Abstract
Graphene nanoribbons as a quasi-one-dimensional form of graphene has attracted intensive attention in energy related devices. Upon oxidation and cutting of multiwall carbon nanotubes (MWCNTs), highly dispersive graphene oxide nanoribbons (GONRs) were obtained, on which Zn2+ and Sn4+ can be homogenously deposited. The reduced graphene oxide nanoribbons (rGONRs)/Zn2SnO4 composite with a homogeneous distribution of nanoparticles on the nanoribbons have been prepared through facile in situ chemical co-reduction process. It is worth noting that the size of Zn2SnO4 particles tightly dispersed on rGONRs is about 15 nm. Benefit from the introduction of rGONRs, the specific surface area and electrode conductivity of rGONRs/Zn2SnO4 can both be effectively enhanced. The as-prepared rGONRs/Zn2SnO4 as anode material for lithium-ion batteries displays desirable electrochemical performance (727.2 mA h/g after 50 cycles at the current density of 100 mA/g), which is mainly attributed to the uniformly distributed Zn2SnO4 nanoparticles and the immobilizing and conducting effects of rGONRs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. INTRODUCTION
Graphene nanoribbons (GNRs), a quasi-one-dimensional form of graphene, elegantly combine the structure and properties of carbon nanotubes and graphene sheets.1–3 Owing to their tunable electrical properties, high carbon aspect ratio, and high surface area, GNRs has attracted intensive attention from researchers in energy related devices.4–7 Recently, GNRs/metal oxide composites [GNRs/SnO2,4 GNRs/Fe2O3,8 GNRs/V2O5 (Ref. 9)] as electrode materials for lithium-ion batteries have been prepared and exhibited desirable capacities and rate performances. The GNRs in above-mentioned composites were prepared through the splitting of multiwalled carbon nanotubes (MWCNTs) utilizing intercalation of Na/K alloy. Nevertheless, it is worth noting that the experiment usually required time-consuming synthetic procedures (3–5 days) and rigorous condition. Therefore, it is highly desirable to develop a simple route for the synthesis of GNRs-based composite.
Up to date, graphene oxide nanoribbons (GONRs) could be obtained via the oxidation of MWCNTs, and then reduced graphene oxide nanoribbons (rGONRs) could be got through further reductive treatment.10,11 Bhardwaj et al. reported that as-prepared GNRs (GONRs or rGONRs) delivered better electrochemical properties than MWCNTs or mesocarbon microbead (MCMB).12 Moreover, GONRs could be charged negatively in aqueous solution due to abundant of expoxy, hydroxyl, and carboxyl groups on basal planes and edges of GONRs,10,11 which is similar to graphene oxide (GO) produced by Hummers’ method.13,14 Hence, GONRs also can be served as excellent substrate to host metal oxide. The negatively charged GONRs and the positively charged metal ions can be favorably bond with each other via electrostatic interactions, which plays an important role in determining the uniform attachment of the metal oxide on GONRs.15,16
Zn2SnO4, as a kind of tin-zinc composite oxide with typical inverse spinel structure, has drawn more attention as anode material for lithium-ion batteries (LIBs) due to its high electrical conductivity and good electron mobility.17–19 However, the practical application of Zn2SnO4 has been limited because of the poor capacity retention.20 During the charge/discharge cycles, Zn2SnO4 material usually exhibits particle aggregation and large volume change.21,22 There are several approaches to circumvent the above-mentioned problems. One solution is to fabricate special nanostructures of Zn2SnO4, such as Zn2SnO4 nanowires,23 Zn2SnO4 nanoflowers24 and so on.25 Another effective method is to form composite of Zn2SnO4 with the carbonaceous materials.26,27 The volume change can be effectively buffered with introducing carbonaceous materials, and also lithium diffusion rate can be enhanced. Hence, Zn2SnO4 hybrid nanocomposites with carbon material always display outstanding electrochemical performances, such as Zn2SnO4 boxes/graphene,28 Zn2SnO4 nanoflowers/graphene,24 Zn2SnO4 nanoparticles/graphene,21 and Zn2SnO4 nanoparticles/carbon.29 To our best knowledge, there is no report about the electrochemical properties of Zn2SnO4/GONRs composite.
In this paper, rGONRs have been firstly used as support material for Zn2SnO4 based anode for lithium-ion batteries. rGONRs/Zn2SnO4 composite has been prepared by a facile situ hydrothermal route utilizing GONRs as precursor. Furthermore, the formation mechanism of composite was also proposed. With this novel structure, the rGONRs/Zn2SnO4 composite shows highly reversible capacity, desirable cycle performance, and rate capability.
II. EXPERIMENTAL SECTION
A. Synthesis of samples
All regents were of analytical grade without further treatment in this study. The raw MWCNTs with 5–15 μm in length and 60–100 nm in diameter were received from Shenzhen Nanotech Port Co., Ltd.
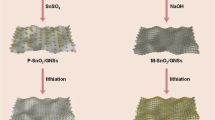
The formation of rGONRs/Zn2SnO4 composite is shown in Fig. 1. Highly dispersive GONRs were obtained by lengthwise unzipping and unraveling of MWCNTs sidewalls through a simple solution-base oxidative process, which usually contain abundant of oxygenic groups on basal planes and edges. So the Zn2+ and Sn4+ cations, formed by the dissolution of ZnCl2 and SnCl4·5H2O in water, can homogenously deposited on GONRs. The composite of rGONRs supported Zn2SnO4 nanoparticles (rGONRs/Zn2SnO4) was further prepared through facile in situ chemical co-reduction. The Zn2SnO4 nanoparticles could be uniformly dispersed and immobilized on the graphene nanoribbons reduced from GONRs.
The details of preparation of GONRs are as follows: 0.15 g raw MWCNTs were mixed with 150 mL of concentrated H2SO4 (98%). At room temperature, the mixture was stirred for 6 h. KMnO4 (700 mg) was then added and the suspension was allowed to stir for another 1 h. The reaction was then heated at 65 °C for 2 h in oil bath. The as-obtained suspension was further poured into 400 mL of ice water containing 6 mL 30% H2O2, and then separated by centrifugation and washed with 10% HCl and deionized water. After dried under vacuum, GONRs were obtained.
RGONRs/Zn2SnO4 composite was synthesized via a simple hydrothermal treatment. In this process, 120 mg GONRs were dispersed in deionized water under ultrasonic treatment for 2 h to obtain a stable suspension. Subsequently, 0.136 g of ZnCl2 and 0.175 g of SnCl4·5H2O were dissolved in deionized water and added dropwise into the above GONRs dispersion under magnetic stirring for 2 h. After that, 245 μL N2H4·H2O was added with stirring for another 30 min. Then the resulting mixture was sealed in a Teflon-lined stainless steel autoclave with a filling capacity of 80% and was hydrothermally treated at 180 °C for 24 h. The hydrothermal product was collected by centrifugation and vacuum dried at 60 °C for 12 h. To improve the crystallinity of Zn2SnO4, the as-obtained product was annealed at 500 °C under Ar atmosphere for 2 h. As a reference, pure rGONRs and bare Zn2SnO4 nanoparticles were prepared under the similar experimental conditions in the absence of chloride or GONRs. The main text of the article should appear here with headings as appropriate.
B. Characterization
The crystalline structures of the as-prepared samples were investigated by X-ray diffraction (XRD) analysis (D/max-Ultima IV, Rigaku Corporation, Tokyo, Japan). The morphology and microstructures of the products were observed utilizing scanning electron microscopy (SEM; Hitachi S-4800, Hitachi Corp., Tokyo, Japan) and transmission electron microscopy (TEM; JEOL-3010, JEOL Ltd., Tokyo, Japan). Nitrogen adsorption–desorption isotherms were determined at 77 K, using a Micromeritics Model ASAP 2020 surface area and porosity analyzer (Micromeritics, Norcross, Georgia). Raman spectrum was collected by LABRAM-010 (Horiba Scientific, Longjumeau, France). The thermogravimetric analysis (TGA; DTA7300, JEOL Ltd.) was carried out from room temperature to 800 °C at a heating rate of 10 °C/min under flowing air.
C. Electrochemical measurements
The working electrodes were prepared by mixing 80 wt% active material, 10 wt% acetylene black, and 10 wt% polyvinylidene fluoride (PVDF) binder dissolved in N-methyl-2-pyrrolidinone (NMP). The obtained slurry was coated onto pure copper foil and dried at 80 °C in vacuum for 10 h to remove the solvent before pressing. Then the electrodes were cut into disks (12 mm in diameter). Electrochemical measurements were carried out via CR2025 coin-type cell with lithium foil as the counter and reference electrode, microporous polypropylene as the separator. The electrolyte consisted of a solution of LiPF6 (1 M) in ethylene carbonate/ethyl methyl carbonate/dimethyl carbonate (1:1:1, v/v/v). Discharge–charge measurements were carried out galvanostatically at various rates over a voltage range from 0.005 V to 3 V (versus Li/Li+) on a LAND battery tester. Cyclic voltammetry (CV) measurement was conducted at 0.5 mV/s between 3.0 and 0.005 V on an electrochemical workstation (CHI 660B). Electrochemical impedance spectra (EIS) were recorded in the frequency range of 100 kHz to 0.01 Hz after the cells had been discharged and charged for three cycles.
III. RESULTS AND DISCUSSION
The structure information and crystal phase of as-prepared samples were obtained by XRD. As shown in Fig. 2(a), raw MWCNTs show a typical peak at 2θ = 26.0°, corresponding to an interlayer spacing about 0.34 nm. Since the numbers of oxygen functionalities have been generated during the oxidation process, which can be effective to intercalate in the plane of the graphene layers and increase the interlayer spacing,30 GONRs show a peak at 2θ = 10.9° with the basal spacing of 0.81 nm. After reduction, the diffraction peak at 2θ = 10.9° completely disappeared, and a broad peak at 25.5° was observed, corresponding to an interlayer spacing of about 0.36 nm, which is slight larger than d-spacing of MWCNTs, indicating the presence of residual oxygen-containing groups or other structural defects on rGONRs. All of the diffraction peaks of the pure Zn2SnO4 could be unambiguously indexed to cubic Zn2SnO4 with a space group Fd3m (JCPDS No. 74–2184).21 A weak characteristic peak of rGONRs (2θ = 25.5°) was observed from the XRD pattern of rGONRs/Zn2SnO4 composite [Fig. 2(b)]. However, this small peak has not been obviously observed due to the small content and the noncrystalline structure of rGONRs in the composite.
The Raman spectra of GONRs, rGONRs, and rGONRs/Zn2SnO4 composite are presented in Fig. 3. The peak at about 1330 cm−1 (D band) is corresponded to the vibration of sp2-bonded carbon atoms in a 2-dimensional hexagonal lattice, while the peak at about 1590 cm−1 (G band) is related to the defect and disorder in the hexagonal graphitic layers.31 The intensity ratio of D to G band (ID/IG) generally reflects the graphitization degree of carbonaceous materials and the defect density.32 Compared with the curves in Fig. 3, the ID/IG of rGONRs and rGONRs/Zn2SnO4 composite are both higher than that of GONRs, which is agreement with some previous reports on chemical reduction of GONRs and GO.11,33 The increased ID/IG could be attributed to the existence of unrepaired defects after the removal of partial oxygen groups during the reduction of GONRs.33
TGA analysis of rGONRs/Zn2SnO4 composite has been carried out in an air atmosphere between 35 and 800 °C. As shown in Fig. 4, the weight loss from 35 to 350 °C is mainly due to the release of the absorbed water and coordinated water.34 While the mass loss ratio of rGONRs/Zn2SnO4 composite between 350 and 600 °C is mainly resulted from the oxidization of rGONRs. At temperatures above 600 °C, the curve is rather stable, indicating rGONRs have been completely disappeared. The amount of rGONRs in the composite is determined to be about 28.3%.
Figure 5 displays SEM and TEM images of the products. It can be seen that bare Zn2SnO4 nanoparticles have a roughly spherical morphology with a size of 15–20 nm [Fig. 5(a)]. MWCNTs can be successfully unzipped via advanced oxidation process, which is confirmed by TEM image of rGONRs [Fig. 5(b)]. It can be seen that ribbon-like graphene structures with the width of carbon nanostructures about 130 nm displays linear edges with partial pristine tube structure remaining. It is stressing that the special structure of rGONRs can significantly provide increased accessible area. Figure 5(c) shows the morphology of the rGONRs/Zn2SnO4 composite, which indicates that the rGONRs can provide an interconnected web for anchoring Zn2SnO4 particles. The Zn2SnO4 particles could be anchoring on the surface of rGONRs without forming free Zn2SnO4 particles even though undergone vigorous ultrasonication treatment. As shown in Figs. 5(d) and 5(e), spherical Zn2SnO4 particles are homogenously distributed on transparent rGONRs, which might be ascribed to the strong interaction between Zn2SnO4 particles and defect sites of GONRs.35 Furthermore, the high-resolution TEM (HRTEM) image of rGONRs/Zn2SnO4 composite is shown in Fig. 5(f), which presents the clear lattice fringe of individual Zn2SnO4 particle on rGONRS. The spacing of the adjacent lattice planes is about 0.31 nm, agreeing well with the plane (220) of Zn2SnO4. The distance of 0.36 nm between the uniform lattice fringes is related to the plane (002) of rGONRs, which is in good agreement with the results from XRD analysis. Above all, it can be concluded from the XRD, Raman and TEM results that rGONRs/Zn2SnO4 composite has been successfully prepared through hydrothermal process.
Figure 6 shows the N2 adsorption–desorption isotherms of the Zn2SnO4 nanoparticles and as-obtained rGONRs/Zn2SnO4 composite. The specific surface area of rGONRs/Zn2SnO4 composite is calculated to be 132.09 m2/g from the nitrogen adsorption–desorption isotherm, which is higher than that of the bare Zn2SnO4 nanoparticles (90.56 m2/g). It indicates that the specific surface area of the composite could be significantly increased because of the addition of rGONRs. Furthermore, the total pore volume values of Zn2SnO4 nanoparticles and rGONRs/Zn2SnO4 composite are 0.216 cm3/g and 0.312 cm3/g, respectively, which were got based on the Barrett–Joyner–Halenda (BJH) pore size distribution analysis (the insets in Fig. 6). The increased pore volume of rGONRs/Zn2SnO4 composite could be mainly attributed to the formation of secondary pores between Zn2SnO4 nanoparticle and rGONRs.36 The high specific surface area and porous nanostructure of rGONRs/Zn2SnO4 could be beneficial to shorten the diffusion paths of Li+, and also can provide a high conductive medium for electron transfer.28,31
Figure 7 shows representative CV curves of the rGONRs/Zn2SnO4 electrode from the 1st to 3rd cycle with the potential range from 0.005 to 3 V versus Li/Li+ at a scanning rate of 0.5 mV/s. The shape of the CV curves indicates the multistep electrochemical lithium reaction process and the lithium insertion.31 It is generally accepted that the electrochemical reaction of Zn2SnO4 as an anode material can be simplified as follows17,19:
First cathodic scan shows a peak at about 1.08 V, which disappeared in the subsequent cycles, suggesting that a small amount of lithium is inserted into the crystal structure of Zn2SnO4 before the structure change occurs.21 Similar lithium intercalation behaviors are mainly attributed to the small particle size of Zn2SnO4.37 Moreover, a clear reduction peak at around 0.51 V and a weak peak at about 0.2 V can be observed in the first discharge process, which can be attributed to the decomposition of Zn2SnO4 and the multistep lithium reaction of Sn and Zn.19,24 During the first anode scan, two peaks at around 0.63 and 1.34 V can be observed, corresponding to the dealloying process and the partial re-oxidation, respectively. In the second and subsequent cycles, the main cathodic peak shifts to 0.78 V (i) and a small cathodic peak is located around 0.30 V (ii), illustrating the different lithium reaction process.38 The redox couple indexed as a/a′ is corresponding to the partially reversible oxidation of Sn and Zn, while another redox couple b/b′ is related to the decomposition of Zn2SnO4 and the multistep lithium reaction of Sn and Zn.37 while another redox couple b/b′ is related to the alloying/dealloying process of LixSn and LiyZn.19 In addition, the third and second CV profiles are similar, indicating highly stabilized electrochemical reactions.39 The galvanostatic charge/discharge measurements were performed at the current density of 100 mA/g with the potential range of 0.005–3 V. The 1st and 30th typical charge/discharge curves of Zn2SnO4 and rGONRs/Zn2SnO4 composite are shown in Fig. 8(a). It can be seen that pure Zn2SnO4 nanoparticles deliver a capacity of 1763.8 mA h/g for the first discharge and a reversible capacity of 691.1 mA h/g. While the first discharge and charge capacity of rGONRs/Zn2SnO4 composite are 1618.7 mA h/g and 842.1 mA h/g. The rGONRs/Zn2SnO4 composite deliver higher reversible capacity and better coulombic efficiency (52.0%) of the first cycle relative to those of Zn2SnO4 nanoparticles (coulombic efficiency 39.1%). The initial capacity losses for both samples could be caused by the formation of solid electrolyte interphase (SEI) and the reduction of metal oxides to metal with Li2O formation.40 And most of the irreversible capacity comes from Zn2SnO4.28 Hence, the higher coulombic efficiency of this composite is likely attributed to the synergetic effect between Zn2SnO4 nanocrystals and the conductive graphene. All these results imply that the synergistic effect between Zn2SnO4 and conductive rGONRs can improve the electrochemical performance of this hybrid composite. Moreover, after 30 cycles, the charge capacity of Zn2SnO4 nanoparticles drops to 612.5 mA h/g. While the charge 794.5 mA h/g after 30 cycles, corresponding to the capacity retention of 94.4%, which is much higher than that of pure Zn2SnO4 nanoparticles (88.6%). All the above results illustrate the electrochemical behaviors of the composite as anode for LIBs can be excellently enhanced due to introduce the rGONRs material. On the one hand, the aggregation problem could be alleviated since the nano-sized Zn2SnO4 nanoparticles can be anchored tightly on the surface of rGONRs. On the other hand, with the addition of rGONRs, the electronic conductivity of rGONRs/Zn2SnO4 composite can be improved.
(a) Voltage profiles of Zn2SnO4 nanoparticles and rGONRs/Zn2SnO4 composite charged-discharged at 100 mA/g. (b) Cycling performance and coulombic efficiency of rGONRs/Zn2SnO4 composite at 100 mA/g. (c) Rate capability of rGONRs/Zn2SnO4 composite at various current densities. (d) EIS of Zn2SnO4 nanoparticles and rGONRs/Zn2SnO4 composite electrodes after three cycles.
Figure 8(b) shows the capacity retention performance of rGONRs/Zn2SnO4 composite. The composite maintains a high reversible charge capacity of 727.2 mA h/g after 50 cycles, which is higher than that of previous reported Zn2SnO4/RGO and Zn2SnO4/C composites.41,42 The columbic efficiency of rGONRs/Zn2SnO4 composite is above 90% after 4 cycles, and stay at above 95% after 8 cycles. The rate performance of rGONRs/Zn2SnO4 composite was also investigated, which is shown in Fig. 8(c). The composite was cycled from 50 mA/g to 1600 mA/g in steps and returned to 50 mA/g. The specific capacities of rGONRs/Zn2SnO4 composite anode are 1184.8 mA h/g, 882.3 mA h/g, 799.5 mA h/g, 629.7 mA h/g, 441.0 mA h/g and 330.4 mA h/g at various current densities of 50 mA/g, 100 mA/g, 200 mA/g, 400 mA/g, 800 mA/g and 1600 mA/g, respectively. When the current density returned to 50 mA/g, the charge capacity can be recovered to 942 mA h/g, which is close to the original value, indicating that the integrity of this electrode can be kept during cycling.21 Taking Zn2SnO4-based materials reported so far for comparison (Table I), the as-prepared rGONRs/Zn2SnO4 composite here displays high specific capacitance, which can be attributed to the homogeneous distribution of Zn2SnO4 nanoparticles as well as the rGONRs networks. The rGONRs with high specific surface area not only can provide better electrolyte penetration, but also can act as mechanical buffers, apparently able to adjust the volume change of Zn2SnO4 during the cycling.4 Moreover, the high conductive rGONRs might be the most important reason for the desirable reversible rate capacity of the composite.
The enhanced electronic conductivity of rGONRs/Zn2SnO4 composite was further verified by EIS measurement. Figure 8(d) shows the EIS analysis of the electrodes of Zn2SnO4 nanoparticles and rGONRs/Zn2SnO4 composite from 0.01 Hz to 100 KHz after 3 cycles. Both impedance curves show a semicircle at middle frequency region and a straight sloping line in the low frequency region. It is believed that the semicircle at middle frequency region is ascribed to the charge-transfer impedance on electrode/electrolyte interface (Rct), and the inclined line is corresponded to the lithium ion diffusion processes (Warburg impedance, W).24 It can be seen that, the rGONRs/Zn2SnO4 composite electrode exhibits smaller semicircle compared with the bare Zn2SnO4. The smaller diameter of the semicircles, the smaller the charge transfer resistance. Thus, the result indicates a lower charge transfer resistance and the greater facilitation of electronic transportation during the electrochemical reactions of rGONRs/Zn2SnO4 composite electrode.43 The electrical conductivity of rGONRs/Zn2SnO4 composite can be enhanced with the introducing rGONRs, which could contribute to the improved rate and cycle properties. Therefore, rGONRs/Zn2SnO4 composite is beneficial for the charge transfer because of the outstanding electric properties of rGONRs, which could result in improving electrochemical performance.
IV. CONCLUSION
In summary, rGONRs/Zn2SnO4 composite has been successfully synthesized through a simple one-pot in situ hydrothermal route, by using GONRs as precursor. The GONRs obtained by oxidative process contain abundant of oxygenic groups on basal planes and edges, which is different from other graphene nanoribbon-based composite. So the Zn2SnO4 particles with a small size about 15 nm could be tightly confined and homogeneously dispersed on rGONRs. The large specific surface area of rGONRs can serve both as a mechanical buffer for Zn2SnO4 nanoparticles and as the separator to inhibit the aggregation of the nanoparticles. Moreover, the results of EIS declare that the incorporation of rGONRs is an effective way to increase the electrode conductivity. Hence, the obtained rGONRs/Zn2SnO4 composite as anode for LIBs retains a high reversible charge capacity of 727.2 mA h/g after 50 cycles at the current density of 100 mA/g and desirable rate capability (330.4 mA h/g at 1.6 A/g).
References
Q. Peng, Y. Li, X. He, X. Gui, Y. Shang, C. Wang, C. Wang, W. Zhao, S. Du, E. Shi, P. Li, D. Wu, and A. Cao: Graphene nanoribbon aerogels unzipped from carbon nanotube sponges. Adv. Mater. 26 (20), 3241 (2014).
X. Li, X. Wang, L. Zhang, S. Lee, and H. Dai: Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 319 (5867), 1229 (2008).
L. Jiao, L. Zhang, X. Wang, G. Diankov, and H. Dai: Narrow graphene nanoribbons from carbon nanotubes. Nature 458 (7240), 877 (2009).
J. Lin, Z. Peng, C. Xiang, G. Ruan, Z. Yan, D. Natelson, and J.M. Tour: Graphene nanoribbon and nanostructured SnO2 composite anodes for lithium ion batteries. ACS Nano 7 (7), 6001 (2013).
O. Hod, V. Barone, J.E. Peralta, and G.E. Scuseria: Enhanced half-metallicity in edge-oxidized zigzag graphene nanoribbons. Nano Lett. 7 (8), 2295 (2007).
B. Luo, S. Liu, and L. Zhi: Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 8 (5), 630 (2012).
B. Genorio, W. Lu, A.M. Dimiev, Y. Zhu, A-R.O. Raji, B. Novosel, L.B. Alemany, and J.M. Tour: In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 6 (5), 4231 (2012).
J. Lin, A-R.O. Raji, K. Nan, Z. Peng, Z. Yan, E.L.G. Samuel, D. Natelson, and J.M. Tour: Iron oxide nanoparticle and graphene nanoribbon composite as an anode material for high-performance Li-ion batteries. Adv. Funct. Mater. 24 (14), 2044 (2014).
Y. Yang, L. Li, H. Fei, Z. Peng, G. Ruan, and J.M. Tour: Graphene nanoribbon/V2O5 cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 6 (12), 9590 (2014).
D.V. Kosynkin, A.L. Higginbotham, A. Sinitskii, J.R. Lomeda, A. Dimiev, B.K. Price, and J.M. Tour: Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458 (7240), 872 (2009).
A.L. Higginbotham, D.V. Kosynkin, A. Sinitskii, Z. Sun, and J.M. Tour: Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 4 (4), 2059 (2010).
T. Bhardwaj, A. Antic, B. Pavan, V. Barone, and B.D. Fahlman: Enhanced electrochemical lithium storage by graphene nanoribbons. J. Am. Chem. Soc. 132 (36), 12556 (2010).
Q. Qu, S. Yang, and X. Feng: 2D sandwich-like sheets of iron oxide grown on graphene as high energy anode material for supercapacitors. Adv. Mater. 23 (46), 5574 (2011).
W.S. Hummers and R.E. Offeman: Preparation of graphitic oxide. J. Am. Chem. Soc. 80 (6), 1339 (1958).
S. Chen, J. Zhu, X. Wu, Q. Han, and X. Wang: Graphene oxide−MnO2 nanocomposites for supercapacitors. ACS Nano 4 (5), 2822 (2010).
H. Wang, H.S. Casalongue, Y. Liang, and H. Dai: Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132 (21), 7472 (2010).
X.J. Zhu, L.M. Geng, F.Q. Zhang, Y.X. Liu, and L.B. Cheng: Synthesis and performance of Zn2SnO4 as anode materials for lithium ion batteries by hydrothermal method. J. Power Sources 189 (1), 828 (2009).
K. Kim, A. Annamalai, S.H. Park, T.H. Kwon, M.W. Pyeon, and M-J. Lee: Preparation and electrochemical properties of surface-charge-modified Zn2SnO4 nanoparticles as anodes for lithium-ion batteries. Electrochim. Acta 76, 192 (2012).
A. Rong, X.P. Gao, G.R. Li, T.Y. Yan, H.Y. Zhu, J.Q. Qu, and D.Y. Song: Hydrothermal synthesis of Zn2SnO4 as anode materials for Li-ion battery. J. Phys. Chem. B 110 (30), 14754 (2006).
Y. Zhao, Y. Huang, W. Zhang, Q. Wang, K. Wang, M. Zong, and X. Sun: Botryoidalis hollow Zn2SnO4 boxes@graphene as anode materials for advanced lithium-ion batteries. RSC Adv. 3 (45), 23489 (2013).
W. Song, J. Xie, W. Hu, S. Liu, G. Cao, T. Zhu, and X. Zhao: Facile synthesis of layered Zn2SnO4/graphene nanohybrid by a one-pot route and its application as high-performance anode for Li-ion batteries. J. Power Sources 229, 6 (2013).
W.S. Yuan, Y.W. Tian, and G.Q. Liu: Synthesis and electrochemical properties of pure phase Zn2SnO4 and composite Zn2SnO4/C. J. Alloys Compd. 506 (2), 683 (2010).
Y.R. Lim, C.S. Jung, H.S. Im, K. Park, J. Park, W.I. Cho, and E.H. Cha: Zn2GeO4 and Zn2SnO4 nanowires for high-capacity lithium- and sodium-ion batteries. J. Mater. Chem. A 4 (27), 10691 (2016).
K. Wang, Y. Huang, Y. Shen, L. Xue, H. Huang, H. Wu, and Y. Wang: Graphene supported Zn2SnO4 nanoflowers with superior electrochemical performance as lithium-ion battery anode. Ceram. Int. 40 (9), 15183 (2014).
H. Fan, Z. Liu, J. Yang, C. Wei, J. Zhang, L. Wu, and W. Zheng: Surfactant-free synthesis of Zn2SnO4 octahedron decorated with nanoplates and its application in rechargeable lithium ion batteries. RSC Adv. 4 (91), 49806 (2014).
Y.J. Hong and Y.C. Kang: Formation of core–shell-structured Zn2SnO4-carbon microspheres with superior electrochemical properties by one-pot spray pyrolysis. Nanoscale 7 (2), 701 (2015).
H.Y. Wang, B.Y. Wang, J.K. Meng, J.G. Wang, and Q.C. Jiang: One-step synthesis of Co-doped Zn2SnO4–graphene–carbon nanocomposites with improved lithium storage performances. J. Mater. Chem. A 3 (3), 1023 (2015).
Y. Zhao, Y. Huang, X. Sun, H. Huang, K. Wang, M. Zong, and Q. Wang: Hollow Zn2SnO4 boxes wrapped with flexible graphene as anode materials for lithium batteries. Electrochim. Acta 120, 128 (2014).
C. Yan, J. Yang, Q. Xie, Z. Lu, B. Liu, C. Xie, S. Wu, Y. Zhang, and Y. Guan: Novel nanoarchitectured Zn2SnO4 anchored on porous carbon as high performance anodes for lithium ion batteries. Mater. Lett. 138, 120 (2015).
H. Wang, Y. Wang, Z. Hu, and X. Wang: Cutting and unzipping multiwalled carbon nanotubes into curved graphene nanosheets and their enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 4 (12), 6827 (2012).
H. Huang, Y. Huang, M. Wang, X. Chen, Y. Zhao, K. Wang, and H. Wu: Preparation of hollow Zn2SnO4 boxes@C/graphene ternary composites with a triple buffering structure and their electrochemical performance for lithium-ion batteries. Electrochim. Acta 147, 201 (2014).
E. Yoo, J. Kim, E. Hosono, H.S. Zhou, T. Kudo, and I. Honma: Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 8 (8), 2277 (2008).
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen, and R.S. Ruoff: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45 (7), 1558 (2007).
C. Zhong, J. Wang, Z. Chen, and H. Liu: SnO2–graphene composite synthesized via an ultrafast and environmentally friendly microwave autoclave method and its use as a superior anode for lithium-ion batteries. J. Phys. Chem. C 115 (50), 25115 (2011).
C. Wang, H. Li, J. Zhao, Y. Zhu, W.Z. Yuan, and Y. Zhang: Graphene nanoribbons as a novel support material for high performance fuel cell electrocatalysts. Int. J. Hydrogen Energy 38 (30), 13230 (2013).
F. Han, W.C. Li, M.R. Li, and A.H. Lu: Fabrication of superior-performance SnO2@C composites for lithium-ion anodes using tubular mesoporous carbon with thin carbon walls and high pore volume. J. Mater. Chem. 22 (19), 9645 (2012).
B.Y. Wang, H.Y. Wang, Y.L. Ma, X.H. Zhao, W. Qi, and Q.C. Jiang: Facile synthesis of fine Zn2SnO4 nanoparticles/graphene composites with superior lithium storage performance. J. Power Sources 281, 341 (2015).
K. Wang, Y. Huang, T. Han, Y. Zhao, H. Huang, and L. Xue: Facile synthesis and performance of polypyrrole-coated hollow Zn2SnO4 boxes as anode materials for lithium-ion batteries. Ceram. Int. 40 (1), 2359 (2014).
Y. Zhao, Y. Huang, Q. Wang, K. Wang, M. Zong, L. Wang, W. Zhang, and X. Sun: Preparation of hollow Zn2SnO4 boxes for advanced lithium-ion batteries. RSC Adv. 3 (34), 14480 (2013).
S. Yuvaraj, W.J. Lee, C.W. Lee, and R.K. Selvan: In situ and ex situ carbon coated Zn2SnO4 nanoparticles as promising negative electrodes for Li-ion batteries. RSC Adv. 5 (82), 67210 (2015).
W. Song, J. Xie, S. Liu, G. Cao, T. Zhu, and X. Zhao: Graphene-induced confined crystal growth of octahedral Zn2SnO4 and its improved Li-storage properties. J. Mater. Res. 27 (24), 3096 (2012).
X. Ji, X. Huang, Q. Zhao, A. Wang, and X. Liu: Facile synthesis of carbon-coated Zn2SnO4 nanomaterials as anode materials for lithium-ion batteries. J. Mater. Res. 2014, 1 (2014).
A.K. Rai, J. Gim, L.T. Anh, and J. Kim: Partially reduced Co3O4/graphene nanocomposite as an anode material for secondary lithium ion battery. Electrochim. Acta 100, 63 (2013).
T. Jiang, X. Tian, H. Gu, H. Zhu, and Y. Zhou: Zn2SnO4@C core–shell nanorods with enhanced anodic performance for lithium-ion batteries. J. Alloys Compd. 639, 239 (2015).
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 51272075, No. 51372080 and No. 51238002). This work was also financially supported by the China Scholarship Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jing, M., Hou, Z., Yang, H. et al. Zn2SnO4 coated reduced graphene oxide nanoribbons with enhanced electrochemical performance for lithium-ion batteries. Journal of Materials Research 31, 3666–3674 (2016). https://doi.org/10.1557/jmr.2016.431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2016.431