Abstract
Ferromagnetic resonance was used to study the temperature dependence of mixed manganese-cobalt ferrite nanoparticles with a size range between 5 and 42 nm synthesized by the hydrothermal method. Structural characterization was carried out using X-ray diffraction, scanning, and transmission electron microscopy. M(H) curves as a function of the Mn2+ content at 2.5 and 300 K, and FMR in the temperature range of 80 < T < 700 K were used for the magnetic characterization. Temperature dependence of the resonance field shows three regions that can be interpreted in terms of agglomeration, dispersion and superparamagnetism in the samples. The HR and \({\Delta H}_{\text{PP}}\) as a function of the temperature and Mn2+ content allows us to elucidate the key characteristics of the deviation from the ideal superparamagnetic behavior observed by magnetic measurements and open up new research possibilities for evaluating interparticle interactions in nanoparticles.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinel ferrite draws its attention to researchers for a long time due to its unique structural and magnetic properties that can be used in transformer cores, magnetic resonance imaging, microwave devices, data storage devices, drug delivery, supercapacitors and biomedical purposes [1, 2]. The suitable magnetic and mechanical properties of ferrites make them worthy for device fabrication. During the development of new technologies, spinel-oxide-type materials are considered competent materials due to their extraordinary physical, chemical, electronic, and magnetic properties [3]. The structural, optical, electrical, and magnetic properties of spinel ferrites also change as the distribution of cations among available A and B sites is modified with doping of impurities, furthermore depending on the physical characteristics, such as particle size, chemical composition, preparation method, surface properties of the particles and interparticle interactions. The magnetic order of especially the surface spins play important role in determining the magnetic properties of a whole ferrite particle due to the high surface-to-volume ratio [4]. Among the various ferrites, cobalt ferrite (CoFe2O4) nanoparticles have fascinated continuous interest owing to their excellent structural, optical, electrical, magnetic, chemical, catalytic, and thermal properties over the last fifteen years. CoFe2O4 nanoparticles are gaining increasing interest as gas sensors, photocatalysts, contrast agents for magnetic resonance imaging, targeted drug delivery, cancer imaging and therapy, magnetic hyperthermia, ferrofluids, theragnostic, lithium-ion batteries, magneto-optical devices, and spintronics [5]. It has been found that among the various factors that affect the magnetic, structural, and electrical behavior of nanoferrites particles are their composition, crystal size, and shape. Badoo et al. states that a fine tune of the stoichiometry or composition of the constituents elements is very important to control this properties with the incorporation of Zn, Cd, Zr, and Cu in the ferrite CoFe2O4 [6,7,8,9]. In addition, manganese ferrite has also been of great interest as it has good chemical stability, low coercivity, electrical properties, mechanical hardness, moderate saturation magnetization, and contrast enhancement agents for MRI technology. Recently, the attention of the researchers is focused on the substitution of manganese in cobalt ferrite as they find distorted structure in the samples. When Mn is added to cobalt ferrite, Mn takes its position in the tetrahedral (A) site, resulting in the movement of Co from the tetrahedral (A) site to the octahedral [B] site, in turn; magnetic properties of the Co1−xMnxFe2O4 system are changed. Currently, interest has been switched towards mixed Mn–Co ferrite nanoparticles with modified structure due to enhanced and emerging properties than that of Mn and Co ferrite individually [10, 11].
Temperature dependence of the Ferromagnetic Resonance Spectra (FMR) provides valuable information on magnetic systems and phase transitions, especially in the assembly of magnetic nanoparticles [12]. For magnetic nanoparticles, a decrease in temperature is accompanied by a shift in the resonance field, an increasingly asymmetric lineshape, and an increased broadening of the resonance lineshape [13]. The FMR of ferrites is important for investigating the magnetic properties at high frequency due to the resonance originates from the interaction between the spin and electromagnetic waves. FMR has been used to study the spin-glass transition in mixed and doped iron oxide nanoparticles. A sudden decrease in resonance field and simultaneous enhancement in spectral linewidth respectively with the decrease in the temperature are accompanied by this characteristics transition [14].
Here, we study the effect of the Mn2+ substitution on the structural properties of the MnxCo1−xFe2O4 ferrite nanoparticles, and the effect of the temperature on the magnetic properties. Moreover, the temperature dependence of the ferromagnetic resonance spectrum for these nanoparticles, with the aim of characterizing the temperature dependence of the spin dynamics of the nanosized system has been focused on. The ferromagnetic resonance spectra were recorded as a function of the Mn2+ content \(\left(0\le x\le 1\right)\) in the temperature range 80 < T < 700 K. Herein, we present a systematic study of the influence of Mn2+ content in the resonance field and the peak-peak linewidth. These measurements as a function of temperature have proved to be a powerful tool to distinguish magnetic contributions that otherwise were not clear from direct current (DC) measurements, which have contradictory interpretations in the literature.
Materials and methods
Methods
MnxCo1−xFe2O4 nanoparticles (x = 0.00; 0.25; 0.50; 0.75 and 1.00) have been synthesized using the hydrothermal method. For hydrothermal reactions, all the reagents were of analytical grade and used as received. A mixture of 1 mmol Fe(NO3)3·9H2O, 0.5 mmol of Co(NO3)3·6H2O, and 0.5 mmol of Mn(NO3)3·4H2O were dissolved in distilled water in a relation of (2:1:1) respectively, followed by the addition of 4 M KOH solution to adjust the pH at 12. The mixture was then transferred into a Teflon-lined stainless-steel autoclave of 100 mL capacity and stirred until the reactants were completely dissolved. The resulting black precipitates were collected by filtration and washed with deionized water and ethanol, and finally dried in an oven at 60 °C for 24 h. Phase identification, purity, relative crystallinity, structure, and the average size of the samples were determined at room temperature in a polycrystalline sample brand X-ray diffractometer model Bruker D8 focus using Cu K radiation (λ = 1.5406 ˚A) and resolution of 0.002 in 2θ. The structural properties were obtained using a Scanning Electron Microscopy (SEM) model Philips XL-30 with an analyzer EDAX DX-4 for elemental analysis and Transmission Electron Microscopy (TEM) JEOL 1200EXII). The magnetic measurements were carried out in a Quantum Design SQUID magnetometer MPMSXL7 operating between 1.8 and 350 K for DC-applied fields ranging from − 7 to 7 T. Ferromagnetic Resonance measurements were recorded using an EMX BRUKER spectrometer working at the X-band in the temperature range 80 ≤ T ≤ 700 K.
Results and discussion
Structural properties
XRD
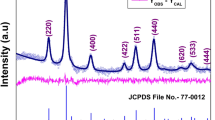
XRD patterns of \({\text{Mn}}_{x}{\text{Co}}_{1-x}{\text{Fe}}_{2}{O}_{4} \left(0\le x\le 1\right)\) are shown in Fig. 1a. The samples show the characteristic peaks indexed to the cubic spinel phase of ferrite material with the most intense peak attributed as (311). The hkl indexes of the samples are (220), (311), (222), (400), (422), (511), and (440), which indicate the presence of a mixed type cubic spinel structure checked with XRD cards of CoFe2O4 NPs (JCPS file: 22-1086) and MnFe2O4 PDF-ICDD, 2012). A displacement of the position of (311) peak with the increase of the concentration of Mn2+, indicates a systematic change of the lattice parameter.
The average particle size, d, was calculated using the Debye–Scherrer Eq. (1) for the most intense peak (311).
where λ is the wavelength of the radiation, θ is the diffraction angle, and β is the FWHM of the diffraction peak. The lattice parameter has been computed using the d-spacing values and the respective (hkl) parameters from the classical formula given in Eq. (2)
In Table 1, we show the average size (d) and the lattice parameter (a), determined using Eqs. (1, 2), as a function of Mn2+ content for MnxCo1−xFe2O4. From the table, an increase with the Mn2+ content is observed, except for x = 0.75, in which a slight decrease in d spacing occurs, maybe due to the rearrangement of the ions in the crystalline structure, that produce a movement of the Mn2+ ions from the octahedral to tetrahedral sites [1, 15]. The calculated average sizes of the particles vary in the range of 5–60 nm and the lattice parameter varies in the range of 0.831–0.843 nm, both have a dependence on the Mn2+ content, as can be seen in Table 1, obeying the Vegard Law [1, 2]. This increase is attributed to the replacement of Co2+ cations having a smaller ionic radius (0.078 nm) by Mn2+ cations having a larger one (0.082 nm). When small-sized Co2+ ions are substituted with large-sized Mn2+ ions, the spinel cobalt ferrite will eventually expand. Doping of Mn2+ ions in spinel-type structure will induce uniform strain in the lattice as the material is elastically deformed.
SEM and TEM analysis
SEM and TEM analysis was made over all the samples with the aim of visualizing the structural properties. TEM images for the MnxCo1−xFe2O4 \(\left(0\le x\le 1\right)\) nanoparticles are shown in Fig. 2. The mean particle size of the samples was obtained using the TEM measurements and the results are shown in Fig. 2. The average particle size distribution shows that the mean size is relatively close for x = 0 and 0.25 and equal to \(\left(5 \pm 1\right)\) and \(\left(6 \pm 1\right) {\text{nm}}\), respectively (Fig. 2a, b). The shape of these nanoparticles is predominantly spherical. For x = 0.5 and 0.75, the size of the nanoparticles increases to 15 ± 1 and 11 ± 1 nm, respectively and the morphology becomes rhomboidal (Fig. 2c, d). For sample x = 1, nanoparticles with the mean size 42 ± 1 nm and large nanoparticles also with a rhomboidal shape could be observed in the TEM image in Fig. 2e. The increase of the size of the nanoparticles with the Mn2+ substitution is in line with that observed from the XRD analysis. The average size of the nanoparticles and lattice parameter increases with the Mn2+ concentrations. Elemental chemical analysis confirms that Co, Mn, Fe, and O ions are present in the nanoparticles and shows that the experimental stoichiometry obtained is similar to the expected one. The SEM micrographs in figure S.1 (Supplementary Information), show the formation of large grains without a defined shape.
Magnetic properties
Magnetization curves
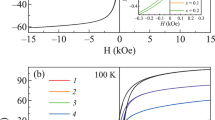
Figure 3a–e show the magnetization curves at 300 K and Fig. 3f–j exhibit the magnetization curves at 2.5 K of MnxCo1−xFe2O4 at different Mn2+ content. The magnetization values at 3 kOe, MS, remanent magnetization, Mr, and the coercive field, Hc, are summarized in Table 1. MS ranges between 50 and 75 emu/g for all the samples at 300 K.
These MS values are lower than the ones of corresponding bulk materials (110 emu/g for MnFe2O4 and 93.9 emu/g for CoFe2O4) [16] probably due to the effects of surface spin disorder or the spins canting [17, 18]. The Magnetization curves, measured at 2.5 K, showed hysteresis loops corresponding to a ferromagnetic state of the samples, and for the measurements, at room temperature, we observe no hysteresis loop in agreement with a superparamagnetic state. At low temperatures, the magnetization curves have two important characteristics, an open hysteresis loop and unsaturation at 3 kOe for all samples except x = 1. The dependence of the coercive field, Hc, as a function of the Mn2+ content (Fig. 4a) shows a maximum in the coercive field for x = 0.5 at 300 K, while it is not present at 2.5 K. It is important to note that the average size of the nanoparticles also has a local maximum at this Mn2+ concentration [4]. At 2.5 K a monotonic decrease in Hc with the Mn2+ content may be observed [19]. Figure 4b displays the dependence of the MS as a function of the Mn2+ content at 2.5 and 300 K. A linear increase of the MS as a function of the Mn2+ content is observed at 300 and 2.5 K. The magnetization increases in all the range studied, except for x = 0.75 at room temperature, due to the substitution of Mn2+ (5.92 µB) ions and Co2+ (3.87 µB) in the octahedral sites [20, 21]. At 2.5 K the saturation of the magnetization is not observed even at a relatively high applied field of 3 kOe, Topkaya et al. suggest the existence of surface spins disorder due to symmetry broken for antiferromagnetic interactions [4]. Due to a strong coupling or pinning between disordered surface spins and ordered core ones, the former are not easily aligned even with a high applied field [4]. Figure 4c shows the remanent magnetization Mr as a function of the Mn2+ content at 300 K and 2.5 K. Small Mr values are observed for all concentrations studied at 300 K similarly to the behavior obtained for the coercive field, these results are in accordance with a superparamagnetic ordering in the nanoparticles at this temperature. When the temperature is lowered to 2.5 K, a slightly linear increase in the remanent magnetization up to x = 0.75, due to changes in the Mn2+ distribution, is observed. At 2.5 K, Mr exhibits an out-of-trend behavior with an increment for sample x = 0.5, besides that, an abrupt decrease for MnFe2O4. Similarly, the remanent magnetization at 300 K shows a linear behavior, except for x = 0.5, where a marked increase is observed may be due to the particle size distribution. The corresponding sizes for x = 0.0, 0.25, and 0.75 are in accordance with particles in the superparamagnetic state, but x = 0.5 are in a monodomain state where the remanence magnetization is greater than for the former [22, 23]. The uniaxial anisotropy constant was determined using the LAS law, Eq. (3) [24].
where the numerical coefficient 8/105 holds for random polycrystalline specimens with cubic anisotropy and K1 is the uniaxial anisotropy constant. The term κH is included to take into consideration of the observed increase in the spontaneous magnetization at high fields, known as the forced magnetization, with the parameter K being the high-field susceptibility. In general, the forced magnetization term is necessary to fit the hysteresis curves at higher temperatures and higher fields [24]. The uniaxial anisotropy constant of MnxCo1−xFe2O4 system as a function of the Mn2+ content at 300 K and 2.5 K, are plotted in Fig. 4d. The calculated uniaxial anisotropy constant is ranging from 0.06 to 0.38 × 106 erg/cm3 at 300 K, and 0.08 to 0.59 × 106 erg/cm3 at 2.5 K. In both cases, low and room temperatures, the uniaxial anisotropy constant is nearly the same for x = 1 sample (MnFe2O4). K1 takes its maximum value at room temperature when x = 0.25, at 2.5 K this maximum shift to x = 0.5. It may be due to the largest contribution of Co2+ ions, located at the octahedral sites B of the spinel structure [4], while for the x > 0.5, the cobalt ions have a smaller contribution to this parameter due to the reduction of the occupation of Co2+ ions on the sites B with a decrease in the anisotropic properties of the Co2+ ions [25].
Ferromagnetic resonance (FMR)
The temperature dependence of the ferromagnetic spectra, in the temperature range 90 < T < 670 K as a function of the Mn2+ content, was investigated to evaluate the effect of the temperature and the concentration of Mn2+ on the dynamic properties of the samples. The FMR condition for magnetic materials is given by:
where ω, γ, and Heff are the angular frequency, the gyromagnetic ratio, and the effective magnetic field, respectively. If the resonance field value (3400 Oe, in g = 2) is different from the one observed, this indicates that there is an interaction between magnetic nanoparticles [4]. Therefore, the effective magnetic field can be described by the sum of two terms:
where the first term of the right side of Eq. (5) represents the applied external magnetic field, this is the resonance field HR, the second term represents the internal magnetic field, defined as follows:
where Hdem is the internal field, due to demagnetization, Hdip is the internal field, due to the dipole–dipole interaction between neighboring magnetic nanoparticles, and \({H}_{\text{dip}}^{^{\prime}}\) is the internal field that arises from the dipole–dipole interaction between agglomerates.
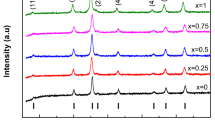
Figure 5 shows the FMR spectra at room temperature for all the Mn2+ concentrations studied. For the CoFe2O4 nanoparticles, a single very broad signal with zero field absorption centered around 150 mT is observed. The doping with Mn2+ gradually produces a narrowing in the FMR spectra up to reach the x = 1 concentration (MnFe2O4) in which a very clear and intense spectrum with two signals, one of them the high field signal around 340 mT, and the low field signal around 250 mT.
Figure 6 shows the temperature dependence of the FMR spectra for samples at x = 0.75 (Fig. 6a) and x = 1 (Fig. 6b) in the temperature range 100 < T < 600 K. Figure 6c and d, exhibit the temperature dependence of the resonance field (HR) and peak-to-peak linewidth \(\left({\Delta H}_{\text{PP}}\right)\) for two samples, x = 0.75 and MnFe2O4 ones. Three regions can be observed in all samples containing Co2+ ions, but not for MnFe2O4. Their analysis shows different behaviors according to temperature. In region III (high field region) a near-constant resonance field is observed, which is in accordance with a superparamagnetic state of the samples where above the blocking temperature the resonance field is temperature independent. In this region, a slow increase of \({\Delta H}_{\text{PP}}\) with temperature decrease, agrees with an increase of dipolar interactions, due to the decrease in thermal energy. In region II (intermediate field region), a decrease in \({H}_{R}\) and an increase in \({\Delta H}_{\text{pp}}\) with the decrease of temperature is observed. This behavior is in accordance with both, the blocking of the magnetic moments of the nanoparticles and with the appearance of dipolar interparticle interactions. This latter increases the dipolar field in Eq. (5) and consequently decreases \({H}_{R}\) (Eq. 4) up to a minimum value, when nanoparticles begin to agglomerate. Resonance field behavior in the III–II regions can be explained using the Landau-Lifshitz dynamics [26] and as suggested by Kodama [27], as the temperature decreases, the surface spins freeze in the direction of the magnetic DC field. The Resonance field behavior at low field can be explained by the surface spin disorder. An exchange coupling between the spins on the surface and spins of the nucleus, which causes unidirectional anisotropy with an easy axis along the direction of the field [28], bringing therefore a decrease in the resonance field [29]. In region I (low field region) an increase in \({H}_{R}\) and \({\Delta H}_{\text{PP}}\) with the decreasing of the temperature up to \(T \sim 180 {\text{K}}\) is observed. The resonance field reaches a minimum value and then increases, which may occur because of the formation of nanoparticles agglomerates. This latter induces an appearance of dipolar interactions between agglomerates in addition to the interparticle dipolar interactions. The former is more weakly than the latter, so the resonance field begins to increase. According to the Landau-Lifshitz model [30] in the temperature range \(180\le T\le 360 K\), \({\Delta H}_{\text{PP}}\) scales as l/T as predicted by the model, which states this behavior for aggregates of particles. Below \(T \sim 180 {\text{K}},\) the resonance field and the peak-to-peak linewidth becomes almost constant showing a paramagnetic like behavior. In the three regions, the linewidth increases continuously, while the temperature decreases. However, a slight change in the slope of the linewidth at the blocking temperature for the NPs and just before the nanoparticles begin to agglomerate. In the MnFe2O4 sample, two signals very close each to other in almost the entire temperature range studied are observed, the high field signal may be associated with a paramagnetic phase of manganese in very low proportion; instead for ferrites containing cobalt, they show a unique and symmetric signal above of 400 K that can be associated to superparamagnetic behavior of the system [31]. MnFe2O4 shows a different behavior than that shown by mixed Mn–Co ferrites. For this sample, the resonance field shows a monotonous growth with the increase in temperature, this behavior is typical of ferrimagnetic systems where the increase in thermal energy gradually decreases the local fields. On the other hand, the linewidth shows a change in slope around T = 430 K. Even when the superparamagnetic blocking temperature is not reached, this slope change must be related to the start of the superparamagnetic behavior of the nanoparticles. It is important to emphasize that the behavior of the resonance field and the linewidth in MnFe2O4 is different from that of mixed ferrites because the size of the former is large enough to be multidomain particles, while the latter is essentially monodomain particles.
Figure 7 shows the dependence of HR and ∆HPP as a function of Mn2+ content for MnxCo1−xFe2O4 at 300 K. A decrease in ∆HPP with the increase in the Mn2+ content is observed, except for sample x = 0.75, which is out of the tendency, in accordance with the results for magnetization and the particle size obtained with XRD. A small saturation magnetization in conjunction with a small particle size presents a favorable situation for interparticle magnetostatic interaction, which results in a broadening of ∆HPP [32]. An abrupt decrease in linewidth is observed for the MnFe2O4 sample, indicating a decrease in dipole interactions of the nanoparticles. The behavior of ∆HPP as a function of the Mn2+ content agrees with those reported by Arda et al. [33] for the Zn1−xCoxO system synthesized by the sol–gel method. The resonance field HR linearly increases due to the occupation of Mn2+ at octahedral sites. The presence of Mn2+ at the octahedral sites causes a decrease in the magnetic moment of sublattice B, which results in a decrease in the total magnetic moment, this in accordance with a decrease in the internal fields, and therefore an increase in HR is observed. An anomalous decrease in the HR behavior for x = 0.5 is observed in agreement with the behavior of the remanent magnetization.
The spin–spin relaxation time constant (τ) as a function of temperature for all samples is shown in Fig. 8. The spin–spin relaxation time constant is calculated with the help of the following equation:
where h is the Planck’s constant (6.626 × 10−34 J s), \(\hslash =h/2\pi\), and β is a Bohr magneton (9.27 × 10−24 J T−1). All mixed Mn2+-Co2+ samples, except the sample x = 0.50, present similar behavior: at low temperatures \((T<400 {\text{K}})\), a quasi-constant behavior of the spin–spin relaxation time with a maximum reached at 225 K for x = 0, and 255 K for x = 0.25 and 0.75, respectively. This maximum may be correlated with the temperature at which the dipolar interaction between agglomerates of the nanoparticles begins to dominate over the dipolar interaction between nanoparticles. Above \(T=400 {\text{K}},\) an abrupt increase in the spin–spin relaxation time is observed. This behavior is correlated with a decrease in the \(\Delta {H}_{\text{PP}}\) in accordance with the decrease in the dipole–dipole interactions. The temperature dependence of the spin–spin relaxation time for x = 0.50 is shown in Fig. 8a. The maximum on this curve at T = 230 K can be correlated with the formation of agglomerates. Then, a continuous decrease in the relaxation time is observed. It can be seen that the spin–spin relaxation time is one order of magnitude higher than those for the other samples in agreement with the results obtained for the magnetization curves. Note that a continuous increase in the spin–spin relaxation time in all temperature range can be observed for MnFe2O4 (Fig. 8c), with a change in the slope at near \(T=400 {\text{K}},\) as for the rest of the samples, except for x = 0.5.
Summary
A series of spherical and rhomboidal ferrites nanoparticles of MnxCo1−xFe2O4 with an average size range 5–42 nm was obtained using the hydrothermal method. Their magnetic properties show a ferromagnetic behavior at low temperatures and a superparamagnetic behavior at room temperature. The temperature dependence of the ferromagnetic resonance (FMR) spectra shows three regions. The resonance field increases when the temperature decreases in region I and the peak to peak linewidth follows a Landau-Lifshitz behavior (product of the agglomeration of the nanoparticles). In region II, the resonance field increases with temperature, following the Landau-Lifshitz model. The linewidth in this region decreases with temperature with a slow change in the slope in agreement with weakening in dipole–dipole interactions. Finally, region III shows a superparamagnetic behavior throughout the range of temperature as a consequence of modification in the magnetic regime (vanishing of the magnetic moments blocking). Here, a decrease in the linewidth with temperature in agreement with a superparamagnetic behavior is observed. The peak-to-peak linewidth shows a slight decrease with the Mn2+ content. This suggests that dipolar interactions become weaker, while the resonance field increases with the concentration of Mn2+. However, sample x = 0.5 presents an exception from this behavior because Mn2+ occupy the octahedral sites, which provides a decrease in the magnetic moment of sublattice B. This situation induces a decrease in the total magnetic moment. Spin–Spin relaxation time indicates the temperature at which the nanoparticles begin to agglomerate and the temperature at which the systems have shown a superparamagnetic behavior.
References
S. Nasrin, F.U.Z. Chowdhury, S.M. Hoque, Study of hyperthermia temperature of manganese-substituted cobalt nano ferrites prepared by chemical co-precipitation method for biomedical application. J. Magn. Magn. Mater. 479, 126–134 (2019). https://doi.org/10.1016/j.jmmm.2019.02.010
L.S. Ghadimi, N. Arsalani, I. Ahadzadeh, A. Hajalilou, E. Abouzari-Lotf, Effect of synthesis route on the electrochemical performance of CoMnFeO nanoparticles as a novel supercapacitor electrode material. Appl. Surf. Sci. 494, 440–451 (2019). https://doi.org/10.1016/j.apsusc.2019.07.183
S.M. Ansari, K.C. Ghosh, R.S. Devan, D. Sen, P.U. Sastry, Y.D. Kolekar, C.V. Ramana, Eco-friendly synthesis, crystal chemistry, and magnetic properties of manganese-substituted CoFe2O4 nanoparticles. ACS Omega 5(31), 19315–19330 (2020). https://doi.org/10.1021/acsomega.9b02492
R. Topkaya, Ö. Akman, S. Kazan, B. Aktas, Z. Durmus, A. Baykal, Surface spin disorder and spin-glass-like behaviour in manganese-substituted cobalt ferrite nanoparticles. J. Nanopart. Res. 14, 1156 (2012)
P. Monisha, P. Priyadharshini, S.S. Gomathi, K. Pushpanathan, Influence of Mn dopant on the crystallite size, optical and magnetic behavior of CoFe2O4 magnetic nanoparticles. J. Phys. Chem. Solids 148, 109654 (2021)
S. Asiri, M. Sertkol, S. Guner, H. Gungunes, K.M. Batoo, T.A. Saleh, H. Sozeri, M.A. Almessiere, A. Manikandan, A. Baykal, Hydrothermal synthesis of CoyZnyMn1–2yFe2O4 nanoferrites: magneto-optical investigation. Ceram. Int. 18(5), 5751–5759 (2018). https://doi.org/10.1016/j.ceramint.2017.12.233
S.S. Desai, S.E. Shirsath, K.M. Batoo, S.F. Adil, M. Khan, S.M. Patange, Influence of Zn-Zr substitution on the crystal chemistry and magnetic properties of CoFe2O4 nanoparticles synthesized by sol-gel method. Phys. B 596, 412400 (2020). https://doi.org/10.1016/j.physb.2020.412400
K.M. Batoo, E.H. Raslan, Y. Yang, S.F. Adil, M. Khan, A. Imran, Y. Al-Douri, Structural, dielectric and low temperature magnetic response of Zn doped cobalt ferrite nanoparticles. AIP Adv. 9, 055202 (2019). https://doi.org/10.1063/1.5078411
N. Boda, K.C. Naidu, K.M. Batoo, G.H. Joice, J.L. Naik, D. Ravinder, Structural, morphological and electronic properties of cadmium cobalt ferrite nanoparticles. Biointerface Res. Appl. Chem. 10(1), 4752–4763 (2020). https://doi.org/10.33263/BRIAC101.752763
A. Jamil, M.F. Afsar, F. Sher, M.A. Rafiq, Temperature and composition-dependent density of states extracted using overlapping large polaron tunneling model in MnxCo1 xFe2O4 (x = 0.25, 0.5, 0.75) nanoparticles. Phys. B 509, 76–83 (2017)
M.M. Hossen, S. Nasrin, M.B. Hossen, Structural, dielectric and magnetic properties of Mn2+ doped cobalt ferrite nanoparticles. J. Magn. Magn. Mater 599, 165726 (2019)
R. Rai, K. Verma, S. Sharma, S.S. Nair, M.A. Valente, A.L. Kholkin, N.A. Sobolev, Study of structural and ferromagnetic properties of pure and Cd doped copper ferrite. J. Phys. Chem. Solids 72(7), 862–868 (2011). https://doi.org/10.1016/j.jpcs.2011.04.002
H. Bayrakdar, O. Yalçın, U. Cengiz, S. Özüm, E. Anigi, O. Topel, Comparison effects and electron spin resonance studies of α-Fe2O4 spinel-type ferrite nanoparticles. Spectrochim. Acta A 374, 696–702 (2014)
M.R. Diehl, J.Y. Yu, J.R. Heath, G.A. Held, H. Doyle, S. Sun, C. Murray, Crystalline, shape and surface anisotropy in two morphologies of superparamagnetic cobalt nanoparticles by ferromagnetic resonance. J. Phys. Chem. 33, 7913–7919 (2001)
A.B. Salunkhe, V.M. Khot, M.R. Phadatare, N.D. Thorat, R.S. Joshi, H.M. Yadav, S.H. Pawar, Low temperature combustion synthesis and magnetostructural properties of Co-Mn nanoferrites. J. Magn. Magn. Mater. 352(1), 91–98 (2014). https://doi.org/10.1016/j.jmmm.2013.09.020
M.K. Shobana, S. Sankar, Characterization of sol-gel-prepared nanoferrites. J. Magn. Magn. Mater. 321(6), 599–601 (2009). https://doi.org/10.1016/j.jmmm.2008.09.040
R.H. Kodama, A.E. Berkowitz, E.J. McNiff Jr., S. Foner, Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett. 77(2), 394–397 (1996)
B. Martínez, X. Obradors, L. Balcells, A. Rouanet, C. Monty, Low-temperature surface spin-glass transition in γ-Fe2O3 nanoparticles. Phys. Rev. Lett. 80(1), 181–184 (1998)
Y. Köseoglu, H. Kavas, Size and surface effects on magnetic properties of Fe3O4 nanoparticles. J. Nanosci. Nanotechnol. 8, 584 (2008)
S.D. Bhame, P.A. Joy, Tuning of the magnetostrictive properties of CoFe2O4 by Mn substitution for Co. J. Appl. Phys. 100, 113911 (2006)
R.C. Kambale, P.A. Shaikh, N.S. Harale, V.A. Bilur, Y.D. Kolekar, C.H. Bhosale, K.Y. Rajpure, Structural and magnetic properties of Co1-xMnxFe2O4(0 {<=} x {<=} 0.4) spinel ferrites synthesized by combustion route. J Alloys Compd 490(1–2), 568–571 (2010). https://doi.org/10.1016/j.jallcom.2009.10.082
M. Angelakeris, Magnetic nanoparticles: a multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta - Gen. Subj. 1861(6), 1642–1651 (2017)
A. Mumtaz, K. Maaz, B. Janjua, S.K. Hasanain, M.F. Bertino, Exchange bias and vertical shift in CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 313(2), 266–272 (2007). https://doi.org/10.1016/j.jmmm.2007.01.007
K.M. Batoo, D. Salah, G. Kumar, A. Kumar, M. Singh, M. Abd El-Sadek, F.A. Mir, A. Imran, D.A. Jameel, Hyperfine interaction and tuning of magnetic anisotropy of cu doped CoFe2O4 ferritenanoparticles. J. Magn. Magn. Mater. 411, 91–97 (2016). https://doi.org/10.1016/j.jmmm.2016.03.058
M. Tachiki, Origin of the magnetic anisotropy energy of cobalt ferrite. Prog. Theor. Phys. 23, 1055 (1960)
G. Dixit, J.P. Singh, R.C. Srivastava, H.M. Agrawal, Magnetic resonance study of Ce and Gd doped NiFe2O4. J. Magn. Magn. Mater. 324(4), 479–483 (2014)
E. de Biasi, C.A. Ramos, R.D. Zysler, Size and anisotropy determination by ferromagnetic resonance in dispersed magnetic nanoparticle systems. J. Magn. Magn. Mater. 262(2), 235–241 (2003)
J. Typek, K. Wardal, N. Guskos, D. Sibera, U. Narkiewicz, FMR and magnetization study of ZnFe2O4 nanoparticles in 0.40Fe2O3/0.60ZnO nanocomposite. IEEE J. Magn. Mater. 50, 6101606 (2014)
D. Shi, B. Aktas, B. Pust, F. Mikailov, Nanostructured Magnetic Materials, and Their Applications (Springer- Verlag Inc., New York, 2003)
C. Vittoria, C.M. Williams, Ferrimagnetic resonance linewidth in single crystal MnZn-Ferrite. J. Magn. Magn. Mater. 54–57(3), 1193–1194 (1986)
H.H. Hamdeh, J.C. Ho, S.A. Oliver, R.J. Willey, G. Oliveri, G. Busca, Magnetic properties of partially-inverted zinc ferrite aerogel powders. Phys. J. Appl. 81, 1851 (1997)
G. Vázquez-Victorio, U. Acevedo-Salas, R. Valenzuela, in Ferromagnetic Resonance - Theory and Applications, ed. By O. Yalçin (IntechOpen, 2013), p. 169
L. Arda, M. Acikgoz, N. Doǧan, D. Akcan, O. Cakiroglu, Synthesis, characterization and ESR studies of Zn1−xCoxO nanoparticles. J. Supercond. Nov. Magn. 27, 799 (2014)
Acknowledgments
Thanks to the FONACIT for the financing of the PEII 2011001368 and MPPCTI- ECOS-Nord (V13PS01). The authors thank the CNRS, the University of Montpellier, and the Venezuelan Institute for Scientific Research (IVIC) for financial support. We also thank the Platform of Analysis and Characterizations of ICGM for measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sánchez, Y., Briceño, S., Larionova, J. et al. Temperature dependence of the ferromagnetic resonance (FMR) for MnxCo1−xFe2O4 (0 ≤ x ≤ 1) nanoparticles. Journal of Materials Research 36, 3329–3338 (2021). https://doi.org/10.1557/s43578-021-00345-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00345-9