurities still exist. This can be corroborated by the predominance of speckled particles on crystalline surfaces by scanning electron microscope (SEM) images [35].
Experimental progress
It is encouraging that Linda et al. [35, 37] reported two new classes of lithium thioborate halides in 2021, namely Li7.5B10S18X1.5 (X = Cl, Br, and I) and Li6B7S13I. Considering the impurities during the synthesis of Li6+2x [B10S18]Sx (x \(\approx \) 2), Linda et al. obtained Li7.5B10S18X1.5 by replacing sulfides with halides, exhibiting ionic conductivities up to 1.4 mS cm−1 [Fig. 8(d)]. The structure of Li7.5B10S18X1.5 is based on [B10S18−6]n, which is connected by corner sulfur anions in B10S20 to form the framework [Fig. 8(a)]. Halide anions with large radii can only reside in large interpenetrating voids. The weak bonding of the anion–cation pairs and unique crystalline framework with large voids containing disordered Li+ or halides results in a system with high ion mobility. Li6B7S13I has a similar synthesis process to Li7.5B10S18X1.5, but it needs to be grounded again during subsequent annealing to reduce particle size and homogenize the components. Li6B7S13I has a three-dimensional framework composed of corner-sharing BS4 tetrahedra that form unique B4S13 anion clusters rather than B10S20 [Fig. 8(b)]. The lithium substructure of Li6B7S13I is highly similar with argyrodites Li6PS5X (X = Cl, Br, or I), where Li ions form cages around disordered halide/sulfur sites. The Li+ ions in Li6B7S13I also form cages around I− ions and adjoining cages are connected by Li sites [Fig. 8(c)]. The experimentally obtained ionic conductivity of Li6B7S13I at room temperature is 0.5 mS cm−1 with an overall activation energy of 0.30 eV, which is lower than the calculated value of 5.2 mS cm−1 [Fig. 8(e)]. Due to the high resistance of grain boundaries, the theoretical prediction is quite different from the reality.
Structure of (a)Li7.5B10S18X1.5 and (b) cubic Li6B7S13I. (c) Li+ cage around I− in Li6B7S13I and argyrodite Li6PS5I; (d) Room-temperature conductivity (25 °C), activation energy, and pre-exponential factor; (e) Arrhenius plots of Li6B7S13I obtained from temperature-dependent AIMD simulations (left) and EIS measurements (right) [35, 37].
Challenges and future perspectives
Since studies on lithium thioborates are very limited, the information on their electrochemical performances, such as lithium-ion conduction mechanism, compatibility with electrode materials, and applications in ASSBs, is urgently needed to be explored at this moment. The following challenges still remain for this research field.
-
(1)
Difficulty in synthesis is the primary problem hindering the development of Li–B–S materials. As mentioned earlier, since boron attacks most common container materials (e.g., silica tubes) at temperatures above 400 °C, the precursor mixture needs to be supported in a glassy carbon quartz tube or graphite crucible. Then the tube needs to be sealed off by a flame torch under vacuum and heated in a tube furnace. To solve this problem, designing new synthetic methods including liquid-phase method, mechanochemical method, and gas-phase method is urgently needed [81].
-
(2)
The impurity of the synthetic product is another important reason for the huge gap between theoretical prediction and experimentally measured values. Li2B2S5, Li3BS3, Li9B19S33, and Li5B7S13 have been synthesized experimentally, but all of them contained impurities [38]. Therefore improving the purity of synthesized products is expected to further improve the ion conductivity. This can be realized by selecting raw materials, optimizing synthesis process and exploring new synthesis method.
-
(3)
In terms of air stability, almost all thioborates containing planar-triangle BS3 units are air and moisture sensitive, probably caused by the electron deficiency of boron atoms. The electronically saturated boron in BS4 polyhedra can be effectively shielded against nucleophilic attack [75, 82], but this phenomenon has not been observed in existing lithium thioborates. Discovering air-stable lithium thioborates would be undoubtedly a huge improvement of this field and facilitate the application of Li–B–S systems.
Summary
SEs are the most critical component of next-generation ASSBs. Sulfide SEs have become the most promising technical routes due to their highest ionic conductivity, desirable mechanical ductility, and good interfacial contact with electrodes. Recently, one type of sulfide SEs, lithium thioborates, has been theoretically predicted to have extremely high lithium-ion conductivity and good electrochemical stability, indicating excellent application promises. In this paper, Li–B–S SEs are comprehensively reviewed in terms of their development history, glassy/crystalline structures, synthesis process/parameters, challenges, and future perspectives. Although crystalline Li–B–S SEs were theoretically predicted to have ultra-high ionic conductivities (~ 62 mS cm−1 for Li5B7S13 and ~ 80 mS cm−1 for Li9B19S33), the experimentally observed data were much lower. Li–B–S glass (30Li2S–25B2S3–45LiI-25SiO2) has the highest experimentally measured ionic conductivity (up to 2 mS cm−1) among all Li–B–S SEs. This unsatisfactory result may be caused by the influence of grain boundary and multiphase mixtures. Moreover, challenges remain in the difficulty during synthesis process. Further attempts and additional investigations are required for synthesis process design, property improvements, and electrochemical performances/mechanism studies.
Data availability
There are no original data associated with this manuscript.
References
J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001)
K. Xu, Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104(10), 4303–4417 (2004)
M.M. Wang, X.H. Zheng, X. Zhang, D.L. Chao, S.Z. Qiao, H.N. Alshareef, Y. Cui, W. Chen, Opportunities of aqueous manganese-based batteries with deposition and stripping chemistry. Adv. Energy Mater. 11(5), 19 (2021)
L.L. Liu, J.R. Xu, S. Wang, F. Wu, H. Li, L.Q. Chen, Practical evaluation of energy densities for sulfide solid-state batteries. eTransportation 1, 16 (2019)
J. Peng, D. Wu, F. Song, S. Wang, Q. Niu, J. Xu, P. Lu, H. Li, L. Chen, F. Wu, High current density and long cycle life enabled by sulfide solid electrolyte and dendrite-free liquid lithium anode. Adv. Funct. Mater. 32, 2105776 (2021)
F. Croce, G.B. Appetecchi, L. Persi, B. Scrosati, Nanocomposite polymer electrolytes for lithium batteries. Nature 394(6692), 456–458 (1998)
D. Zhou, D. Shanmukaraj, A. Tkacheva, M. Armand, G.X. Wang, Polymer electrolytes for lithium-based batteries: advances and prospects. Chemistry 5(9), 2326–2352 (2019)
J.C. Bachman, S. Muy, A. Grimaud, H.H. Chang, N. Pour, S.F. Lux, O. Paschos, F. Maglia, S. Lupart, P. Lamp, L. Giordano, Y. Shao-Horn, Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116(1), 140–162 (2016)
X.Y. Yao, B.X. Huang, J.Y. Yin, G. Peng, Z. Huang, C. Gao, D. Liu, X.X. Xu, All-solid-state lithium batteries with inorganic solid electrolytes: review of fundamental science. Chin. Phys. B 25(1), 14 (2016)
M. Matsuo, S. Orimo, Lithium fast-ionic conduction in complex hydrides: review and prospects. Adv. Energy Mater. 1(2), 161–172 (2011)
X.N. Li, J.W. Liang, N. Chen, J. Luo, K.R. Adair, C.H. Wang, M.N. Banis, T.K. Sham, L. Zhang, S.Q. Zhao, S.G. Lu, H. Huang, R.Y. Li, X.L. Sun, Water-mediated synthesis of a superionic halide solid electrolyte. Angew. Chem.-Int. Ed. 58(46), 16427–16432 (2019)
X.N. Li, J.W. Liang, J. Luo, M.N. Banis, C.H. Wang, W.H. Li, S.X. Deng, C. Yu, F.P. Zhao, Y.F. Hu, T.K. Sham, L. Zhang, S.Q. Zhao, S.G. Lu, H. Huang, R.Y. Li, K.R. Adair, X.L. Sun, Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energy Environ. Sci. 12(9), 2665–2671 (2019)
M. Tatsumisago, A. Hayashi, Sulfide glass-ceramic electrolytes for all-solid-state lithium and sodium batteries. Int. J. Appl. Glass Sci. 5(3), 226–235 (2014)
Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba, R. Kanno, High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 7 (2016)
P.S. Lu, L.L. Liu, S. Wang, J.R. Xu, J. Peng, W.L. Yan, Q.C. Wang, H. Li, L.Q. Chen, F. Wu, Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity. Adv. Mater. 33(32), 13 (2021)
J.R. Xu, Y.X. Li, P.S. Lu, W.L. Yan, M. Yang, H. Li, L.Q. Chen, F. Wu, Water-stable sulfide solid electrolyte membranes directly applicable in all-solid-state batteries enabled by superhydrophobic Li+-conducting protection layer. Adv. Energy Mater. 12, 2102348 (2022)
Y. Wang, Y. Lv, Y.B. Su, L.Q. Chen, H. Li, F. Wu, 5V-class sulfurized spinel cathode stable in sulfide all-solid-state batteries. Nano Energy 90, 12 (2021)
Y.J. Wu, S. Wang, H. Li, L.Q. Chen, F. Wu, Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat 3(8), 827–853 (2021)
R. Pathak, K. Chen, A. Gurung, K.M. Reza, B. Bahrami, F. Wu, A. Chaudhary, N. Ghimire, B. Zhou, W.H. Zhang, Y. Zhou, Q.Q. Qiao, Ultrathin bilayer of graphite/SiO2 as solid interface for reviving Li metal anode. Adv. Energy Mater. 9(36), 10 (2019)
L. Lilu, W. Fan, L. Hong, C. Liquan, Advances in electrochemical stability of sulfide solid-state electrolyte. J. Chin. Ceram. Soc. 47(10), 1367–1385 (2019)
W.L. Yan, F. Wu, H. Li, L.Q. Chen, Application of Si-based anodes in sulfide solid-state batteries. Energy Storage Sci. Technol. 10, 821 (2021)
F. Wu, W. Fitzhugh, L. Ye, J. Ning, X. Li, Advanced sulfide solid electrolyte by core-shell structural design. Nat. Commun. 9(1), 1–11 (2018)
A. Vasylenko, J. Gamon, B.B. Duff, V.V. Gusev, L.M. Daniels, M. Zanella, J.F. Shin, P.M. Sharp, A. Morscher, R.Y. Chen, A.R. Neale, L.J. Hardwick, J.B. Claridge, F. Blanc, M.W. Gaultois, M.S. Dyer, M.J. Rosseinsky, Element selection for crystalline inorganic solid discovery guided by unsupervised machine learning of experimentally explored chemistry. Nat. Commun. 12(1), 12 (2021)
W. Fitzhugh, F. Wu, L.H. Ye, H.Q. Su, X. Li, Strain-stabilized ceramic-sulfide electrolytes. Small 15(33), 14 (2019)
W. Fitzhugh, F. Wu, L.H. Ye, W.Y. Deng, P.F. Qi, X. Li, A high-throughput search for functionally stable interfaces in sulfide solid-state lithium ion conductors. Adv. Energy Mater. 9(21), 12 (2019)
F. Bianchini, H. Fjellvag, P. Vajeeston, A first-principle investigation of the Li diffusion mechanism in the super-ionic conductor lithium orthothioborate Li3BS3 structure. Mater. Lett. 219, 186–189 (2018)
A.D. Sendek, E.R. Antoniuk, E.D. Cubuk, B. Ransom, B.E. Francisco, J. Buettner-Garrett, Y. Cui, E.J. Reed, Combining superionic conduction and favorable decomposition products in the crystalline lithium-boron-sulfur system: a new mechanism for stabilizing solid Li-ion electrolytes. ACS Appl. Mater. Interfaces 12(34), 37957–37966 (2020)
H. Park, S. Yu, D.J. Siegel, Predicting charge transfer stability between sulfide solid electrolytes and Li metal anodes. ACS Energy Lett. 6(1), 150–157 (2021)
A. Levasseur, R. Olazcuaga, M. Kbala, M. Zahir, P. Hagenmuller, Synthesis and electrical properties of new sulfide galss with high ionic conductivity. Comptes Rendus De L Academie Des Sciences Serie Ii 293(8), 563–565 (1981)
H. Wada, M. Menetrier, A. Levasseur, P. Hagenmuller, Preparation and ionic conductivity of new B2S3-LI2S-LiI glasses. Mater. Res. Bull. 18(2), 189–193 (1983)
F. Hiltmann, P. Zumhebel, A. Hammerschmidt, B. Krebs, Li5B7S13 and Li9B19S33: two lithium thioborates with novel highly polymeric anion networks. Z. Anorg. Allg. Chem. 619(2), 293–302 (1993)
J. Kuper, C. Jansen, B. Krebs, Na2B2S5 and Li2B2S5: two novel perthioborates with planar 1,2,4-trithia-3,5-diborolane rings. Abstr. Pap. Am. Chem. Soc. 210, 507 (1995)
F. Hiltmann, C. Jansen, B. Krebs, Li3BS3 and LiSrBS3: new orthothioborates with trigonal planar boron coordination. Z. Anorg. Allg. Chem. 622(9), 1508–1514 (1996)
P. Zumhebel, B. Krebs, M. Grune, W. Mullerwarmuth, Preparation, crystal-structure and 7Li NMR of Li6+2X [B10S18]Sx (x≈2). Solid State Ion. 43, 133–142 (1990)
K. Kaup, K. Bishop, A. Assoud, J. Liu, L.F. Nazar, Fast ion-conducting thioboracite with a perovskite topology and argyrodite-like lithium substructure. J. Am. Chem. Soc. 143(18), 6952–6961 (2021)
K. Kaup, J.D. Bazak, S.H. Vajargah, X.H. Wu, J. Kulisch, G.R. Goward, L.F. Nazar, A lithium oxythioborosilicate solid electrolyte glass with superionic conductivity. Adv. Energy Mater. 10(8), 9 (2020)
K. Kaup, A. Assoud, J. Liu, L.F. Nazar, Fast Li-ion conductivity in superadamantanoid lithium thioborate halides. Angew. Chem.-Int. Ed. 60(13), 6975–6980 (2021)
Z. Zhang, J.H. Kennedy, J. Thompson, S. Anderson, D.A. Lathrop, H. Eckert, Competitive network modification in non-oxide chalcogenide glasses structural and motional properties of glasses in system Li2S-P2S5-B2S3 studied by multinuclear NMR techniques. Appl. Phys. A-Mater. Sci. Process. 49(1), 41–54 (1989)
C. Puttmann, H. Diercks, B. Krebs, Synthesis, crystal-structure and properties of M3B3S6 (M = Na, K, Rb) and LiSrB3S6. Phosphorus Sulfur Silicon Relat. Elem. 64–5(1–4), 179–182 (1992)
P. Vinatier, M. Menetrier, A. Levasseur, Structure and ionic conduction in lithium thioborate glasses and crystals. Phys. Chem. Glasses 44(2), 135–142 (2003)
M. Tatsumisago, A. Hayashi, Superionic glasses and glass-ceramics in the Li2S-P2S5 system for all-solid-state lithium secondary batteries. Solid State Ion. 225, 342–345 (2012)
S. Kondo, K. Takada, Y. Yamamura, New lithium ion conductors based on Li2S-SiS2 system. Solid State Ion. 53, 1183–1186 (1992)
M.D. Ingram, Superionic glasses: theories and applications. Curr. Opin. Solid State Mater. Sci. 2(4), 399–404 (1997)
Z.M. Zhang, J.H. Kennedy, Synthesis and characterization of the B2S3-Li2S, the P2S5-Li2S and the B2S3-P2S5-Li2S glasses systems. Solid State Ion. 38(3–4), 217–224 (1990)
M. Menetrier, C. Estournes, A. Levasseur, K.J. Rao, Ionic-conduction in B2S3-Li2S-LiI glasses. Solid State Ion. 53, 1208–1213 (1992)
W. Burckhardt, M. Makyta, A. Levasseur, P. Hagenmuller, Fast Li+ ion-transport in iodine-thioborate glasses. Mater. Res. Bull. 19(8), 1083–1089 (1984)
Y. Seino, K. Takada, B.C. Kim, L.Q. Zhang, N. Ohta, H. Wada, M. Osada, T. Sasaki, Synthesis and electrochemical propeities of Li2S-B2S3-Li4SiO4. Solid State Ion. 177(26–32), 2601–2603 (2006)
F. Mizuno, A. Hayashi, K. Tadanaga, M. Tatsumisago, High lithium ion conducting glass-ceramics in the system Li2S-P2S5. Solid State Ion. 177(26–32), 2721–2725 (2006)
M. Grune, H. Meierkord, W. Mullerwarmuth, P. Zumhebel, B. Krebs, M. Wulff, 7Li NMR spinp-lattice relaxation and ionic-diffusion in lithium thioborate glasses. Ber. Bunsen-Ges. Phys. Chem. Chem. Phys. 93(11), 1313–1317 (1989)
K.S. Suh, A. Hojjaji, G. Villeneuve, M. Menetrier, A. Levasseur, 11B NMR-studies of the local environment of boron in B2S3-Li2S-LiI glasses. J. Non-Cryst. Solids 128(1), 13–17 (1991)
M. Menetrier, A. Hojjaji, C. Estournes, A. Levasseur, Ionic-conduction in the B2S3-Li2S glass system. Solid State Ion. 48(3–4), 325–330 (1991)
R. Mercier, J.P. Malugani, B. Fahys, G. Robert, Superionic conduction in Li2S-P2S5-LiI-glasses. Solid State Ion. 5, 663–666 (1981)
S. Ujiie, T. Inagaki, A. Hayashi, M. Tatsumisago, Conductivity of 70Li2S-30P2S5 glasses and glass-ceramics added with lithium halides. Solid State Ion. 263, 57–61 (2014)
J.H. Kennedy, Z.M. Zhang, H. Eckert, Ionically conductive sulfide-based lithium glasses. J. Non-Cryst. Solids 123(1–3), 328–338 (1990)
M. Menetrier, A. Hojjaji, A. Levasseur, M. Couzi, K.J. Rao, Structural hypotheses and Raman-spectroscopy investigations of glasses belonging to the B2S3-Li2S-LiI system. Phys. Chem. Glasses 33(6), 222–227 (1992)
J. Deppe, M. Balkanski, R.F. Wallis, Statistical mechanical theory of ionic-conductivity in sulfur glasses. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 15(3), 255–259 (1992)
P. Vinatier, M. Menetrier, A. Levasseur, Aggregation of the doping salt in B2S3-Li2S-LiI glasses, effect on the dynamical properties. Solid State Ion. 116(1–2), 35–45 (1999)
S. Balasubramanian, K.J. Rao, A molecular-dynamics study of atomic correlations in glassy B2S3. J. Phys. Chem. 98(37), 9216–9221 (1994)
M. Kbala, M. Makyta, A. Levasseur, P. Hagenmuller, Characterization and electrical behavior of new lithium chalcoborate thin-films. Solid State Ion. 15(2), 163–169 (1985)
M. Menetrier, A. Levasseur, C. Delmas, J.F. Audebert, P. Hagenmuller, New secondary batteries for room-temperature applications using a vitreous electrolyte. Solid State Ion. 14(3), 257–261 (1984)
M. Menetrier, C. Delmas, A. Levasseur, On the behavior of intercalation compounds in solid-state batteries. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 15(1), 101–104 (1992)
D.Y. Seung, A. Levasseur, M. Couzi, Preparation, infrared and Raman characterisation and electrical properties of Li2S-B2S3-As2S3 based glasses. Phys. Chem. Glasses 39(5), 295–300 (1998)
A. Hammerschmidt, P. ZumHebel, F. Hiltmann, B. Krebs, Synthesis and crystal structures of Li4–2xSr2+xB10S19 (x≈0.27) and Na6B10S18: two novel thioborates with highly polymeric macro-tetrahedral networks. Z. Anorg. Allg. Chem. 622(1), 76–84 (1996)
A. Hammerschmidt, C. Jansen, J. Kuper, C. Koster, B. Krebs, Syntheses, crystal structures, and properties of the novel thioborates KBa4(BS3)3 and K4Ba11(BS3)8S. Z. Anorg. Allg. Chem. 627(4), 669–674 (2001)
J. Kuchinke, C. Jansen, A. Lindemann, B. Krebs, Syntheses and crystal structures of the novel ternary thioborates Na3BS3, K3BS3, and Rb3BS3. Z. Anorg. Allg. Chem. 627(5), 896–902 (2001)
J. Kuchinke, J. Kuper, B. Krebs, Novel thioborates of cesium: CS3BS3 and Li2CsBS3. Z. Naturforsch. (B) 57(12), 1433–1438 (2002)
J. Kuchinke, A. Lindemann, C. Koster, A. Hammerschmidt, M. Doch, T. Pruss, B. Krebs, Li6-xCsxB10Q18 (Q=S, Se; x≈1): New polymeric layered anion networks in chalcogenoborates. Phosphorus Sulfur Silicon Relat. Elem. 168, 605–608 (2001)
B. Krebs, H. Diercks, Ag6B10S18-A noval thioborate with tetrahedral coordination of boron. Z. Anorg. Allg. Chem. 518(11), 101–114 (1984)
A. Haffner, A.K. Hatz, I. Moudrakovski, B.V. Lotsch, D. Johrendt, Fast sodium-ion conductivity in supertetrahedral phosphidosilicates. Angew. Chem.-Int. Ed. 57(21), 6155–6160 (2018)
R. Bertermann, W. Muller-Warmuth, C. Jansen, F. Hiltmann, B. Krebs, NMR studies of the lithium dynamics in two thioborate superionic conductors: Li9B19S33 and Li4–2xSr2+xB10S19 (x≈0.27). Solid State Ion. 117(3–4), 245–255 (1999)
A. Haffner, A.K. Hatz, O.E.O. Zeman, C. Hoch, B.V. Lotsch, D. Johrendt, Polymorphism and fast potassium-ion conduction in the T5 supertetrahedral phosphidosilicate KSi2P3. Angew. Chem.-Int. Ed. 60(24), 13641–13646 (2021)
M. Grune, W. Mullerwarmuth, P. Zumhebel, B. Krebs, Unusual lithium dynamics and NRM relaxation in a novel crystalline thioborate-sulfide. Solid State Ion. 66(1–2), 165–173 (1993)
M. Grune, W. Mullerwarmuth, P. Zumhebel, B. Krebs, Complex lithium dynamics in the novel thioborate Li5B7S13 revealed by relaxation and lineshape studies. Solid State Ion. 78(3–4), 305–313 (1995)
D. Gonbeau, H. Bouih, L. Benoist, G. Pfister-Guillouzo, M. Menetrier, P. Vinatier, A. Levasseur, XPS analyses of B2S3-Li2S glasses. Experimental and theoretical study. Phys. Chem. Glasses 39(3), 167–172 (1998)
Y.K. Lian, L.M. Wu, L. Chen, Thioborates: potential nonlinear optical materials with rich structural chemistry. Dalton Trans. 46(13), 4134–4147 (2017)
J. Kuchinke, A. Hammerschmidt, B. Krebs, Rb8B12(BS3)6 and Cs8B12(BS3)6: the first thioborato-closo-dodecaborates. Solid State Sci. 5(1), 189–196 (2003)
T. Kajiki, Y. Hayashi, H. Takizawa, High-pressure synthesis of a new copper thioborate, CuBS2. Mater. Lett. 61(11–12), 2382–2384 (2007)
H.Y. Guo, Q. Wang, A. Stuke, A. Urban, N. Artrith, Accelerated atomistic modeling of solid-state battery materials with machine learning. Front. Energy Res. 9, 25 (2021)
F.J. Chen, S.Q. Cheng, J.B. Liu, S.N. Li, W.H. Ouyang, B.X. Liu, Insights into the electrochemical stability and lithium conductivity of Li4MS4 (M = Si, Ge, and Sn). ACS Appl. Mater. Interfaces 13(19), 22438–22447 (2021)
A.D. Sendek, Q. Yang, E.D. Cubuk, K.A.N. Duerloo, Y. Cui, E.J. Reed, Holistic computational structure screening of more than 12 000 candidates for solid lithium-ion conductor materials. Energy Environ. Sci. 10(1), 306–320 (2017)
J. Xu, L. Liu, N. Yao, F. Wu, H. Li, L. Chen, Liquid-involved synthesis and processing of sulfide-based solid electrolytes, electrodes, and all-solid-state batteries. Mater. Today Nano 8, 37 (2019)
B. Krebs, Thio-compounds and seleno-compounds of main group elements-novel inorganic oligomers and polymers. Angew. Chem.-Int. Ed. Engl. 22(2), 113–134 (1983)
Acknowledgments
This work is supported by the Key Program-Automobile Joint Fund of National Natural Science Foundation of China (Grant No. U1964205), Key R&D Project funded by Department of Science and Technology of Jiangsu Province (Grant No. BE2020003), General Program of National Natural Science Foundation of China (Grant No. 51972334), General Program of National Natural Science Foundation of Beijing (Grant No. 2202058), Cultivation project of leading innovative experts in Changzhou City(CQ20210003), National Overseas High-level Expert recruitment Program (Grant No. E1JF021E11), Talent Program of Chinese Academy of Sciences, “Scientist Studio Program Funding” from Yangtze River Delta Physics Research Center and Tianmu Lake Institute of Advanced Energy Storage Technologies (Grant No. TIES-SS0001), and Science and Technology Research Institute of China Three Gorges Corporation (Grant 202103402).
Author information
Authors and Affiliations
Contributions
ZX and ZZY performed literature research and wrote the manuscript. WF conceived the idea, supervised the project, and revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Zhu, X., Zhang, Z., Chen, L. et al. Progress in lithium thioborate superionic conductors. Journal of Materials Research 37, 3269–3282 (2022). https://doi.org/10.1557/s43578-022-00592-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00592-4