Abstract
The Ag3PO4/C3N4 composites with improved photocatalytic activity were prepared by a facile in situ deposition of Ag3PO4 particles on the surface of C3N4 sheets and characterized by x-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy, x-ray photoelectron spectroscopy, UV–vis diffuse reflectance absorption spectra, Fourier transform infrared spectra, and photoluminescence spectra. The photocatalytic degradation of Rhodamine B (RhB) over the Ag3PO4/C3N4 composites was investigated and optimized, indicating that the optimal amount of Ag3PO4 in the composites was 90 wt%. The remarkably improved photocatalytic activity of Ag3PO4/C3N4 composites could be attributed to the effective separation of photogenerated charge carriers. The photoelectrochemical measurements confirmed that the charge separation efficiency was improved for the formation of composites. Moreover, the tests of radical scavengers demonstrated that h+ and ·O2− were the main active species for the degradation of RhB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. INTRODUCTION
Semiconductor photocatalysis driven by visible light has been paid more attention in the past few decades because of its potential applications in environmental remediation and hydrogen energy production by utilization of solar light energy.1,2 Design and synthesis of novel visible-light-responsive photocatalysts are still the focus in this field.3–5 In recent years, silver orthophosphate (Ag3PO4) and carbon nitrides (C3N4) have been respectively reported as visible-light-active photocatalysts for the photodecomposition of organic compounds.6–10 However, bare Ag3PO4 is prone to photocorrosion and decomposing to weakly active Ag during the photocatalytic reaction process, which could deteriorate its photocatalytic activity.11,12 On the other hand, the photocatalytic performance of pristine C3N4 is limited owing to the high recombination rate of photogenerated electron–hole pairs.13 Therefore, it is necessary to develop an efficient strategy to overcome these drawbacks of single Ag3PO4 or C3N4 as photocatalysts. Coupling two semiconductors to form composites is considered as an effective approach toward improving the separation efficiency of charge carriers, and then enhancing the photocatalytic activity and stability.14–24 In this case, various Ag3PO4-based and C3N4-based coupled systems have been explored such as AgBr/Ag3PO4,14 Ag3PO4/TiO2,15 Ag3PO4/ZnO,16 Ag3PO4/SnO2,17 Bi2MoO6/Ag3PO4,18 Bi2WO6/Ag3PO4,19 TiO2/C3N4,20 ZnO/C3N4,21 ZnWO4/C3N4,22 CdS/C3N4,23 BiOBr/C3N4,24 and so on. The photocatalytic reaction results indicated that the formation of composites could enhance the photocatalytic activity of Ag3PO4 or C3N4.
According to previous reports,25,26 the conduction band (CB) and valence band (VB) potentials of Ag3PO4 [∼0.24 and ∼2.69 eV versus normal hydrogen electrode (NHE), respectively] are both lower than those of C3N4 (∼−1.12 and ∼1.73 eV versus NHE, respectively). This staggered energy band structure favors the separation of photogenerated charge carriers when Ag3PO4 combines with C3N4 to form composites. Very recently, several groups have reported the synthesis of Ag3PO4/C3N4 composites with enhanced photocatalytic activity and stability.25,27–31 As a promising hybrid material for photocatalysis, the exploration of Ag3PO4/C3N4 composites still has a long way to go, especially through a facile, environmentally friendly, and economical method. In this work, Ag3PO4/C3N4 composites were prepared by a facile in situ deposition of Ag3PO4 particles on the surface of C3N4 sheets at room temperature and applied into photocatalytic degradation of Rhodamine B (RhB) solution under visible light irradiation. Remarkably, enhanced photocatalytic activity was achieved compared with single Ag3PO4 or C3N4. Moreover, the effect of mass ratios of Ag3PO4 in the Ag3PO4/C3N4 composites on photocatalytic activity was investigated comparatively. Compared with the previous reports, our work provides some new and meaningful results on the radical scavenger and photoelectric conversion measurement experiments, which indicate the main species in the catalytic system and support the possible photocatalytic mechanism.
II. EXPERIMENTAL SECTION
A. Materials preparation
C3N4 was synthesized by thermal treatment of 5 g urea (AR, Sinopharm Chemical Reagent Co. Ltd., China) in an alumina crucible with a cover. After being dried at 80 °C for 12 h, the urea was heated to 500 °C and held for 3 h with a heating rate of 5 °C min−1. The resultant light yellow powders were collected for use without further treatment.
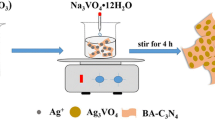
Ag3PO4/C3N4 composites were prepared as follows: an appropriate amount of C3N4 was added into 30 mL of deionized water with sonicating for 30 min to make C3N4 dispersion totally. 0.26 g of AgNO3 was dissolved in 1.5 mL concentrated ammonia to form a transparent silver-ammino complex solution. Afterward, the later solution was introduced into the former one and stirred for 30 min before the addition of 0.183 g Na2HPO4·12H2O. The pH value of a mixture was adjusted to 7.30 by 0.1 M HNO3 solution. The suspensions were stirred for 4 h in the dark at room temperature. The resultant powders were washed with ethanol and deionized water several times, dried in a vacuum at 60 °C for 12 h. For comparison, a series of Ag3PO4/C3N4 composites with different theoretical mass ratios of Ag3PO4 (from 50 to 92 wt%) in the composites were fabricated by changing C3N4 amounts. Similarly, the pristine Ag3PO4 was also prepared by following the same procedure as above except the addition of C3N4.
B. Material characterization
The samples were characterized by x-ray diffraction (XRD) patterns using a Bruker D8 Advance x-ray diffractometer with Cu Kα irradiation (λ = 0.154178 nm) at 40 kV and 40 mA. The morphology of samples was investigated by a JSM-6700F microscope (Japan Electron Optics Laboratory Co., Ltd., Japan). The transmission electron microscopy (TEM) measurement was conducted using a JEOL JEM 2100F microscope (Japan Electron Optics Laboratory Co., Ltd., Japan) working at 200 kV. The x-ray photoelectron spectroscopy (XPS) measurement was performed on a VG Multilab 2000 (Thermo Electron Corporation, USA) with Al Kα source operation at 300 W. The UV–vis diffuse reflectance absorption spectra (DRS) were obtained by a Shimadzu UV-3600 spectrophotometer (Shimadzu Corporation, Japan) equipped with an integrating sphere using BaSO4 as the reference sample. The Fourier transform infrared spectra (FTIR) spectra of samples were recorded on a Thermo Nicolet Avatar 360 spectrometer using conventional KBr pellets. The photoluminescence (PL) spectra were measured at room temperature on a varian cary eclipse fluorescence spectrophotometer with the excitation wave length at 340 nm.
C. Photocatalytic activity measurement
The photocatalytic activities of Ag3PO4/C3N4 composites were evaluated by the degradation of RhB aqueous solution under visible light irradiation. 50 mg of photocatalysts were added into 100 mL of RhB solution with the initial concentration of 1.0 × 10−5 mol·L−1. A 500 W xenon lamp (Changzhou Yuyu Electro-Optical Device Co. Ltd., China) with a 420 nm cutoff filter provided visible light irradiation. Prior to irradiations, the suspensions were magnetically stirred in the dark for 30 min to ensure the establishment of an adsorption–desorption equilibrium. At given irradiation time intervals, about 4 mL of the suspensions were collected and then centrifuged at 10,000 rpm for 10 min to remove the photocatalysts. The TU-1810 spectrophotometer (Beijing Purkinje General Instrument Co. Ltd., China) was used to measure the concentration changes of RhB solution with the wave length of 554 nm.
D. Photoelectric conversion measurement
The transient photocurrent responses and electrochemical impedance spectra (EIS) were performed on an electrochemical system (CHI 660E, China). A standard three-electrode cell with a working electrode (as-prepared photocatalysts), a platinum plate as the counter electrode, and Ag/AgCl electrode (saturated KCl) as the reference electrode were used in the photoelectric studies. 0.1 mol L−1 Na2SO4 was used as the electrolyte solution. For the preparation of working electrodes, a certain amount of photocatalysts were suspended in 1 mL ethanol with 0.1 mL Nafion aqueous solution (5 wt%), the mixtures were ultrasonically scattered for 30 min to form a homogeneous solution. Then, 0.1 mL solution was dropped on the indium tin oxide (ITO) glass (1 cm × 1 cm). After evaporation of the ethanol in air, the catalyst was attached on the ITO glass surface. Photocurrent and EIS tests were irradiated under visible light through a 300 W Xe lamp with a 420 nm cut-off filter.
III. RESULTS AND DISCUSSION
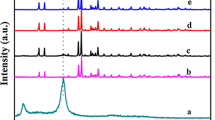
The XRD patterns of C3N4, Ag3PO4, and 90 wt% Ag3PO4/C3N4 composite are shown in Fig. 1. As could be seen from the diffraction pattern of C3N4, there is a broad peak at about 27.6°, which is characteristic peak of C3N4 corresponding to the (002) plane.26 As for pristine Ag3PO4, all of the diffraction peaks could be well indexed to the cubic phase of Ag3PO4 (JCPDS, No. 06-0505), in agreement with previous reports.27,28 The intense diffraction peaks of Ag3PO4 indicate that the as-prepared sample would be well crystallized. In addition, the 90 wt% Ag3PO4/C3N4 composite exhibits a similar XRD pattern to that of bare Ag3PO4. No diffraction peak assigning to C3N4 is observed, which may be due to the relatively low diffraction intensity of C3N4 in the composite.
Figures 2(a)–2(c) show the field emission scanning electron microscopy (FESEM) images of C3N4, Ag3PO4, and 90 wt% Ag3PO4/C3N4 composite. It is seen that C3N4 presents obvious two-dimensional (2D) lamellar structure, which is consistent with the previous report.26 Pristine Ag3PO4 exhibits irregular particle morphology with the diameters of 100–500 nm together with smooth surface. For the 90 wt% Ag3PO4/C3N4 composite, it is constituted with the layer-like C3N4 and granular Ag3PO4. The TEM image [Fig. 2(d)] of 90 wt% Ag3PO4/C3N4 further confirms that Ag3PO4 particles are intimately attached on the surface of C3N4, in agreement with the FESEM observation.
The XPS measurement was performed to determine the chemical composition and valence state of various species. The peak positions in all of the XPS spectra are calibrated with C 1s at 284.6 eV. Figure 3(a) displays the XPS survey spectrum of 90 wt% Ag3PO4/C3N4 composite. As expected, it contains Ag, P, O, C, and N elements. The peaks [Fig. 3(b)] with a binding energy of 368.5 and 374.5 eV in the high resolution XPS spectrum of Ag 3d are assigned to Ag 3d5/2 and Ag 3d3/2, indicating the existence of Ag+ in the composite. The XPS peak of P 2p is found at 133.3 eV [Fig. 3(c)], which could be attributed to P5+ in PO43−.32 The O 1s XPS spectrum illustrated in Fig. 3(d) could be deconvoluted into two peaks at 530.6 and 531.6 eV. The former corresponds to the crystal lattice oxygen of Ag3PO4,32 the latter could be derived from the chemisorbed oxygen of surface hydroxyls or incompletely reacted oxygen-containing intermediates during the preparation of C3N4.10 The C 1s [Fig. 3(e)] XPS spectra have two distinct peaks at 284.6 and 288.2 eV. The peak at 288.2 eV is identified as sp2-bonded carbon (N–C=N). The peak located at 284.6 eV could be assigned to adventitious carbon and graphitic carbon.10,26 As shown in Fig. 3(e), the N 1s peaks at 398.5 and 399.2 eV correspond to sp2 hybridized aromatic N bonded to carbon atoms (C=N–C) and the tertiary N bonded to carbon atoms in the form of N–(C)3 or H–N–(C)2, while the peak at 400.7 eV could be ascribed to N–H side groups, which are consistent with the reported results on N 1s XPS spectra of C3N4.26 The XPS results verify the coexistence of Ag3PO4 and C3N4 in the composite.
Figure 4 depicts the FTIR spectra of C3N4, Ag3PO4, and 90 wt% Ag3PO4/C3N4 composite. The broad peaks at about 3420 cm−1 in all of the spectra correspond to the stretching vibration mode of adsorbed H2O molecules. As for bare C3N4, the absorption band at 1635 cm−1 could be ascribed to C=N stretching vibration modes, whereas the four peaks at 1253, 1328, 1420, and 1574 cm−1 are assigned to aromatic C–N stretching. The peak at 812 cm−1 is related to the s-triazine ring. A shoulder band near 3168 cm−1 is attributed to the stretching modes of terminal N–H groups.26 In the case of Ag3PO4, the peak at 1650 cm−1 is assigned to the –OH bending vibration. The peak at around 540 cm−1 is ascribed to O=P–O bending vibration, while the peaks at 850 and 1097 cm−1 are due to the symmetric and asymmetric stretching vibrations of P–O–P rings. One band featured at 1383 cm−1 derives from the stretching vibration of doubly bonded oxygen (P=O) and the harmonics of the above modes.33 For the 90 wt% Ag3PO4/C3N4 composite, the characteristic bands for Ag3PO4 and C3N4 coexist. In addition, the characteristic peak assigned to the stretching vibration of the P–O–P group shifts to a higher wave number of 1125 cm−1 compared with that of 1097 cm−1 in bare Ag3PO4, suggesting that the interaction between the Ag3PO4 and C3N4 has already appeared.33 The interaction between Ag3PO4 and C3N4 may benefit the photogenerated charge carriers’ transfer and then enhance the photocatalytic activity of composites.
Figure 5 shows the UV–vis DRS of C3N4, Ag3PO4, and 90 wt% Ag3PO4/C3N4 composite. As can be seen, single C3N4 and Ag3PO4 present the strong absorption edges at about 435 and 506 nm, which are attributed to the intrinsic band gap absorption. The band gaps of C3N4 and Ag3PO4 are estimated to be 2.85 and 2.45 eV according to the equation of Eg = 1240/λg, where Eg is the band gap energy of semiconductor and λg is the optical absorption edge of semiconductor. The 90 wt% Ag3PO4/C3N4 composite shows a combination of these two DRS contributing from Ag3PO4 and C3N4.
Figure 6 displays the photocatalytic activities of C3N4, Ag3PO4, and Ag3PO4/C3N4 composites with different mass ratios for the degradation of RhB solution. For comparison, the blank test was also conducted under the same reaction conditions. It can be seen that the degradation of RhB is negligible in the absence of photocatalysts under visible light irradiation for 40 min. Pristine Ag3PO4 exhibits better photocatalytic activity with the decolorization percentage of 75.4% after 40 min of visible light irradiation although it has a poor adsorption capacity. Bare C3N4 presents a best adsorption performance, and about 23.3% RhB molecules are adsorbed on its surface before illumination. Under visible light irradiation for 40 min, the decrease of RhB concentration reaches 65.3%. The adsorption abilities of Ag3PO4/C3N4 composites lie between Ag3PO4 and C3N4. Moreover, the adsorption capacity declines with increasing the amount of Ag3PO4 in the composites. The decolorization percentages of RhB are about 57.7, 62.7, 98.5, and 95.7% for the 50, 70, 90, and 92 wt% Ag3PO4/C3N4 composites for 40 min visible light illumination, respectively. As for a series of Ag3PO4/C3N4 photocatalysts, the photocatalytic activities of the composites improve with increasing the Ag3PO4 contents and the 90 wt% Ag3PO4/C3N4 composite shows the highest photocatalytic performance. Whereas further enhancing the amount of Ag3PO4 in the composites, the photocatalytic activity decreases, which is due to the excessive Ag3PO4 contents leading to the decrease of interfaces in the composites.
The photocatalytic degradation of RhB can be considered as a pseudo-first-order reaction with low concentration, and its kinetics can be expressed by the equation of −ln(C/C0) = kt, where k is the degradation rate constant, C0 and C are the absorption equilibrium concentration of RhB and the concentration at a reaction time of t, respectively.30 As shown in Fig. 7, the rate constants (k) of different samples are 0.03514, 0.01586, 0.01975, 0.10445, 0.07819, and 0.02080 min−1 for the pristine Ag3PO4, 50, 70, 90, 92 wt% Ag3PO4/C3N4 composites, and C3N4, respectively. For the degradation of RhB, the kinetic constant of 90 wt% Ag3PO4/C3N4 is about 3.0 and 5.0 times as large as those of pristine Ag3PO4 and C3N4.
To test the stability of photocatalysts, the 90 wt% Ag3PO4/C3N4 composite was reused for photocatalytic reaction under the same conditions, and the result is shown in Fig. 8. The photocatalytic decolorization efficiency still reaches 90.8% after three runs of photodegradation of RhB, indicating that the composite has considerable photostability.
To investigate the reaction mechanism, isopropanol (IPA), triethanolamine (TEOA), and p-benzoquinone (BQ) were respectively introduced as the scavengers of hydroxyl radicals (·OH), holes (h+), and superoxide radicals (·O2−) to examine the effects of reactive species on the photocatalytic degradation of RhB. The concentrations of IPA, TEOA, and BQ in the reaction system were 10, 10, and 1 mmol·L−1, respectively. From Fig. 9, it can be seen that the BQ and TEOA lead to an obvious suppression of the decolorization efficiency of RhB. Whereas the IPA exhibits weakly restraining effect on the decolorization efficiency. The results suggest that h+ and ·O2− are the main active species for the degradation of RhB in the current system.
Based on the above results, a possible mechanism is proposed to explain the improvement of photocatalytic activity of Ag3PO4/C3N4 composite. As is well known, the efficient separation of photogenerated charge carriers is crucial for the enhancement of photocatalytic activity. As for the semiconductor composites, the migration direction of photogenerated charge carriers depends on the band edge potential positions. According to previous reports,25,26 the CB and VB potential of C3N4 are −1.12 and 1.73 eV, respectively (versus NHE), which are more negative than those of the CB (0.24 eV versus NHE) and VB (2.69 eV versus NHE) potentials of Ag3PO4. This well-matched energy band structure facilitates the separation of charge carriers. As shown in Fig. 10, when the Ag3PO4/C3N4 composite is illuminated under visible light, both Ag3PO4 and C3N4 are excited to generate electron–hole pairs. The electrons in the CB of C3N4 can smoothly transfer into the CB of Ag3PO4, meanwhile the holes in the VB of Ag3PO4 can conveniently migrate into the VB of C3N4. As a result, the electron–hole pairs are efficiently separated between Ag3PO4 and C3N4, which benefits to improving the photocatalytic activity. Since the CB potential of Ag3PO4 is more positive than the single electron reduction potential of O2/·O2− (−0.33 eV versus NHE),14 it cannot reduce O2 to yield ·O2− on the surface of Ag3PO4. However, the photogenerated electrons from C3N4 can reduce the surface chemisorbed O2 to give ·O2− through one electron reaction. On the other hand, the VB potentials of Ag3PO4 and C3N4 are not more positive than the redox potentials of ·OH/H2O (2.72 eV versus NHE).14 This suggests that the photogenerated holes on the VB of Ag3PO4 and C3N4 could not react with H2O to form ·OH. Consequently, the degradation of RhB would be the reaction with photogenerated holes directly.
To confirm the effective separation of photogenerated electrons and holes in the Ag3PO4/C3N4 composites, the transient photocurrent responses and electrochemical impedance spectroscopy (EIS) spectra have been used to investigate the interfacial charge separation efficiency over pure Ag3PO4, C3N4, and 90 wt% Ag3PO4/C3N4 composite. As shown in Fig. 11(a), the photocurrent density obtained over the 90 wt% Ag3PO4/C3N4 composite is obviously enhanced compared with that of pure Ag3PO4 and C3N4. Photocurrent is formed mainly by transferring photogenerated electrons to the counter electrode; the higher photocurrent indicates more effective separation and longer lifetime of the photogenerated electrons on it. In addition, EIS Nyquist analysis was performed to study the interfacial electrons’ transfer [Fig. 11(b)]. The diameter of the Nyquist circle of the 90 wt% Ag3PO4/C3N4 composite is smaller than that of single Ag3PO4 and C3N4, suggesting that the 90 wt% Ag3PO4/C3N4 composite has a relatively lower charge transfer resistance at the contact interface between the electrode and electrolyte solution. This may prove that the 90 wt% Ag3PO4/C3N4 composite exhibits more efficient activity compared with pure Ag3PO4 and C3N4.
The better separation of photogenerated electrons and holes in the Ag3PO4/C3N4 composite is also elucidated by PL emission spectra of single C3N4 and 90 wt% Ag3PO4/C3N4 composite. It is well known that the PL signal of semiconductor material results from the recombination of photogenerated charge carriers. In generally, the lower PL intensity indicates the decrease in recombination rate of photogenerated charge carriers. As shown in Fig. 12, the main emission peak is centered at about 440 nm for the bare C3N4, which is approximately equal to the band gap of C3N4. In contrast, the quenching of PL peak is observed for the 90 wt% Ag3PO4/C3N4 composite. The result of PL verifies that the Ag3PO4/C3N4 composite can effectively separate photogenerated electron–hole pairs.
IV. CONCLUSIONS
In summary, the visible-light-responsive Ag3PO4/C3N4 composites were prepared by a facile in situ deposition of Ag3PO4 particles on the surface of C3N4 sheets and applied into photocatalytic degradation of RhB solution. The improved photocatalytic activity of Ag3PO4/C3N4 composites could be ascribed to the effective separation of photogenerated charge carriers. Photoelectrochemical and PL measurements confirmed the effective separation of photogenerated charges in the Ag3PO4/C3N4 composites. Moreover, the tests of radical scavengers indicated that h+ and ·O2− were the main active species for the degradation of RhB. The resultant Ag3PO4/C3N4 composites may be promising efficient photocatalysts for the degradation of organic pollutants in the industrial and engineering field.
References
X.L. Hu, G.S. Li, and J.C. Yu: Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 26, 3031 (2010).
A. Kubacka, M. Fernández-García, and G. Colón: Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 112, 1555 (2012).
Y. Sang, L. Kuai, C.Y. Chen, Z. Fang, and B.Y. Geng: Fabrication of a visible-light-driven plasmonic photocatalyst of AgVO3@AgBr@Ag nanobelt heterostructures. ACS Appl. Mater. Interfaces 6, 5061 (2014).
G.C. Xi and J.H. Ye: Synthesis of bismuth vanadate nanoplates with exposed {001} facets and enhanced visible-light photocatalytic properties. Chem. Commun. 46, 1893 (2010).
J.J. Wu, F.Q. Huang, X.J. Lü, P. Chen, D.Y. Wan, and F.F. Xu: Improved visible-light photocatalysis of nano-Bi2Sn2O7 with dispersed s-bands. J. Mater. Chem. 21, 3872 (2011).
Y.P. Bi, S.X. Ouyang, N. Umezawa, J.Y. Cao, and J.H. Ye: Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 133, 6490 (2011).
W.G. Wang, B. Cheng, J.G. Yu, G. Liu, and W.H. Fan: Visible-light photocatalytic activity and deactivation mechanism of Ag3PO4 spherical particles. Chem. Asian J. 7, 1902 (2012).
H. Wang, L. He, L.H. Wang, P.F. Hu, L. Guo, X.D. Han, and J.H. Li: Facile synthesis of Ag3PO4 tetrapod microcrystals with an increased percentage of exposed {110} facets and highly efficient photocatalytic properties. CrystEngComm 14, 8342 (2012).
Y.J. Cui, J.H. Huang, X.Z. Fu, and X.C. Wang: Metal-free photocatalytic degradation of 4-chlorophenol in water by mesoporous carbon nitride semiconductors. Catal. Sci. Technol. 2, 1396 (2012).
J.H. Liu, T.K. Zhang, Z.C. Wang, G. Dawson, and W. Chen: Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 21, 14398 (2011).
P.Y. Dong, Y.H. Wang, B.C. Cao, S.Y. Xin, L.N. Guo, J. Zhang, and F.H. Li: Ag3PO4/reduced graphite oxide sheets nanocomposites with highly enhanced visible light photocatalytic activity and stability. Appl. Catal., B 132–133, 45 (2013).
Z. Wang, L. Yin, M. Zhang, G.W. Zhou, H. Fei, H.X. Shi, and H.J. Dai: Synthesis and characterization of Ag3PO4/multiwalled carbon nanotube composite photocatalyst with enhanced photocatalytic activity and stability under visible light. J. Mater. Sci. 49, 1585 (2014).
J.X. Sun, Y.P. Yuan, L.G. Qiu, X. Jiang, A.J. Xie, Y.H. Shen, and J.F. Zhu: Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 41, 6756 (2012).
J. Cao, B.D. Luo, H.L. Lin, B.Y. Xu, and S.F. Chen: Visible light photocatalytic activity enhancement and mechanism of AgBr/Ag3PO4 hybrids for degradation of methyl orange. J. Hazard. Mater. 217–218, 107 (2012).
W.F. Yao, B. Zhang, C.P. Huang, C. Ma, X.L. Song, and Q.J. Xu: Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 22, 4050 (2012).
W. Liu, M.L. Wang, C.X. Xu, S.F. Chen, and X.L. Fu: Ag3PO4/ZnO: An efficient visible-light-sensitized composite with its application in photocatalytic degradation of rhodamine B. Mater. Res. Bull. 48, 106 (2013).
L.L. Zhang, H.C. Zhang, H. Huang, Y. Liu, and Z.H. Kang: Ag3PO4/SnO2 semiconductor nanocomposites with enhanced photocatalytic activity and stability. New J. Chem. 36, 1541 (2012).
Y.S. Xu and W.D. Zhang: Monodispersed Ag3PO4 nanocrystals loaded on the surface of spherical Bi2MoO6 with enhanced photocatalytic performance. Dalton Trans. 42, 1094 (2013).
G.K. Fu, G.N. Xu, S.P. Chen, L. Lei, and M.L. Zhang: Ag3PO4/Bi2WO6 hierarchical heterostructures with enhanced visible light photocatalytic activity for the degradation of phenol. Catal. Commun. 40, 120 (2013).
B. Chai, T.Y. Peng, J. Mao, K. Li, and L. Zan: Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 14, 16745 (2012).
Y.J. Wang, R. Shi, J. Lin, and Y.F. Zhu: Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 4, 2922 (2011).
L.M. Sun, X. Zhao, C.J. Jia, Y.X. Zhou, X.F. Cheng, P. Li, L. Liu, and W.L. Fan: Enhanced visible-light photocatalytic activity of g-C3N4-ZnWO4 by fabricating a heterojunction: Investigation based on experimental and theoretical studies. J. Mater. Chem. 22, 23428 (2012).
J. Fu, B.B. Chang, Y.L. Tian, F.N. Xi, and X.P. Dong: Novel C3N4-CdS composite photocatalysts with organic-inorganic heterojunctions: In situ synthesis exceptional activity, high stability and photocatalytic mechanism. J. Mater. Chem. A 1, 3083 (2013).
L.Q. Ye, J.Y. Liu, Z. Jiang, T.Y. Peng, and L. Zan: Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal., B 142–143, 1 (2013).
H. Katsumata, T. Sakai, T. Suzuki, and S. Kaneco: Highly efficient photocatalytic activity of g-C3N4/Ag3PO4 hybrid photocatalysts through Z-scheme photocatalytic mechanism under visible light. Ind. Eng. Chem. Res. 53, 8018 (2014).
B. Chai, X. Liao, F.K. Song, and H. Zhou: Fullerene modified C3N4 composites with enhanced photocatalytic activity under visible light irradiation. Dalton Trans. 43, 982 (2014).
Z.L. Xiu, H. Bo, Y.Z. Wu, and X.P. Hao: Graphite-like C3N4 modified Ag3PO4 nanoparticles with highly enhanced photocatalytic activities under visible light irradiation. Appl. Surf. Sci. 289, 394 (2014).
S. Kumar, T. Surendar, A. Baruah, and V. Shanker: Synthesis of a novel and stable g-C3N4-Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. A 1, 5333 (2013).
D.L. Jiang, J.J. Zhu, M. Chen, and J.M. Xie: Highly efficient heterojunction photocatalyst based on nanoporous g-C3N4 sheets modified by Ag3PO4 nanoparticles: Synthesis and enhanced photocatalytic activity. J. Colloid Interface Sci. 417, 115 (2014).
F.J. Zhang, F.Z. Xie, S.F. Zhu, J. Liu, J. Zhang, S.F. Mei, and W. Zhao: A novel photofunctional g-C3N4/Ag3PO4 bulk heterojunction for decolorization of Rh.B. Chem. Eng. J. 228, 435 (2013).
P.Z. He, L.M. Song, S.J. Zhang, X.Q. Wu, and Q.W. Wei: Synthesis of g-C3N4/Ag3PO4 heterojunction with enhanced photocatalytic performance. Mater. Res. Bull. 51, 432 (2014).
M. Ge, N. Zhu, Y.P. Zhao, J. Li, and L. Liu: Sunlight-assisted degradation of dye pollutants in Ag3PO4 suspension. Ind. Eng. Chem. Res. 51, 5167 (2012).
Q.H. Liang, Y. Shi, W.J. Ma, Z. Li, and X.M. Yang: Enhanced photocatalytic activity and structural stability by hybridizing Ag3PO4 nanospheres with graphene oxide sheets. Phys. Chem. Chem. Phys. 14, 15657 (2012).
ACKNOWLEDGMENT
This work was supported by Science and Technology Investigation Project of Educational Committee of Hubei Province, China (No. Q20141708).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chai, B., Zou, F. & Chen, W. Facile synthesis of Ag3PO4/C3N4 composites with improved visible light photocatalytic activity. Journal of Materials Research 30, 1128–1136 (2015). https://doi.org/10.1557/jmr.2015.91
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.91