Abstract
The oxidation behavior of nonstoichiometric Ti2AlCx (x = 0.69) powders synthesized by combustion synthesis was investigated in flowing air by means of simultaneous thermal gravimetric analysis-differential thermal analysis, X-ray diffraction, X-ray photoelectron spectroscopy, and scanning electron microscope/energy dispersive spectroscopy, with an effect of powder size. The oxidation of the fine Ti2AlC powders with the size of about 1 μm starts at 300 °C and completes at 980 °C, while with increasing the powder size around 10 μm the corresponding temperature increases to 400 and 1040 °C, respectively. The oxidation of nonstoichiometric Ti2AlCx (x = 0.69) powders is controlled by surface reaction in 400–600 °C, and mainly diffusion in 600–900 °C, with the corresponding oxidation activation energy of 2.35 eV and 0.12 eV, respectively. In other words, the critical temperature of changing oxidation controlling step is around 600 °C. The oxidation products were mainly rutile-TiO2 and α-Al2O3. The tiny white flocculent particles of α-Al2O3 appeared on the surface of fine Ti2AlC powders and increased with increasing the oxidation temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. INTRODUCTION
Ti2AlC belongs to a family of Mn+1AXn ternary compounds (MAX phases for short, where M represents early transition metal, A is group IIIA or IVA element, and X means C/or N, with n = 1–3),1 which displays a unique combination of the advantageous attributes of both metals and ceramics. Like metals, Ti2AlC has good thermal conductivity [46 W/(m K)],2 high damage tolerance,1 microscale plastic deformation,3 and excellent thermal shock resistance.4 Like ceramics, it exhibits low density (4.11 g/cm3),1 high Young’s modulus (277.6 GPa),5 and excellent high-temperature oxidation resistance.6 These unusual properties make Ti2AlC for potential structural and functional applications.7
Due to the great difficulty in the preparation of high-purity Ti2AlC, although early in 1963 the material was experimentally discovered by Jeitschko et al.,8 it has been ignored for a long time. Around 2000, Barsoum et al.9,10 fabricated polycrystalline high-purity Ti2AlC by reactive hot isostatic pressing and first identified its unusual mechanical and electrical properties. However, whether it is the long reaction time or high processing temperature will limit the future application of this technologically important material. To respond this problem, by the use of self-propagating high temperature combustion synthesis with pseudohot isostatic pressing (SHS/PHIP), Bai et al. have succeeded in the synthesis11,12 and characterization13,14 of high-purity Ti2AlC bulk, with the advantages of energy saving, short fabrication time, simple operation, and no external heat source. The resultant sample mainly contains typical plate-like nonstoichiometric Ti2AlCx (x = 0.69), which is rich in the lattice defects. Of importance, the fine microstructures with a mean grain size of 2.5–3 µm12 due to the short fabrication time of SHS/PHIP process contribute to the excellent mechanical properties of the derived Ti2AlC, which is evidenced in the very high flexural strength of 620 ± 20 MPa.14
Considering the potential use of Ti2AlC in the high-temperature applications, it is important and timely to get some more insights into its oxidation behavior. Indeed, the oxidation behavior of Ti2AlC has attracted much attention in the previous work,6,15–23 including oxidation kinetics, oxide scale, microstructure evolution, and crack healing in a relatively large oxidation time range from 20 h to 3000 h and temperature range from 500 °C to 1400 °C, since the first report on the oxidation behavior of Ti2AlC bulk by Barsoum et al.16 Wang and Zhou6,17 found that Ti2AlC had an excellent oxidation resistance owing to the formation of a protective α-Al2O3 scale, of importance where the oxidation kinetics were better described by cubic kinetics. This is also confirmed in the following work by Byeon et al.,18 Yang et al.,22 and Basu et al.23 Moreover, the excellent match between the thermal expansions of Ti2AlC and alumina in turn minimizes thermal residual stresses and concomitant propensity of spallation.18 Combining other properties such as lower density, Ti2AlC is by far the most attractive for practical applications.15
Materials in the form of powders are widely used in the industry as raw materials in powder metallurgy or thermal spray. In the latter, the high temperature (>2100 °C) in the center of plasma or flame will cause the Ti2AlC powders to be melted and softened before reaching the substrate.24 However, few research on the oxidation of Ti2AlC powders is conducted although it will contribute to well understanding the oxidation behavior of Ti2AlC at different scales. The only relevant report is from Zhang et al.24: they investigated the oxidation of Ti2AlC-based composite powders containing 68.3 wt% Ti2AlC, 14.0 wt% Ti3AlC2, 7.9 wt% TiC, and 9.8 wt% Ti1.2Al0.8 in the temperature range from 200 °C to 1000 °C, with the mean particle size of 45–100 µm.
Therefore, it is timely and important to investigate the oxidation behavior of high-purity Ti2AlC powders, especially for the nonstoichiometric Ti2AlC that may exhibit a different oxidation behavior from the stoichiometric one. The present work is to study the calefactive and isothermal oxidation behavior of Ti2AlC powders from 400 to 900 °C in flowing air, which would shed some light on the intrinsic oxidation behavior of Ti2AlC powders.
II. EXPERIMENTAL
A. Material preparation
The fabrication details can be found elsewhere.11 In short, commercially available elemental powders of Ti (99.2%, 36 µm), Al (99.5%, 12 µm), and carbon black (99.0%, 1 µm) with the Ti:Al:C molar ratios of 2.9:2:1 were selected as raw materials. Considering the volatilization of aluminum in the raw materials at high temperature, the Ti/Al/C molar ratios of 2.9:2:1 were close to 2:1:0.69 of nonstoichiometric Ti2AlCx (x = 0.69). At first, the porous Ti2AlC was synthesized from the Ti/Al/C powder mixture by combustion synthesis (SHS) without any pressure, and the as-synthesized Ti2AlC was rich in the lattice defects.12 After pulverizing, the Ti2AlC powders with different particle sizes used in this study were obtained by controlling milling time and sieving. The measured mean particle sizes of fine and coarse Ti2AlC powders by a laser particle size analyzer (Mastersizer 2000, Malvern Instruments, Malvern, United Kingdom) were around 1 µm and 10 µm, respectively.
B. Oxidation tests and characterization methodologies
Simultaneous thermal gravimetric analysis-differential thermal analysis (TG-DTA) was used to study the oxidation behavior of Ti2AlC powders. Calefactive oxidation tests of fine and coarse Ti2AlC powders were measured by using a SDTA851 thermal analyzer (Mettler-Toledo, Zurich, Switzerland) with Al2O3 crucibles in flowing air of 40 mL/min with a heating rate of 10 °C/min from room temperature (RT) to 1200 °C. Isothermal oxidation examinations of fine Ti2AlC powders with Al2O3 crucibles were performed over the temperature range from 400 to 800 °C in flowing air of 40 mL/min for 2 h, respectively. Due to the limitation of the instrument performance, the isothermal oxidation at 900 °C was carried out only for 1 h. The powders were protected in argon before reaching the required temperature. When the required temperature was reached, the computer automatically introduced flowing air into the furnace. Prior to each TG-DTA test, the corresponding baseline was drawn by heating the clean Al2O3 crucible to remove the false weight changes during the experiment. In this work, the thermal analysis results were calibrated by subtracting the corresponding baselines.
The phase analysis was conducted by X-ray diffraction (XRD; D/max-rB, Rigaku Corporation, Tokyo, Japan) using Cu Kα radiation with a step of 0.02°. X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, Thermo Fisher Scientific, Waltham, Massachusetts) employing a 500 µm monochromatic Al Kα X-ray beam was used to characterize the surface chemistry of powders after oxidation at different temperatures. The morphology surface observation of oxidation powders was conducted by scanning electron microscope (SEM; Quanta 200FEG, FEI Company, Hillsboro, Oregon) equipped with an energy dispersive spectroscopy (EDS) system. A thin layer of gold was sputtered on the surface of the oxidized powders prior to SEM.
III. RESULTS AND DISCUSSION
A. Simultaneous TG-DTA in flowing air
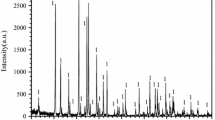
To investigate the effect of particle size on the oxidation behavior, the Ti2AlC powders with the particle sizes of 1 µm and 10 µm were used. Figure 1 shows the XRD patterns of fine and coarse Ti2AlC powders synthesized by SHS. It is seen that minor TiAl is detected for both the fine and coarse powders, and the coexistence of TiAl with Ti2AlC is probably because of the surplus of titanium in the raw materials.11 However, the intensity of diffraction peaks for TiAl in the powders is very weak. It can be concluded that high-purity Ti2AlC powders are obtained by SHS and the subsequent milling process.
Simultaneous TG-DTG-DTA curves for heating of Ti2AlC powders up to 1200 °C at a rate of 10 °C/min in flowing air are shown in Fig. 2. Upon heating, the mass gain of fine powders starts at 300 °C and completes at 980 °C [Fig. 2(a)], while with increasing the powder size around 10 µm the corresponding temperature increases to 400 °C and 1040 °C [Fig. 2(b)], respectively. In addition, the highest peak in the DTG curve, which represents the largest mass gain rate, appears at 580 °C of fine powders and 920 °C of coarse powders, respectively. It can be seen that the typical oxidation temperatures of coarse powders are always higher than those of fine powders, indicating that the oxidation resistance has been improved with increasing particle size. Clearly, this should be attributed to the high chemical activity of fine powders due to the high specific surface area. Moreover, the oxide powder size increases with the increase of the used Ti2AlC particle size, but the final oxidation products are both rutile-TiO2 and α-Al2O3 (Fig. 3).
The oxidation behavior of some MAX phase powders has attracted some attention in the previous work.24–27Table I lists and compares the temperatures corresponding to the starting (TS), completing (TC), and highest oxidation rate (TL) in Ti3AlC2, Ti2SnC, Ti2SC, and Cr2AlC. For Ti2AlC-based composite powders with the mean size of 45–100 µm, TS, TC, and TL are determined to be 400 °C, >1298.8 °C, and 947.7 °C,24 in line with the present work. Interestingly, TS of the Ti-containing MAX phases all falls into a narrow temperature range of 300–400 °C, where the A-group elements have no significant effect. However, TS increases greatly at about 800 °C when replacing Ti with Cr. This could be understood by the fact that in the Ti-containing MAX phases Ti (or Sn, S) tends to be oxidized in the lower temperature, while for Cr2AlC, Al is the earliest oxidized element (Al2O3) in the higher temperature, because TS depends on the starting-oxidation temperature of the earliest oxidized element. In fact, the formation temperature of Al2O3 is usually over 800 °C in the present and previous work,17,26,28 and Cr2O3 is not observed in the oxidation of Cr2AlC.26 Notably, the oxidation of Ti2SnC powders25 and Ti2SC powders27 lasts a narrow temperature range due to the lack of protective oxidation scale, which leads to the limited oxidation resistance of the two compounds.
B. Isothermal oxidation at 400–900 °C
Figure 4 shows the relative mass gains as a function of exposure time for the fine Ti2AlC powders at temperatures of 400–900 °C in flowing air. At 400 and 500 °C, the whole oxidation process was kept up slowly, while increasing temperature to 600–900 °C, a rapid oxidation in first five minutes and a following slow oxidation in the rest of the time are observed. Figure 5(a) shows the temperature dependence of the final mass gains in the temperature range 400–900 °C. The final mass gains vary from 2 to 42% and increase with the increase of the oxidation temperature, which is very close to Ti3AlC2 powders.28 It should be noted that the ultimate mass gains increase with a significantly larger extent from 400 to 600 °C, which is consistent with the variation of TG-DTA curves in this temperature range [Fig. 2(a)]. Of much interest, an abnormal oxidation behavior of Ti2AlC bulk with higher kinetics at lower temperatures of 500 and 600 °C was observed in the previous work,17 however, which is absent in the present work.
In the previous work on the oxidation of Ti2AlC bulk and powders,6,15,17–21,23,24,29,30 the cubic law was found to be more accurate to describe the oxidation kinetics, which can be expressed as
where k is the cubic rate constant, k0 is a constant, Ea is the cubic oxidation activation energy, kB is Boltzmann’s constant, and T is the absolute temperature. Based on eq. (1), a linear relation should exist between ln(Δm/m0) and 1/T,
The ln(Δm/m0)–1/T plot is illustrated in Fig. 5(b). Interestingly, although the linear relation is not observed for the whole plot, it is indeed present in two temperature ranges of 400–600 °C and 600–900 °C, where by fitting the plot using Eq. (2) the calculated Ea are 2.35 eV and 0.12 eV, respectively. The different Ea indicates the changing oxidation mechanism when increasing the temperature from 400–600 °C to 600–900 °C and will be discussed latter. In addition, the calculated Ea of Ti2AlC based composite powders is 1.20 eV,24 which is much higher than that in the 600–900 °C temperature range, and much lower than that in the 400–600 °C temperature range. Overall, the oxidation of powder Ti2AlC is less affected by temperature than the bulk one where the much higher Ea was observed (2.69 eV).6
C. Composition and surface morphology
Figure 6 shows the XRD patterns of the fine Ti2AlC powders before and after oxidation at 400–900 °C in flowing air, respectively. The phase composition of oxidation products of Ti2AlC is summarized in Table II. Overall, the similar oxides with previous work17,24 are found in the present work. After oxidized at 400 °C for 2 h, the intensity of Ti2AlC peaks begins to decrease. It is worth noting that the appearance of TiO at this temperature is not previously found in the study of oxidation of Ti2AlC.15,17,24 At 500 °C, small peaks related to TiO2 (anatase and rutile) emerge, indicating that Ti suffered further oxidation. At 600 °C, the intensity of oxide peaks is significantly greater than that of 500 °C, which is in good agreement with the largest rate of oxidation at 580 °C [Fig. 2(a)]. As the oxidation temperature increases to 800 °C, anatase-TiO2 has been completely converted to rutile-TiO2. Increasing temperature to 900 °C, only rutile-TiO2 and α-Al2O3 are observed. In comparison with the oxidation of Ti2AlC bulk,17 the phase transformation of Al2O3 does not appear in the oxidation process of Ti2AlC powders.
As shown in Fig. 7(a), XPS was used to determine the nature of chemical bonding in the survey spectrum for fine Ti2AlC powders after oxidation at 400, 600, 800, and 900 °C, respectively. The XPS signals from elements Al, C, Ti, and O can be detected in the survey region (0–600 eV). The signals of Al, Ti, and O increase with increasing the oxidation temperature, which is a clear indication of the formation of TiO2 and Al2O3, in good agreement with XRD results (Fig. 6). Figures 7(b)–7(d) show the high-resolution XPS in the Ti 2p, Al 2p, and O 1s regions after oxidation in flowing air of fine Ti2AlC powders at 400 and 600 °C, respectively. After oxidation at 400 °C, the top surface layer has fully oxidized into TiO2 and Al2O3 [Figs. 7(b) and 7(c)], which were not detected by XRD due to the small amount, suggesting that the bulk structure of the powder is more stable. Oxidation at 600 °C has led a shift of oxidation peaks toward lower-binding energy [Figs. 7(b)–7(d)], suggesting that the oxidation is easier to carry out with the increase of temperature. For brevity, the XPS spectra of 800 and 900 °C are not presented here because they are similar to those at 400 °C.
Figure 8 shows the typical surface morphologies of the fine Ti2AlC powders oxidized in flowing air at different temperatures. It is obvious that the surface of fine Ti2AlC powders changes with the increase of the oxidation temperature: the characteristic lamellar structure of the surface of Ti2AlC can be observed clearly up to 400 °C [Fig. 8(a)]. At 500 °C, there is a layer of uniform, dense white substance covered on the surface [Fig. 8(b)], which is TiO2 analyzed by EDS. It is worth noting that there are no oxidation-induced cracks on the grains at 600 °C [Fig. 8(c)], which have been observed after oxidation of 45–100 µm Ti2AlC based composite powders24 as well as Ti2AlC bulk17 at the same temperature, and contributed to the abnormal oxidation behavior, e.g., the weight gain unusually decreases with increasing oxidation temperature. Wang and Zhou17 attributed the cracks to the stress developed resulting from the volume expansion associated with the oxidation of Ti2AlC into anatase-TiO2. Considering the 1 µm particles used in this study, the absence of abnormal oxidation behavior of fine Ti2AlC powders caused by oxidation-induced cracks could be related to the size effect of particles.
As the temperature rises to 800 °C, the lamellar structure of the surface was almost invisible and tiny white flocculent particles appeared instead [Fig. 8(e)]. At 900 °C, the nano needle-like protrusions are present on these particles [Fig. 8(f)]. Elemental analysis using EDS revealed that the tiny white flocculent particles and nano needle-like protrusions were both Al2O3. Notably, the white cotton-like Al2O3 was observed on top of the heated Ti3AlC2 powders in argon with a low oxygen content (12 ppm).31 The formation of nano needle-like protrusions is due to the local accumulation of α-Al2O3 at the specific location on the surface. Combined with the above analysis results, the formation of protective α-Al2O3 on the surface of Ti2AlC at this temperature would enable Ti2AlC powders to have a good oxidation resistance.
It can be seen that only TiO or TiO2 is observed in the low temperature range, which indicates that Ti is the earliest oxidized element. For Ti2AlC, the theoretical insights using first principles32 show that Ti is bonded to lattice by the strong covalent Ti–C bond. The used nonstoichiometric Ti2AlCx (x = 0.69) synthesized by SHS/PHIP contains a large number of C vacancies,12 which means that a lot of Ti atoms cannot be bonded to C atoms, therefore with the high chemical activity of Ti atoms and easy diffusion in the Ti2AlC lattice. This results in the easy oxidation in the low temperature range of 400–600 °C and the formation of TiO at 400 °C.
The oxidation rate will be controlled by the surface reaction between O2 and Ti2AlC if no continuous oxide scale is formed on the top layer. This mechanism can explain the oxidation behavior in the low temperature range and early stage of high temperature range (Fig. 4), and contribute to the high temperature dependence (high Ea) because the controlling step of the surface reaction is much dependent of temperature. With increasing temperature to 600–900 °C, after the starting-step quick oxidation (Fig. 4) the top Ti atoms are all oxidized, and the left Al atoms can be reacted with O2 to form protective Al2O3, which greatly decreases the oxidation rate as a result of the harder diffusion of O2 through Al2O3 scale into the Ti2AlC substrate. Notably, the above oxidation process is the controlling step in this stage. It can be concluded that the critical temperature of the changing oxidation controlling step is around 600 °C.
IV. CONCLUSION
The oxidation behavior of nonstoichiometric Ti2AlCx (x = 0.69) powders synthesized by combustion synthesis has been investigated, with an effect of particle size. The fine Ti2AlC powders start oxidation at 300 °C and complete oxidation at 980 °C, while the corresponding temperatures of coarse Ti2AlC powders are 400 and 1040 °C, respectively, indicating that the oxidation resistance has been improved with increasing particle size. In isothermal oxidation, at 400 and 500 °C the whole oxidation process was kept up slowly, while increasing temperature to 600–900 °C, a rapid oxidation in first five minutes and a following slow oxidation in the rest of the time are observed. The critical temperature of the changing oxidation controlling step from the surface reaction to diffusion of O2 is around 600 °C. At 400 °C, the top surface layer has fully oxidized into TiO2 and Al2O3, which were not detected by XRD, suggesting that the bulk structure of the powder is more stable. The final oxide products are mainly rutile-TiO2 with a small amount of anatase-TiO2, while powder particles are covered with white α-Al2O3. The tiny white flocculent particles of α-Al2O3 appear on the surface of fine Ti2AlC powders and increase with increasing the oxidation temperature. At 900 °C, the nano needle-like protrusions of α-Al2O3 were present on these particles, while the formation of protective α-Al2O3 would enable Ti2AlC powders with a high oxidation resistance.
References
M.W. Barsoum: The MN +1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 28, 201 (2000).
M.W. Barsoum, I.I. Salama, T. Elraghy, J. Golczewski, H.J. Seifert, F. Aldinger, W. Porter, and H. Wang: Thermal and electrical properties of Nb2AlC, (Ti,Nb)2AlC and Ti2AlC. Metall. Mater. Trans. A 33, 2775 (2002).
Y.C. Zhou and X.H. Wang: Deformation of polycrystalline Ti2AlC under compression. Mater. Res. Innovations 5, 87 (2001).
V. Adamaki, T. Minster, T. Thomas, G. Fourlaris, and C.R. Bowen: Study of the mechanical properties of Ti2AlC after thermal shock. Mater. Sci. Eng., A 667, 9 (2016).
M. Radovic, M.W. Barsoum, A. Ganguly, T. Zhen, P. Finkel, S.R. Kalidindi, and E. Laracurzio: On the elastic properties and mechanical damping of Ti3SiC2, Ti3GeC2, Ti3Si0.5Al0.5C2 and Ti2AlC in the 300–1573 K temperature range. Acta Mater. 54, 2757 (2006).
X.H. Wang and Y. Zhou: High-temperature oxidation behavior of Ti2AlC in air. Oxid. Met. 59, 303 (2003).
M.W. Barsoum and M. Radovic: Elastic and mechanical properties of the MAX phases. Annu. Rev. Mater. Res. 41, 195 (2011).
W. Jeitschko, H. Nowotny, and F. Benesovsky: Ternary carbide (H-phase). Monatsh. Chem. 94, 672 (1963).
M.W. Barsoum, D. Brodkin, and T. Elraghy: Layered machinable ceramics for high temperature applications. Scr. Mater. 36, 535 (1997).
M.W. Barsoum, T. Elraghy, and M.F. Ali: Processing and characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5. Metall. Mater. Trans. A 31, 1857 (2000).
Y. Bai, X. He, Y. Li, C. Zhu, and S. Zhang: Rapid synthesis of bulk Ti2AlC by self-propagating high temperature combustion synthesis with a pseudo-hot isostatic pressing process. J. Mater. Res. 24, 2528 (2009).
Y. Bai, H. Zhang, X. He, C. Zhu, R. Wang, Y. Sun, G. Chen, and P. Xiao: Growth morphology and microstructural characterization of nonstoichiometric Ti2AlC bulk synthesized by self-propagating high temperature combustion synthesis with pseudo hot isostatic pressing. Int. J. Refract. Met. Hard Mater. 45, 58 (2014).
Y. Bai, X. He, C. Zhu, and G. Chen: Microstructures, electrical, thermal, and mechanical properties of bulk Ti2AlC synthesized by self-propagating high-temperature combustion synthesis with pseudo hot isostatic pressing. J. Am. Ceram. Soc. 95, 358 (2012).
Y. Bai, X. He, R. Wang, Y. Sun, C. Zhu, S. Wang, and G. Chen: High temperature physical and mechanical properties of large-scale Ti2AlC bulk synthesized by self-propagating high temperature combustion synthesis with pseudo hot isostatic pressing. J. Eur. Ceram. Soc. 33, 2435 (2013).
D.J. Tallman, B. Anasori, and M.W. Barsoum: A critical review of the oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in air. Mater. Res. Lett. 1, 115 (2013).
M.W. Barsoum and T. Elraghy: A progress report on Ti3SiC2, Ti3GeC2, and the H-phases, M2BX. J. Mater. Synth. Process. 5, 197 (1997).
X.H. Wang and Y.C. Zhou: Intermediate-temperature oxidation behavior of Ti2AlC in air. J. Mater. Res. 17, 2974 (2002).
J. Byeon, J. Liu, M. Hopkins, W. Fischer, N. Garimella, K.B. Park, M.P. Brady, M. Radovic, T. Elraghy, and Y.H. Sohn: Microstructure and residual stress of alumina scale formed on Ti2AlC at high temperature in air. Oxid. Met. 68, 97 (2007).
H. Yang, Y.T. Pei, J. Rao, J.T.M. De Hosson, S.B. Li, and G.M. Song: High temperature healing of Ti2AlC: On the origin of inhomogeneous oxide scale. Scr. Mater. 65, 135 (2011).
B. Cui, D.D. Jayaseelan, and W.E. Lee: TEM study of the early stages of Ti2AlC oxidation at 900 °C. Scr. Mater. 67, 830 (2012).
B. Cui, D.D. Jayaseelan, and W.E. Lee: Microstructural evolution during high-temperature oxidation of Ti2AlC ceramics. Acta Mater. 59, 4116 (2011).
H. Yang, Y.T. Pei, J. Rao, and J.T.M. De Hosson: Self-healing performance of Ti2AlC ceramic. J. Mater. Chem. 22, 8304 (2012).
S. Basu, N. Obando, A. Gowdy, I. Karaman, and M. Radovic: Long-term oxidation of Ti2AlC in air and water vapor at 1000–1300 °C temperature range. J. Electrochem. Soc. 159, C90–C96 (2012).
Z. Zhang, S.H. Lim, D.M.Y. Lai, S.Y. Tan, X.Q. Koh, J. Chai, S.J. Wang, H. Jin, and J.S. Pan: Probing the oxidation behavior of Ti2AlC MAX phase powders between 200 and 1000 °C. J. Eur. Ceram. Soc. 37, 43 (2017).
H.Y. Dong, C.K. Yan, S.Q. Chen, and Y.C. Zhou: Solid–liquid reaction synthesis and thermal stability of Ti2SnC powders. J. Mater. Chem. 11, 1402 (2001).
Z.J. Lin, M.S. Li, J.Y. Wang, and Y.C. Zhou: High-temperature oxidation and hot corrosion of Cr2AlC. Acta Mater. 55, 6182 (2007).
S.R. Kulkarni, M. Merlini, N. Phatak, S.K. Saxena, G. Artioli, S. Amini, and M.W. Barsoum: Thermal expansion and stability of Ti2SC in air and inert atmospheres. J. Alloys Compd. 469, 395 (2009).
X.H. Wang and Y.C. Zhou: Oxidation behavior of Ti3AlC2 powders in flowing air. J. Mater. Chem. 12, 2781 (2002).
J.L. Smialek: Environmental resistance of a Ti2AlC-type MAX phase in a high pressure burner rig. J. Eur. Ceram. Soc. 37, 23 (2017).
J.L. Smialek: Kinetic aspects of Ti2AlC MAX phase oxidation. Oxid. Met. 83, 351 (2015).
X.H. Wang and Y.C. Zhou: Stability and selective oxidation of aluminum in nano-laminate Ti3AlC2 upon heating in argon. Chem. Mater. 15, 3716 (2003).
Y. Bai, X. He, R. Wang, S. Wang, and F. Kong: Effect of transition metal (M) and M-C slabs on equilibrium properties of Al-containing MAX carbides: An ab initio study. Comput. Mater. Sci. 91, 28 (2014).
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (Grant No. 11302061), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (Grant No. 11421091), Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20132302120024), China Postdoctoral Science Foundation funded project (Grant No. 2013M531033), and the Fundamental Research Funds for the Central Universities. Dr. Yuelei Bai gratefully acknowledges the financial support from International Postdoctoral Exchange Fellowship Program (Grant No. 20130004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, F., Feng, K., Bai, Y. et al. Oxidation behavior of high-purity nonstoichiometric Ti2AlC powders in flowing air. Journal of Materials Research 32, 2747–2754 (2017). https://doi.org/10.1557/jmr.2017.83
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2017.83