Abstract
The effect of magnesium nitrate on the thermo-foaming of powder dispersions in molten sucrose for the preparation of alumina foams has been studied. The magnesium nitrate decreases the melting point of sucrose from 180 to 160 °C and acts as a blowing and setting agent. The foaming time and setting time decreases with an increase in foaming temperature as well as an increase in magnesium nitrate concentration. The rapid foaming followed by foam collapse is observed beyond 140 °C. The accelerated foaming and setting is due to an increase in the rate of–OH condensation because of the catalytic effect of H+ generated by the hydrolysis of magnesium nitrate. The porosity of alumina foam increases while cell size and grain size decrease with an increase in magnesium nitrate concentration. A change in foam structure, from partially interconnected cellular to completely interconnected reticulate-like, occurs when the magnesium nitrate concentration increase from 4 to 8 wt%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
I. INTRODUCTION
Ceramic foams are getting renewed interest due to their wide spread applications as high temperature thermal insulation, filter media for molten metal’s, catalyst support, bio-implant and pre-form for metal-ceramic and polymer-ceramic composites.1–5 The polymer foam replication, foaming, and setting of ceramic powder suspensions, emulsion-based methods and freeze-casting are well studied for the preparation of ceramic foams.6–30 In polymer foam replication method, a ceramic replica of a reticulated polyurethane foam is prepared by coating the webs of the polyurethane foam with a ceramic powder suspension followed by drying, burnout of the polymer foam template, and sintering.6–9 The ceramic foams with interconnected cellular structure are prepared by foaming and setting of aqueous ceramic powder suspensions. In this, the foaming of powder suspensions is accomplished by stabilizing gas bubbles in the slurry medium with the help of a surfactant or a particle with suitable wetting characteristics. The aqueous foams thus obtained are set either by in situ polymerization of organic monomers or by coagulation of the ceramic particles. The wet foams obtained are then dried and sintered to produce ceramic foams.10–18 In the emulsion-based method, fine droplets of immiscible liquid (oil) are dispersed in an aqueous ceramic powder suspension with the help of an emulsifying agent to produce high internal phase emulsions. The emulsions thus obtained are subsequently dried, oil-removed, and sintered to produce ceramic foams.19–25 In the freeze-casting, ceramic powder suspensions cast in a mold is set by freezing the suspension medium. The subsequent removal of the frozen medium by sublimation and sintering of the resultant porous body produces ceramic foams.26–30
Recently we have reported a thermo-foaming of alumina powder dispersion in molten sucrose for the preparation of alumina foams. In this, the sucrose polymer–alumina composite foams obtained by the thermo-foaming of alumina powder dispersions in molten sucrose are subjected to polymer burnout and sintering to produce alumina foams.31 The method is simple and uses nontoxic materials for the preparation of alumina foams. An intermediate pyrolysis of the sucrose polymer in the foam body before burnout facilitates preparation of large alumina foam bodies without any crack.32 The porosity and pore size of alumina foams prepared by this method are controlled by controlling the alumina powder to sucrose weight ratio as well as the foaming temperature.33 The main shortcomings of this process are relatively long time for the foaming and the setting of the alumina powder dispersions in molten sucrose at lower foaming temperatures and large cell size at higher foaming temperatures.33 Incorporation of an additive which catalyzes the –OH condensation is expected to accelerate the foaming and setting of the alumina powder dispersion in molten sucrose and modify the porosity and pore size characteristics of the alumina foam. In the present work, magnesium nitrate is used as a blowing and setting agent for the preparation of alumina foams from alumina powder dispersions in molten sucrose. In addition, MgO produced from the magnesium nitrate can act as sintering aid for alumina. The foaming and setting characteristics of the alumina powder dispersion in molten sucrose and porosity, pore size, and compressive strength of the alumina foams are studied as a function of magnesium nitrate concentration.
II. EXPERIMENTAL
α-Alumina powder (A16SG, ACC Alcoa, Kolkata, India) with an average particle size of 0.34 μm and specific surface area of 10.4 m2/g was used. The analytical reagent grade sucrose (CAS No. 1.94953.5021), magnesium nitrate hexahydrate [Mg(NO3)2·6H2O, CAS No. 1.93709.0521], and acetone (CAS No.1.07021.2521) used were procured from Merck India Ltd. (Mumbai, India). The sucrose (200 g), alumina powder (200 g) and various concentrations of the magnesium nitrate were mixed by planetary ball milling in acetone medium using zirconia grinding media of 10 mm diameter and zirconia grinding jar of 500 mL capacity for 2 h at a speed of 200 RPM. The sucrose to acetone and sucrose to zirconia balls weight ratios used were 1:3 and 1:6, respectively. The slurries thus obtained were dried in a glass tray at 80 °C. The sucrose–alumina–magnesium nitrate mixtures thus obtained were heated in an air oven at 185 °C to melt the sucrose and stirred with a glass rod to achieve uniform dispersion of alumina powder in the molten sucrose. The alumina powder dispersions were further heated in the air oven at the temperatures of 130 and 140 °C for foaming and setting. The time at which the foam reaches its maximum height was noted as the foaming time. The time at which the soft foam turned to a hard one was taken as the foam setting time. The solid organic foam bodies were cut into rectangular pieces of size 10 × 10 × 6 cm and heated in an argon atmosphere furnace at 900 °C for 2 h. The heating rate used was 0.5 °C/min. The samples were unloaded after cooling the furnace to room temperature. The pyrolyzed bodies were heated in an electrically heated sintering furnace in air at 1600 °C for carbon removal and sintering. The heating rates used were 0.5 °C/min up to 600 °C and 1 °C/min from 600 to 1600 °C. A holding time of 2 h was given at 600 and 1600 °C. The shrinkage of the foam bodies was calculated from their initial and final dimensions. The density was obtained from the weight and volume of the rectangular foam bodies. Porosity was calculated from the foam density and theoretical density of alumina (3.98 g/cm3).
The torque-time measurements were carried out using a torque rheometer (Brabender Plasti-Corder, GmbH, Duisburg, Germany). About 50 cm3 of the sucrose–alumina–magnesium nitrate mixture containing various concentrations of magnesium nitrate prepared by the planetary ball milling was loaded in to the internal mixing unit of the rheometer at a temperature of 185 °C and at a rotor speed of 10 RPM. The temperature was lowered to 130 °C after 10 min at which the sucrose in the mixture melts and properly mixes with the alumina powder. The torque data was collected with time until the torque reaches 100 Nm. The differential scanning calorimetric (DSC; TA Instruments, New Castle, Delaware) measurements of sucrose, magnesium nitrate and sucrose-magnesium nitrate mixtures were carried out at a heating rate of 5 °C/min in a nitrogen atmosphere. The sucrose–magnesium nitrate mixture containing various concentrations of magnesium nitrate for DSC measurements were prepared by the planetary ball milling. The microstructure of the alumina foams was observed using a scanning electron microscope (SEM; Hitachi S-2400, Hitachi Corporation, Tokyo, Japan). The samples for SEM analysis were prepared by cutting the sintered alumina foam samples using a knife. The grain size was calculated from the high magnification SEM image of the struts using the linear intercept method. The cell size of the foam samples was measured on the magnified image of the alumina foams observed using a vision inspection system with a charge-coupled device (CCD) color camera (Vision 300 GL, TESA Technologies, Renens, Switzerland). The values reported were average of at least 50 measurements. The compressive strength was measured using an universal testing machine (Instron 5500, Instron, Norwood, Massachusetts) at a loading rate of 0.5 mm/min using the sintered alumina foam samples of 25 × 25 × 12 mm size (ASTM standard C365/C365-05).
III. RESULTS AND DISCUSSION
The thermo-foaming and setting of alumina powder dispersions in molten sucrose is due to the polymerization of glucose and fructose anhydride formed from sucrose through –OH to –OH condensation.31 That is, the water vapor produced as a result of the –OH condensation is responsible for the nucleation and growth of bubbles in the dispersion leading to its foaming. The foam setting is due to the viscosity increase as a result of the above polymerization and cross-linking. The foaming time, setting time, and foam volume depends on the rate of the –OH condensation. The effect of magnesium nitrate concentration on the foaming and setting time of the alumina powder dispersion in molten sucrose is shown in Fig. 1. At both the studied foaming temperatures, the foaming time and setting time show a drastic decrease when the magnesium nitrate concentration increases from 0 to 4 wt%. The foaming time and setting time show a gradual decrease with a further increase in magnesium nitrate concentration to 16 wt%. For example, at 130 °C, the foaming time and setting time decreases from 9.5 and 44 h to 2.5 and 11.3 h, respectively, when the magnesium nitrate concentration increases from 0 to 4 wt%. Further increase in magnesium nitrate concentration to 16 wt% decreases the foaming and setting time to 1.25 and 3.5 h, respectively. The decrease in foaming and setting time observed at 140 °C is from 5.75 to 0.5 and 38.75 to 1.75 h, respectively, when the magnesium nitrate concentration increases from 0 to 16 wt%. At both the studied foaming temperatures, the foam rise, a measure of foam volume increases with an increase in magnesium nitrate concentration up to 12 wt%. Further increase in magnesium nitrate concentration to 16 wt% does not produce any insignificant change in the foam rise. At 130 °C, the foam rise increases from 3.8 to 9 when the magnesium nitrate concentration increases from 0 to 16 wt%. The corresponding increase in foam rise at 140 °C is from 5.8 to 13. Attempts made to foam the alumina powder dispersions containing magnesium nitrate at 150 °C leads to much faster foaming and setting, however, results in foam collapse. The foam collapse is due to the rupture of bubbles before setting. It is well known that the bubble rupture takes place when the pressure inside the bubbles is greater than the strength of cell walls. At 150 °C, the rate of formation of water vapor inside the bubbles is so rapid that the pressure inside the bubbles exceeds the cell wall strength, leading to the bubble rupture and foam collapse. The effect of magnesium nitrate concentration on the foam rise is shown in Fig. 2.
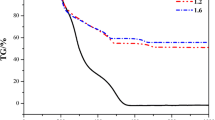
The function of a blowing agent in a foaming process is the generation of gaseous molecules in the fluid medium to facilitate nucleation and growth of bubbles leading to the foaming.34,35 Generally, a blowing agent functions either by evaporation at the foaming temperature or by its thermal decomposition.34,35 A decrease in foaming time and an increase in foam volume with an increase in magnesium nitrate concentration shows that the magnesium nitrate acts as a blowing agent. The thermo-gravimetric analysis (TGA) graph of magnesium nitrate is shown in Fig. 3. A weight loss of nearly 32% observed up to 185 °C (melting temperature of sucrose) is corresponding to the loss of nearly 4 molecules of water of hydration. The weight loss (42 wt%) corresponding to the complete removal of water of hydration from magnesium nitrate takes place at 300 °C. The decomposition of magnesium nitrate starts only at 350 °C, which rules out the contribution of decomposition products of magnesium nitrate toward the foaming of alumina powder dispersion at 130 and 140 °C. It is well known that H+ ions are very good catalyst for the –OH condensation.36 The magnesium nitrate undergoes hydrolysis to form magnesium hydroxide and nitric acid, as given in Eq. (1). The H+ produced by the ionization of the nitric acid catalyzes the –OH condensation. The water required to initiate the hydrolysis of magnesium nitrate is obtained either from the water of hydration of magnesium nitrate or from the initial slow –OH condensation which takes place in the absence of a catalyst. As the concentration of magnesium nitrate increases, the amount of H+ produced in the system increases, which enhances the rate of –OH condensation. This in turn increases the rate of formation of water vapor leading to a decrease in foaming time and an increase in the foam volume. The decrease in foam setting time with an increase in magnesium nitrate concentration shows that the magnesium nitrate acts as a foam setting agent. The faster setting of the thermo-foamed alumina powder dispersion is due to the faster polymerization and cross-linking through –OH condensation catalyzed by the H+ generated by the hydrolysis of magnesium nitrate.
The addition of magnesium nitrate not only decreases the foaming and setting time but also makes the preparation of alumina powder dispersions in molten sucrose easier as the magnesium nitrate decreases the melting point of sucrose. It is well known that the addition of a solute (magnesium nitrate) decreases the melting point of a solvent (sucrose).37–39 The decrease in melting point of sucrose by the addition of magnesium nitrate is evidenced from the DSC graph shown in Fig. 4. The sucrose and magnesium nitrates show endothermic peaks corresponding to their melting at 180 and 89 °C, respectively. Up on the addition of magnesium nitrate the endothermic peak corresponding to the melting of sucrose shifted to a lower temperature of nearly 160 °C. That is, when heated at 185 °C, the sucrose in sucrose–alumina powder–magnesium nitrate mixtures melts and achieves a uniform dispersion much faster than the sucrose–alumina powder mixture.
The increase in the rate of polymerization through –OH condensation, responsible for faster foaming and setting, with an increase in magnesium nitrate concentration is further evidenced from the torque-time measurements. Figure 5 shows the torque-time plots of alumina powder dispersions in molten sucrose containing various concentrations of the magnesium nitrate measured at 130 °C. The mixtures show slightly higher torque value during melting and mixing. After achieving uniform dispersion, the alumina dispersion in molten sucrose without magnesium nitrate show a low and steady torque of nearly 0.2 Nm up to 500 min. The low torque value even at 500 min is due to the sluggish polymerization in the absence of a catalyst and the same was also evidenced in the long foam setting time of 44 h. On the other hand, the alumina powder dispersion containing magnesium nitrate does not show any considerable torque increase up to a certain time, followed by a slow increase and then a rapid increase. The time at which the rapid increase in torque observed, decreases with an increase in magnesium nitrate concentration. The time at which the torque value reaches 100 Nm decreases from 300 to 190 min when the magnesium nitrate concentration increases from 4 to 16 wt%. The early increase in torque at higher magnesium nitrate concentration is due to the faster increase in molecular weight of sucrose polymer by accelerated –OH condensation because of H+ generated by the hydrolysis of the magnesium nitrate.
The thermo-foamed bodies prepared at various magnesium nitrate concentrations do not show any crack or deformation during pyrolysis of the sucrose polymer and subsequent carbon burnout and sintering. The photograph of a large rectangular alumina foam body of size 18 × 15 × 3.5 cm prepared by thermo-foaming at a magnesium nitrate concentration of 8 wt% is shown in Fig. S1, supplementary information. The shrinkage of foam bodies observed during pyrolysis of sucrose polymer and total shrinkage (sum of shrinkage during pyrolysis and sintering) decreases with an increase in magnesium nitrate concentration. The foam bodies prepared at a foaming temperature of 130 °C and at the magnesium nitrate concentrations in the range of 0–16 wt% shows shrinkage in the range of 39.2–32.4 vol% during pyrolysis of the sucrose polymer. The corresponding shrinkage observed in the foam bodies prepared at a foaming temperature of 140 °C is in the range of 38.8–27.7 vol%. The total shrinkage observed in the foam bodies prepared at foaming temperatures of 130 and 140 °C are in the ranges of 68.3–65.1 vol% and 67.5–60.1 vol%, respectively. Our previous work on thermo-foaming of alumina powder dispersions without magnesium nitrate shows nearly same shrinkage for the foam bodies prepared at various foaming temperatures from powder dispersion of a particular alumina powder to sucrose weight ratio in spite of the fact that the foam volume increases with an increase in foaming temperature.33 That is, the shrinkage depends on the alumina powder packing in the thermo-foamed body and is independent of the cell size.33 In the present case, it appears that the magnesium ions interact with the sucrose polymer due to co-ordination with the –OH groups. These interactions are expected to bring the polymer molecules closer and thereby increase the density of the sucrose polymer. The interaction between the magnesium ions and –OH groups in sucrose polymer is evidenced from a slight shift in the absorption peak corresponding to –OH stretching vibrations. The sucrose polymer prepared without and with magnesium nitrate shows peaks corresponding to –OH stretching vibrations at 3407 and 3382 cm−1, respectively (Fig. S2, supplementary information). The decrease in sintering shrinkage with an increase in magnesium nitrate concentration is attributed to an increase in packing of alumina particles in the thermo-foamed body due to a decrease in volume (increase in density) of the polymer layer present in between the particles.
The effect of magnesium nitrate concentration and foaming temperature on porosity of alumina foam is shown in Fig. 6. At the both studied foaming temperatures, the addition of magnesium nitrate blowing agent shows an initial rapid increase in porosity of the alumina foam. At a foaming temperature of 130 °C, the porosity increase from 87.3 to 92.7 vol% when the magnesium nitrate concentration increases from 0 to 4 wt%. Further increase in magnesium nitrate concentration up to 16 wt% slowly increases the porosity to 93.8 vol%. Similarly, at a foaming temperature of 140 °C, the porosity increases from 90.7 to 95.6 vol% when the magnesium nitrate concentration increases from 0 to 16 wt%. The increase in porosity with an increase in magnesium nitrate concentration and foaming temperature is in accordance with the observed foam rise and sintering shrinkage.
The alumina foams prepared without magnesium nitrate blowing agent show cellular microstructure with nearly spherical cells, which are partially interconnected through circular openings. The foams prepared at a magnesium nitrate concentration of 4 wt% also show similar microstructure. However, a clear transition in the microstructure of the foam is observed, irrespective of the foaming temperature, when the magnesium nitrate concentration increases from 4 to 8 wt%. That is, the microstructure changes from partially interconnected cellular to completely open reticulate-like when the magnesium nitrate concentration increases from 4 to 8 wt%. The foam microstructure remains similar at magnesium nitrate concentration in the range of 8–16 wt%. Figure 7 shows SEM photomicrographs of alumina foams prepared at various magnesium nitrate concentrations. The struts of the foams are nonporous as evidenced from the high magnification images shown as insets in each SEM photomicrographs.
The magnesium nitrate used as a blowing agent produces 0.63 to 2.51 wt% of MgO in the alumina during burnout and sintering. The MgO is a well-known sintering additive used to suppress the grain growth of alumina ceramics.40 We have observed a decrease in average grain size of alumina foams from 2.14 to 1.16 μm when the magnesium nitrate concentration increases from 0 to 16 wt%. The high magnification images of alumina foams prepared without and with 16 wt% magnesium nitrate blowing agent are shown in Fig. 8. The variation of average grain size with magnesium nitrate concentration is shown in Fig. S3, supplementary information.
Figure 9 shows the effect of magnesium nitrate concentration on the average cell size of the alumina foams. The average cell size of the alumina foams decreases with an increase in magnesium nitrate concentration. At a foaming temperature of 130 °C, the average cell size decreases from 0.65 to 0.45 mm when the magnesium nitrate concentration increases from 0 to 16 wt%. The corresponding decrease in average cell size observed at the foaming temperature of 140 °C is from 0.75 to 0.4 mm. In general, the cell size increases with an increase in foaming temperature.33 However, in presence of the magnesium nitrate, the foams prepared at a foaming temperature of 140 °C shows lower cell sizes compared to that prepared at 130 °C. The cell size of foam depends on the nucleation and growth of bubbles in the powder dispersion. It appears that, the faster –OH condensation in the presence of magnesium nitrate and at higher foaming temperature results in a high concentration of water vapor in the dispersion which is responsible for the nucleation of a large number of bubbles. That is, the number of nucleated bubbles increases with an increase in magnesium nitrate concentration. These bubbles grow with the incorporation of water vapor produced due to the continued –OH condensation. As the number of bubbles nucleated in a given volume of the dispersion increases their growth decreases as the total volume of water vapor generated from a given amount of sucrose remains nearly constant. It is also well known that the viscous forces restrict the growth of bubbles in a medium. That is, faster increase in viscosity due to the increase in rate of –OH condensation with an increase in magnesium nitrate concentration and foaming temperature also restricts the bubble growth. The lower cell size observed at a higher foaming temperature (140 °C) in the presence of magnesium nitrate is due to the higher rate of bubble nucleation and restricted bubble growth due to higher concentration of water vapor and faster increase in viscosity, respectively, due to the higher rate of –OH condensation. The high standard deviation observed indicates relatively broad distribution of cell sizes in the foams. This shows that it is hard to control the cell sizes to a narrow range by the thermo-foaming process.
Figure 10 shows the effect of magnesium nitrate concentration and foaming temperature on the compressive strength and Young’s modulus of the alumina foams. The compressive strength and Young’s modulus of brittle foam materials mainly depend on the porosity. In general, the compressive strength and Young’s modulus of brittle foams decrease with an increase in porosity. At a constant porosity, the compressive strength and Young’s modulus decreases with an increase in cell size and increase in cell interconnectivity. The compressive strength of the alumina foams prepared at a foaming temperature of 130 °C drastically decreases from 5.02 to 1.55 MPa when the magnesium nitrate concentration increases from 0 to 4 wt%. This is due to a drastic increase in porosity from 87.3 to 92.7 vol%. Further increase in magnesium nitrate concentration to 16 wt% slowly increases the porosity to 93.8 vol% and hence registers a slow decrease in compressive strength to 1.06 MPa. The alumina foam prepared at the foaming temperature of 140 °C shows a similar trend in compressive strength with an increase in magnesium nitrate concentration, but the values (3.26–0.38 MPa) are lower than that of alumina foams prepared at a foaming temperature of 130 °C. The lower compressive strength is due to the higher porosity (90.7–95.6 vol%) achieved by foaming at a higher temperature of 140 °C. The Young’ modulus shows a similar trend as compressive strength with an increase in magnesium nitrate concentration. The Young’s modulus of alumina foams prepared at the foaming temperatures of 130 and 140 °C decreases from 480 to 70 MPa and 252 to 2.25 MPa, respectively, when the magnesium nitrate concentration increases from 0 to 16 wt%. The observed decrease in compressive strength and Young’s modulus with an increase in magnesium nitrate concentration and foaming temperature, in spite of a decrease in cell size, is due to the increase in porosity and increase in cell interconnectivity. The compressive strength of ceramic foams of wide range of porosities prepared by various processing routes has been compiled by Gauckler and co-workers in comparison with the theoretically predicted values based on Gibson and Ashby model.17,41–43 The compressive strength of alumina foams reported in the present work is comparable with that reported for alumina foams of similar porosities obtained by other processing methods.
IV. SUMMARY
The magnesium nitrate is used as a blowing and setting agent for the thermo-foaming of alumina powder dispersions in molten sucrose. The magnesium nitrate decreases the melting point of sucrose from 180 to 160 °C which makes the preparation of the powder dispersion easy. A remarkable decrease in foaming time (5.75–0.5 h) and setting time (38.75–1.75 h) and an increase in the foam rise (5.8–13) are observed with an increase in magnesium nitrate concentration from 0 to 16 wt%. The faster thermo-foaming and setting of the alumina powder dispersions in the presence of magnesium nitrate is due to an increase in the rate of –OH condensation because of the catalytic effect of H+ produced by the hydrolysis of the magnesium nitrate. The porosity increases (90.7 to 95.6 vol%) and cell size decreases (0.75 to 0.4 mm) with an increase in magnesium nitrate concentration from 0 to 16 wt%. A clear transition from partially interconnected cellular to completely interconnected reticulate-like foam structure occurs when the magnesium nitrate concentration increases from 4 to 8 wt%. The compressive strength and Young’s modulus observed are comparable with that reported for alumina foams of similar porosities. The method is amenable to the fabrication of large alumina foam bodies.
References
J. Sagggio-Woyansky and C.E. Scott: Processing of porous ceramics. Am. Ceram. Soc. Bull. 71, 1674 (1992).
M. Takahashi, R.L. Menchavez, M. Fuji, and H. Takegami: Opportunities of porous ceramics fabricated by gelcasting in mitigating environmental issues. J. Eur. Ceram. Soc. 29, 823 (2009).
J. Binner, H. Chang, and R. Higginson: Processing of ceramic–metal interpenetrating composites. J. Eur. Ceram. Soc. 29, 837 (2009).
H. Haugen, J. Will, A. Köhler, U. Hopfner, J. Aigner, and E. Wintermantel: Ceramic TiO2-foams: Characterization of a potential scaffold. J. Eur. Ceram. Soc. 24, 661 (2004).
R. Faure, F. Rossignol, T. Chartier, C. Bonhomme, A. Maître, and G. Etchegoyen: Alumina foam catalyst supports for industrial steam reforming processes. J. Eur. Ceram. Soc. 31, 303 (2011).
K. Schwartzwalder and A.V. Somers: Method of making porous ceramic article. US Patent 3090 094, May 21, 1963.
J.W. Brockmeyer: Process for preparing ceramic foam. US Patent 4610 832, September 9, 1986.
X.W. Zhu, D.L. Jiang, and S.H. Tan: Impregnating process of reticulated porous ceramics using polymeric sponge as template. J. Inorg. Mater. 16, 1144 (2001).
F.A.C. Oliveira, S. Diasa, M.F. Vazb, and J.C. Fernandes: Behaviour of open-cell cordierite foams under compression. J. Eur. Ceram. Soc. 26, 179 (2006).
P. Sepulveda: Gelcasting of foams for porous ceramics. Am. Ceram. Soc. Bull. 76, 61 (1997).
P. Sepulveda and J.G.P. Binner: Processing of cellular ceramics by foaming and in situ polymerization of organic monomers. J. Eur. Ceram. Soc. 19, 2059 (1999).
J.G.P. Binner: Production and properties of low density engineering ceramic foam. Br. Ceram. Trans. 96, 247 (1997).
X. Mao, S. Shimai, and S. Wang: Gelcasting of alumina foams consolidated byepoxy resin. J. Eur. Ceram. Soc. 28, 217 (2008).
F.S. Ortega, P. Sepulveda, and V.C. Pandolfelli: Monomer systems for the gel-casting of foams. J. Eur. Ceram. Soc. 22, 1395 (2002).
K. Prabhakaran, N.M. Gokhale, S.C. Sharma, and R. Lal: A novel process for low-density alumina foams. J. Am. Ceram. Soc. 88, 2600 (2005).
U.T. Gonzenbach, A.R. Studart, E. Tervoort, and L.J. Gauckler: Ultrastable particle-stabilized foams. Angew. Chem., Int. Ed. 45, 3526 (2006).
U.T. Gonzenbach, A.R. Studart, D. Steinlin, E. Tervoort, and L.J. Gauckler: Processing of particle-stabilized wet foams into porous ceramics. J. Am. Ceram. Soc. 90, 3407 (2007).
W. Liu, J. Xu, Y. Wang, H. Xu, X. Xi, and J. Yang: Processing and properties of porous PZT ceramics from particle-stabilized foams via gel casting. J. Am. Ceram. Soc. 96, 1827 (2013).
M.A. Alves-Rosa, L. Martins, S.H. Pulcinelli, and C.V. Santilli: Design of microstructure of zirconia foams from the emulsion template properties. Soft Matter 9, 550 (2013).
S. Barg, E.G. de Moraes, D. Koch, and G. Grathwohl: New cellular ceramics from high alkane phase emulsified suspensions (HAPES). J. Eur. Ceram. Soc. 29, 2439 (2009).
S. Barg, C. Soltmann, M. Andrade, D. Koch, and G. Grathwohl: Cellular ceramics by direct foaming of emulsified ceramic powder suspensions. J. Am. Ceram. Soc. 91, 2823 (2008).
E.M.M. Ewais, S. Barg, G. Grathwohl, A.A. Garamoon, and N.N. Morgan: Processing of open porous zirconia via alkane-phase emulsified suspensions for plasma applications. Int. J. Appl. Ceram. Technol. 8, 85 (2011).
S. Vijayan, R. Narasimman, and K. Prabhakaran: Freeze gelcasting of hydrogenated vegetable oil-in-aqueous alumina slurry emulsions for the preparation of macroporous ceramics. J. Eur. Ceram. Soc. 34, 4347 (2014).
S. Vijayan, R. Narasimman, and K. Prabhakaran: Effect of emulsion composition on gel strength and porosity in the preparation of macroporous alumina ceramics by freeze gelcasting. J. Asian Ceram. Soc. 3, 279 (2015).
S. Vijayan, R. Narasimman, and K. Prabhakaran: Freeze gelcasting of naphthalene-in-aqueous alumina slurry emulsions for the preparation of macroporous alumina ceramics. Ceram. Int. 41, 1487 (2015).
S. Deville: Freeze-casting of porous ceramics: A review of current achievements and issues. Adv. Eng. Mater. 10, 155 (2008).
T. Fukasawa, M. Ando, T. Ohji, and S. Kanzaki: Synthesis of porous ceramics with complex pore structure by freeze dry processing. J. Am. Ceram. Soc. 84, 230 (2001).
K. Araki and J.W. Halloran: Porous ceramic bodies with interconnected pore channels by a novel freeze casting technique. J. Am. Ceram. Soc. 88, 1108 (2005).
R. Chen, C.A. Wang, Y. Huang, L. Ma, and W. Lin: Ceramics with special porous structures fabricated by freeze-gelcasting: Using tert-butyl alcohol as a template. J. Am. Ceram. Soc. 90, 3478 (2007).
Y. Koh, J. Song, E. Lee, and H. Kim: Freezing dilute ceramic/camphene slurry for ultra-high porosity ceramics with completely interconnected pore networks. J. Am. Ceram. Soc. 89, 3089 (2006).
S. Vijayan, R. Narasimman, C. Prudvi, and K. Prabhakaran: Preparation of alumina foams by the thermo-foaming of powder dispersions in molten sucrose. J. Eur. Ceram. Soc. 34, 425 (2014).
S. Vijayan, R. Narasimman, and K. Prabhakaran: Fabrication of large alumina foams by pyrolysis of thermo-foamed alumina-sucrose. J. Mater. Res. 31, 302 (2016).
S. Vijayan, P. Wilson, and K. Prabhakaran: Porosity and cell size control in alumina foam preparation by thermo-foaming of powder dispersions in molten sucrose. J. Asian. Ceram. Soc. 4, 344–350 (2016).
E. Aram and S. Mehdipour-Ataei: A review on the micro- and nanoporous polymer foam: Preparation and properties. Int. J. Polym. Mater. Polym. Biomater. 65, 358 (2016).
D. Klempner and K.C. Frisch: Handbook of Polymeric Foams and Foam Technology (Oxford University Press, New York, 1991).
I.L. Finar: Organic Chemistry, Vol. 2: Stereochemistry and The Chemistry of Natural Products, 5th ed. (Pearson Education, Delhi, 2007); p. 335.
P. Atkins and J.D. Paula: Physical Chemistry, 8th ed. (Oxford University Press, New York, 2006); pp. 150–155.
R.G. Mortimer: Physical Chemistry, 3rd ed. (Elsevier Academic Press, Burlington, MA, USA, 2004); pp. 292–299.
R.S. Berry, S.A. Rice, and J. Ross: Physical Chemistry, 2nd ed. (Oxford University Press, New York, USA, 2001); pp. 708–711.
S.J. Bennison and M.P. Harmer: Effect of MgO solute on the kinetics of grain growth in A12O3. J. Am. Ceram. Soc. 66, C–90 (1983).
U.T. Gonzenbach, A.R. Studart, E. Tervoort, and L.J. Gauckler: Macroporous ceramics from particle-stabilized wet foams. J. Am. Ceram. Soc. 90, 16 (2007).
A.R. Studart, U.T. Gonzenbach, E. Tervoort, and L.J. Gauckler: Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 89, 1771 (2006).
L.J. Gibson and M.F. Ashby: Cellular Solids: Structure and Properties, 2nd ed. (Cambridge University Press, Cambridge Solid State Press, 1997); pp. 183–200.
ACKNOWLEDGMENTS
The authors are thankful to Dr. K.S. Dasgupta, Director, Dr. Kuruvilla Joseph, Dean Student Activities, and Dr. Nirmala Rachel James, Head, Department of Chemistry, IIST for their encouragement.
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
Rights and permissions
About this article
Cite this article
Vijayan, S., Wilson, P. & Prabhakaran, K. Low density ceramic foams from alumina-sucrose using magnesium nitrate as a blowing and setting agent. Journal of Materials Research 31, 3027–3035 (2016). https://doi.org/10.1557/jmr.2016.300
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2016.300