Abstract
Alzheimer’s disease is a neurodegenerative disorder marked by cognitive decline and brain pathology involving amyloid plaques and neurofibrillary tangles. Current drug development focuses on disease-modifying therapies, primarily antibodies targeting amyloid or tau. However, the blood-brain barrier (BBB) poses a challenge for drug delivery to the brain. Pre-and early clinical data suggests that Focused Ultrasound (FUS) technology safely enhances BBB permeability without damaging brain tissue, enabling drug delivery. This systematic review discusses the application of FUS to open the BBB for the treatment of Alzheimer’s disease (AD). We review the safety, efficacy, and potential biological effects of FUS-mediated BBB opening in AD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improving therapeutic strategies for Alzheimer’s disease (AD) is arguably one of the most important scientific and public health issues facing society. Most drugs entering the AD treatment pipeline have failed, despite some amyloid beta (Aβ) monoclonal antibodies showing modest benefits in recent phase 3 clinical trials (1). The development of therapeutics for AD has encountered significant challenges, partly due to the limited ability of peptides and antibodies to access the central nervous system (CNS) without assisted transport through the blood brain barrier (BBB) (2). This might be one potential reason contributing, in part, to failed past trials and why only modest benefits have been observed in successful ones to date. Moreover, the cost-effectiveness of Aβ antibodies compared to the current standard of care remains uncertain (3). Thus, there is an urgent need to broaden our approach to treating AD. This involves not only enhancing our understanding of the neurobiology and exploring rational drug combinations targeting multiple disease pathways, but also finding new methods to deliver these drugs to the CNS while ensuring cost-effectiveness.
The BBB poses a system of high biological impedance and low paracellular and transcellular permeability that results in selective permeability to ions and small lipid-soluble molecules less than 400 Daltons (Da) in size (4). It comprises of capillary endothelium with tight junctions, its basement membrane and astrocyte foot processes (5). Other components include various efflux proteins, including ATP-binding cassette transporters, P-glycoproteins and solute carrier family transporters that use active transcellular transport to remove compounds from the CNS (6). Moreover, AD-related pathological changes at the level of the BBB further compromise drug transport into the brain (10). These pathological changes impair interstitial fluid flow and thereby transport of solutes, potentially trapping AD therapeutic agents in enlarged perivascular spaces and preventing them from reaching their neuronal-glial targets (7, 8). Typically, only 0.1% of intravenously-administered Aβ antibodies reach the brain (9). The blood brain barrier can thus be regarded as a potential bottleneck for the delivery of therapeutic agents into the CNS.
One promising non-invasive tool for BBB opening is focused ultrasound (FUS) technology, which has demonstrated diverse applications in treating neurodegenerative disorders based on the strength and type of stimulus used, as well as the CNS target. High-intensity FUS has proven effective in managing essential tremor and tremor-dominant Parkinson’s disease through its ablative effect on disease-related neurocircuits. Conversely, low-intensity pulsed stimulus with microbubble injection holds potential for localized blood-brain barrier (BBB) opening, regulating the passage of circulating molecules into the brain parenchyma, without causing mechanical or thermal harm to vessels or brain tissue. Studies have shown a synergistic effect due to combined therapy with FUS-mediated BBB opening and anti-amyloid drugs, specifically Aducanumab (10), as well as glycogen synthase kinase-3 inhibitors (11), permitting a much lower dose of the drug to be delivered to the CNS with potential therapeutic effects. This may make treatments more cost-effective. It could also improve the safety profile by reducing the dose, frequency, and duration of treatment necessary.
Therefore, FUS presents several appealing aspects that warrant consideration for further exploration as a therapeutic tool in AD: i) efficacy of FUS technology in specific neurological disorders with available technical instrumentation in multiple centers, potentially scalable to more centers; ii) some preclinical and early clinical data indicating safety and efficacy of FUS alone or combined with drugs in biological and behavioral outcomes; and iii) emerging data (elaborated in this article) suggesting the safety and multiple potential biological effects of low intensity FUS on neural circuit modulation and metabolism, enhancement of stem cell differentiation and inhibition of neural inflammation as it potentially relates to AD (12–14).
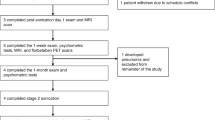
The FUS procedure workflow is outlined in Figure 1. Contrast-enhanced MRI, as a non-ionizing imaging modality, has been extensively used to evaluate the extent of BBB opening, as well as to assess the presence of hemorrhage. Contrast agents like gadolinium leak out from the vascular space through the disrupted BBB, and enhance T1-weighted MR signals. The MR images before and after FUS are compared, and the areas showing signal enhancement indicate regions where successful BBB opening occurred. An additional contrast-enhanced MR study at subsequent time intervals, usually at 24 hours post-procedure and then at one week can be used to confirm the duration of blood brain barrier opening.
In addition to changes seen with structural neuroimaging, other potentially important biological effects of FUS-BBB opening can be explored by using alternative imaging modalities such as resting state functional MRI to explore the implications on functional connectivity, and fluorodeoxyglucose-Positron Emission Tomography (FDG-PET) to study changes in neuronal glucose metabolism. With respect to AD specifically, amyloid and Tau PET could be used to respectfully study the effects on amyloid clearance and reductions in Tau burden.
Given the importance of developing disease-modifying therapies for AD and the potential for FUS-BBB opening to aid drug delivery, we systematically reviewed the literature to synthesize the current evidence on the safety, efficacy, biological effects and potential clinical applications of FUS-BBB opening in patients with AD.
Methods
We conducted a database search on PubMed, MEDLINE, EMBASE, and PsychInfo on 6th March 2023 (see Supplementary Material for search strategy). We followed The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria for this systematic review.
Two authors (A.P. and T.W.) independently screened all abstracts and full-text articles using the Covidence systematic review tool (www.covidence.org), resolving any discrepancies through discussion. We included all studies that: were published within the last 20 years, included patients with AD, applied transcranial focused ultrasound (tFUS) or MRI-guided focused ultrasound (MRIgFUS) for the purpose of blood brain barrier opening, and reported at least one outcome measurement pertaining to safety, efficacy and/or biological effects of the procedure. We excluded review articles, editorials, expert opinion or commentary and animal studies.
Two authors (A.P. and T.W.) independently extracted data from the studies using a standardized template. We extracted the following variables: first author, study year, country, sample size, mean age, sex ratio, baseline minimental state examination (MMSE) score, baseline amyloid positivity for inclusion in the study, FUS device type, FUS target, FUS protocol (frequency, power and duration), follow-up duration, psychometric tests used to evaluate the effects of FUS, evidence of BBB opening, safety, and, where reported, measures indicating neural plasticity, amyloid or tau clearance, improvement in neural activity and functional connectivity.
Results
We identified a total of ten papers comprising of data from six clinical trials involving 42 patients (Figure 2) (15–19). Trial characteristics are outlined in Table 1. All trials were phase 1 studies with the primary aim of examining safety and efficacy of FUS-BBB opening. Trial sample sizes ranged from five to nine patients. Five out of six trials included amyloid positron emission tomography (PET) scans to confirm biomarker evidence of AD. Five trials used a transcranial approach and one used an implantable device to deliver FUS. Only one study applied FUS to multiple sites, targeting whole brain networks (20).
Safety and Efficacy of Blood Brain Barrier opening
The first trial to test the safety and feasibility of FUS-BBB opening in patients with AD was published in 2018, with five subsequent similar trials (18). To date, no trials have been completed that used FUS to deliver an AD therapeutic agent in humans, although one trial of FUS-BBB opening after intravenous aducanumab infusion is ongoing (NCT05469009). Target volumes for BBB opening varied widely, from 1cm3 (18) in the earliest study, to 21cm3 in a later study (17). BBB opening was inferred via the presence of gadolinium enhancement in the sonicated regions. Five of the six trials reported successful opening of the BBB in all patients and confirmed closure at the target site within 24 hours (Table 1). All the AD subjects had treatment tolerability without major clinical side effects and tissue damage (e.g., hemorrhages and/ or edema) on MRI during the follow-up period. The one reported serious adverse event involved transient delirium in the context of rebleeding of a microbleed (19). A second study reported transiently increased confusion in two patients without imaging correlates (20). A second sonication session was abandoned in one study after the patient developed systemic infection, as the safety of FUS-BBB opening is not known under these circumstances (18).
Cognitive, neuroimaging and fluid biomarker effects of FUS-mediated BBB opening
Other important aspects of BBB opening relating to biological or clinical effects of FUS were described in some of the six studies mentioned above and also reported separately in other papers (21–24).
Cognitive Outcomes
Four studies did not show any change in post-procedure psychometric testing during follow up assessments (16–18, 20). One study compared cognition to that of Alzheimer’s disease Neuroimaging Initiative (ADNI) controls, demonstrating no difference in the anticipated trajectory of decline. A transient improvement in the Neuropsychiatric Inventory (NPI) score at 2 weeks after the second procedure was observed in one study (17). However, this returned to the baseline at the three-month evaluation. Despite the failure to achieve BBB opening in the study by Jeong et al (15), measures of immediate recall (z = 2.21, p = 0.03) and recognition memory on the Seoul Verbal Learning Test (z = 2.35, p = 0.02) were found to improve post-sonication at the three week follow up evaluation. However, this study lacked a longer follow up period.
Changes in functional connectivity
One study evaluated changes in bilateral frontoparietal networks (FPN) on resting state functional MRI from five subjects after BBB opening in the right frontal lobe (23). In the primary study, the BBB opening target was the right frontal lobe, a region associated with complex attention and executive function, and the authors hypothesized the FPN would be involved given its proximity to the FUS target. A functional connectivity decrease in the ipsilateral frontoparietal network was detected that recovered at 24 hours.
Changes in glucose metabolism
Two studies reported changes in regional cerebral glucose metabolic rates (rCMRglu) measured via FDG-PET. In the study by Epelbaum et al. no differences in the longitudinal change were observed compared to the neighboring or contralateral regions or to the change observed in the same region in ADNI controls (19). However, Jeong et al (15) observed increased rCMRglu in the right hippocampus (z = 3.07; p = 0.001) compared to non-sonicated regions.
Amyloid Clearance
Five studies compared amyloid PET standardized uptake value ratios (SUVR) pre- and post- procedure (17–21). Lipsman et al. found no changes in SUVR values post-sonication (18). However, subsequent studies demonstrated a decrease in amyloid accumulation after FUS-BBB opening. D’Haese et al. (21) using the same cohort as Rezai (16) and Park et al. (17) demonstrated a statistically significant decrease in amyloid burden, while Epelbaum et al. showed a non significant decrease at four months (19). In the single study that applied multisite FUS-BBB opening, a significant decrease in amyloid SUVR was present in the right parahippocampal and inferior temporal lobe clusters (20)
Glymphatics Visualisation
Meng et al. reported patterns of contrast distribution after the procedure (24). Extravasation of contrast was found in the perivascular space, subarachnoid space, and space surrounding large veins draining toward the dural sinuses on fluid-attenuated inversion recovery following FUS-BBB opening, indicating possible radiological evidence of glymphatic efflux. Mehta et al. demonstrated enhanced permeability at sites further downstream of the BBB, up to one week after sonication, even after closure of the BBB at the target site (26).
Fluid biomarker changes
Meng et al. (20) report a follow up to their original study (18) examining both CSF and plasma biomarkers at baseline and post-FUS-BBB opening in nine patients with AD. This included four additional patients from their original study (N=5). There was no change in CSF Aβ42/40 ratio and phosphorylated tau-181 post-procedure. However, CSF total tau increased post-FUS. Plasma Aβ42, Aβ40 and total tau were unchanged. There was no significant change in biomarkers of BBB integrity, such as plasma PDGFR-β and CSF-to-plasma albumin ratio. CSF and plasma neurofilament light chain (NfL) increased, and the plasma NfL levels returned to baseline at six months in three patients.
Discussion
We identified six trials of FUS-BBB opening in AD, the majority of which demonstrated successful blood brain barrier opening. The procedure appeared to be relatively safe clinically although all studies were substantially underpowered due to small sample sizes included due to their phase 1 nature. Whereas the focus of these phase 1 studies surrounded safety of the procedure, a range of biological and clinical effects were also described including cognitive outcomes, visualization of glymphatics, functional connectivity, cerebral glucose metabolism, amyloid clearance, and fluid biomarker changes.
Safety
Potential deleterious effects of FUS BBB opening may include microhemorrhage, overt hemorrhage from vascular rupture, ischemia from vasoconstriction, cerebral edema and/or inflammation from protein leakage, as well as direct cellular injury from thermal or mechanical injury. Our review suggests that FUS-mediated BBB opening is a relatively safe procedure, with three out of 42 patients across all trials experiencing transient confusion, of which one was considered a serious adverse event as it occurred in the context of rebleeding of a microbleed.
Evidence from animal studies suggests that BBB opening can cause increased brain uptake of blood constituents such as albumin and immunoglobulins, resulting in a sterile inflammatory response (27). However, this response appears to be self-limiting and does not progress to chronic inflammation or neurodegeneration even after repeated FUS administration (29).
Thus, we now have in-human evidence of safety of FUS-BBB opening via clinical, behavioural and radiological metrics, albeit with small sample sizes. However, the clinical relevance of the finding of elevated CSF neurofilament light chain and tau levels in humans following FUS requires further exploration, as both are considered markers of neurodegeneration (20). More caution may need to be exercised in terms of safety in patients with concomitant small vessel disease or those who have prior evidence of microhemorrhages on MRI, as suggested by the Epelbaum study (19). This may represent an AD-related vasculopathy, specifically, cerebral amyloid angiopathy.
Efficacy
The present review suggests that FUS-BBB opening using standardized technical parameters is usually successful in causing transient opening of the blood brain barrier. Five of six studies demonstrated successful opening of the BBB. Only one study attempted BBB opening at multiple target sites, an important consideration for AD therapeutics, given the widespread nature of the pathology.
Only one study failed to demonstrate BBB opening (15). Compared to other trials, this study employed a lower sonication power, at thresholds likely not high enough to cause stable cavitation of microbubbles. Technical factors such as transducer frequency, ultrasound pressure amplitude, exposure duration, burst parameters, microbubble size and dosage are known to affect degree of BBB opening (25). Heterogeneity in the technical FUS parameters between trials may contribute to differences in degree of BBB opening achieved, adverse effect profile as well as secondary outcomes such as cognitive improvement. Therefore, standardization of FUS protocols will be important in improving efficacy of the procedure and in comparing outcomes across trials.
While BBB opening has been shown to resolve in 24 hours in all patients across studies, additional observations by Mehta et al. (26) and Meng et al. (24) point towards potentially longer lasting effects of BBB opening away from the target site. The contrast enhancement in the perivenous space that was observed in these studies, even after the original insonated area had BBB closure, may reflect the lasting permeability in the affected regions of postcapillary venules and meningeal veins, a unit termed as the blood-meningeal barrier. It has been proposed that secondary neuroimmunological responses following BBB opening such as elevations in heat shock protein 70, interleukins and tumour necrosis factor may underlie the enhanced blood-meningeal barrier permeability (27).
Although these studies (24, 26) provide useful insights into the pathways that an AD therapeutic drug may take when delivered via FUS-mediated BBB opening, it should be borne in mind that gadolinium tracers are only proxy markers, and not the intended therapeutic molecule. Pharmacokinetic characteristics of the therapeutic molecule will affect its transport across the disrupted BBB and onward towards neuronal targets.
Cognitive, radiological and fluid biomarker outcomes
Cognitive Outcomes
The phase I studies were not powered to detect small changes in AD-related psychometric measures and none had a control group. However, it is reassuring that no studies demonstrated long-term negative cognitive sequelae.
Cerebral Glucose metabolism
Reduced parietal-temporal and frontal lobe glucose metabolism (rCMRglu) measured via FDG-PET studies has been found in advanced AD. Our review found that the effect of FUS-BBB opening on cerebral glucose metabolism is divergent, with enhanced FDG-PET uptake reported by one study (15), while this was not observed in another study (19). The study by Jeong et al. (15) demonstrated that hippocampal sonication improved the rCMRglu in the temporal, cingulate and frontal cortices. This indicates that low intensity FUS may have biological effects beyond the sonicated region, possibly through its effects on neuronal circuitry and possible modulation of the hippocampal-prefrontal pathway. Therefore, the way in which FUS-BBB opening affects cerebral glucose metabolism and whether this has clinical relevance is yet to be ascertained.
Amyloid Clearance
The removal of amyloid is a key therapeutic target in AD, as evidenced by the modest reduction in cognitive decline with lecanemab (30). Our review found the effects of FUS on amyloid clearance was variable across studies. While the initial study by Lipsman et al. failed to demonstrate a significant group-wise change in amyloid binding on PET (18), subsequent studies did report a significant effect on amyloid clearance in the sonicated regions. This may be related to the volume of brain tissue that was sonicated. As amyloid pathology in AD is widespread, a larger volume of BBB opening may be needed to produce a clinically detectable change in amyloid uptake in target regions.
Amyloid removal is a complex process with multiple stages involving plaque degradation, transport across the BBB, interstitial fluid bulk flow and subsequent absorption into circulatory and lymphatic systems (31). Pre-clinical studies have provided insight into the mechanisms by which FUS-BBB opening may be affecting amyloid clearance. BBB opening may increase the permeability of the glymphatic system and facilitate opsonization and clearance by glial cells (32, 33).
It is therefore unlikely that FUS will be effective in achieving clinically meaningful amyloid clearance when used alone. Evidence from animal studies suggests that combining FUS-BBB opening with an amyloid removing agent could be an effective method of clearing amyloid. The use of FUS-BBB opening with intravenous immunoglobulin (IVIg) in a mouse model of cerebral β-amyloidosis has been shown to promote hippocampal neurogenesis, but the use of FUS or IVIg alone was not sufficient (34). Other mouse studies have demonstrated improved drug delivery and/or efficacy of several other potential AD therapeutic agents such as anti-pyroglutamate-3 Aβ antibody that targets a toxic form of amyloid-beta (35) and glycogen synthase kinase-3 inhibitors that can reduce amyloid production (12).
Therapeutic agents that could be combined with FUS could include anti-amyloid monoclonal antibodies that have shown some modest impact in early AD, e.g., lecanemab or donanemab. IVIg and antisense oligonucleotides represent additional promising therapeutic strategies, which could theoretically benefit from BBB opening for delivery. Since this systematic review was completed, results of a phase I human clinical trial (NCT05469009) of FUS-BBB opening in combination with intravenous aducanumab involving three patients with AD have been published (36). This study showed that there was a greater reduction in amyloid-beta load at 26 weeks post-treatment in regions that received FUS-BBB opening prior to Aducanumab infusions, as compared to areas in the contralateral hemisphere that did not receive FUS. Moreover, a lower dose of Aducanumab (escalating from 1 mg/kg to 6mg/kg) was used, rather than the on-label dose of 10mg/kg. This is the first study to demonstrate the potential of combination therapy of FUS and a biologic agent in patients with AD. IVIg contains naturally occurring antibodies against Aβ and animal studies have shown that FUS BBB opening could deliver IVIg to the brain and promote hippocampal neurogenesis (37). Finally, application of FUS BBB opening may be extended to improve AD diagnostics as well. The BBB may also block the release of potential AD biomarkers into the bloodstream, and localized BBB opening with FUS may cause release of phosphorylated tau species and neurofilament light chain into the blood, facilitating the diagnosis of neurodegenerative disorders (38).
In a recent mini-review, Zhou et al. (39) summarized the technical parameters and biological effects of low intensity FUS on Alzheimer’s disease. Based on the findings of Zhou et al. (39) and the present systematic review, we would like to propose the following recommendations and considerations for future clinical trials:
-
i)
Inclusion and exclusion criteria: We currently recommend performing FUS BBB opening in mild to moderate stages of the disease (Mild Cognitive Impairment and early AD) confirmed via imaging or CSF AD-specific biomarker testing. Baseline FDG-PET could also be considered. A multi-modal 3T MRI to rule out patients with comorbid white matter disease (Fazekas grade 3) on T2-weighted imaging and microbleeds on susceptibility-weighted imaging is also recommended. Current guidelines for management of Amyloid-Related Imaging Abnormalities (ARIA) as with anti-Aβ monoclonal antibody infusions or contraindications (>4 microbleeds) should be implemented for FUS BBB opening as well (40).
-
ii)
Combination with drugs: As discussed, Aβ monoclonal antibodies and IVIg currently are the most common contenders for studying in combination with FUS BBB opening. Combination with agents targeting tau should also be considered in the future.
-
iii)
Volume of sonication: A volume of at least 40 cc may be needed to produce meaningful clinical outcomes. Efforts are being made to devise technology to target widespread brain regions or the whole brain.
-
iv)
FUS procedure and devices: There needs to be standardization of reported parameters in the literature. This includes cavitation dose, duration, frequency and type of microbubbles used.
Limitations of Current Studies
So far, the evidence for FUS-BBB opening in AD patients, although encouraging, is limited to phase I studies. These studies have small sample sizes and so lack statistical power to explore efficacy in detail. No studies had a control or sham group. The procedure appears safe clinically and radiologically, but the effect of BBB opening on biomarkers of neuroinflammation and neurodegeneration requires further evaluation. The ability of BBB opening alone to improve amyloid clearance, which has been shown robustly in rodent models of AD, has shown equivocal results in the currently available studies in AD patients. Current clinical trials have used FUS BBB opening to treat up to a volume of 40 cc per session, but the optimal volume and number of FUS procedures necessary for AD treatment is unknown. The optimal interval for BBB opening for patients with AD is also unknown. In animal trials, 1 to 2 weeks has been shown to be effective, but this has not been repeated in humans. Another unknown is the appropriate number of times the BBB should be opened in human patients. Results from larger, controlled trials on combined use of a therapeutic agent with FUS-BBB opening in patients with AD are awaited. Given the widespread pathology observed in AD, the targeting of BBB opening at multiple brain locations may be required to achieve meaningful therapeutic outcomes. To date, only a single study has assessed the efficacy and safety of FUS-BBB opening at multiple brain sites.
Conclusion and Future Directions
Evidence from phase I studies suggests that focused ultrasound-mediated blood brain barrier opening is a safe procedure and has a range of theoretical beneficial biological effects that may be exploited further towards achieving disease modification in patients with AD. The unique aspects of FUS technology to open BBB (Box 1) could represent a paradigm shift in the treatment of AD and other neurodegenerative disorders, particularly if used in combination with AD therapeutic agents. Larger studies with sham control groups and standardization of FUS protocols are needed.
References
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023 Jan 5;388(1):9–21. doi: https://doi.org/10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.
Gribkoff VK, Kaczmarek LK. The Need for New Approaches in CNS Drug Discovery: Why Drugs Have Failed, and What Can Be Done to Improve Outcomes. Neuropharmacology 2017;120:11. doi:https://doi.org/10.1016/J.NEUROPHARM.2016.03.021
Ross EL, Weinberg MS, Arnold SE. Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US. JAMA Neurol. 2022 May 1;79(5):478–487. doi: https://doi.org/10.1001/jamaneurol.2022.0315. PMID: 35344024; PMCID:PMC8961406.
Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS. 2020 17:1 2020;17:1–24. doi:https://doi.org/10.1186/S12987-020-00230-3
Sweeney MD, Sagare AP, Zlokovic B v. Blood–brain barrier breakdown in Alzheimer’s disease and other neurodegenerative disorders. Nat Rev Neurol 2018;14:133. doi:https://doi.org/10.1038/NRNEUROL.2017.188
Kusuhara H, Sugiyama Y. Efflux transport systems at the blood–brain barrier and blood CSF barrier. Int Congr Ser 2005;1277:111–22. doi:https://doi.org/10.1016/J.ICS.2005.02.015
Vilor-Tejedor N, Ciampa I, Operto G, et al. Perivascular spaces are associated with tau pathophysiology and synaptic dysfunction in early Alzheimer’s continuum. Alzheimers Res Ther 2021;13:1–13. doi:https://doi.org/10.1186/S13195-021-00878-5/TABLES/4
Miners JS, Schulz I, Love S. Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer’s disease. Journal of Cerebral Blood Flow and Metabolism 2018;38:103–15. doi:https://doi.org/10.1177/0271678X17690761
Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid beta protein antibody across the blood-brain barrier in a mouse model of Alzheimer’s disease. Peptides. 2002;23(12):2223–2226. doi:https://doi.org/10.1016/s0196-9781(02)00261-9
Kong C, Yang EJ, Shin J, Park J, Kim SH, Park SW, Chang WS, Lee CH, Kim H, Kim HS, Chang JW. Enhanced delivery of a low dose of aducanumab via FUS in 5×FAD mice, an AD model. Transl Neurodegener. 2022 Dec 27;11(1):57. doi: https://doi.org/10.1186/s40035-022-00333-x. PMID: 36575534; PMCID: PMC9793531.
Hsu PH, Lin YT, Chung YH, Lin KJ, Yang LY, Yen TC, Liu HL. Focused Ultrasound-Induced Blood-Brain Barrier Opening Enhances GSK-3 Inhibitor Delivery for Amyloid-Beta Plaque Reduction. Sci Rep. 2018 Aug 27;8(1):12882. doi: https://doi.org/10.1038/s41598-018-31071-8. PMID: 30150769; PMCID: PMC6110796.
Ren C, Li JM, Lin X. LIPUS enhance elongation of neurites in rat cortical neurons through inhibition of GSK-3β. Biomed Environ Sci. (2010) 23:244. doi:https://doi.org/10.1016/S0895-3988(10)60059-1
Chen SF, Su WS, Wu CH, Lan TH, Yang FY. Transcranial ultrasound stimulation improves long-term functional outcomes and protects against brain damage in traumatic brain injury. Mol Neurobiol. (2018) 55:7079–89. doi: https://doi.org/10.1007/s12035-018-0897-z
El-Bialy T, Alhadlaq A, Wong B, Kucharski C. Ultrasound effect on neural differentiation of gingival stem/progenitor cells. Ann Biomed Eng. (2014) 42:1406–12. doi: https://doi.org/10.1007/s10439-014-1013-9
Jeong H, Im JJ, Park JS, et al. A pilot clinical study of low-intensity transcranial focused ultrasound in Alzheimer’s disease. Ultrasonography 2021;40:512–9. doi:https://doi.org/10.14366/USG.20138
Rezai AR, Ranjan M, D’Haese PF, et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc Natl Acad Sci U S A 2020;117:9180–2. doi:https://doi.org/10.1073/PNAS.2002571117
Park SH, Baik K, Jeon S, et al. Extensive frontal focused ultrasound mediated blood-brain barrier opening for the treatment of Alzheimer’s disease: a proof-of-concept study. Transl Neurodegener 2021;10. doi:https://doi.org/10.1186/S40035-021-00269-8
Lipsman N, Meng Y, Bethune AJ, et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 2018;9:1–8. doi:https://doi.org/10.1038/s41467-018-04529-6
Epelbaum S, Burgos N, Canney M, et al. Pilot study of repeated blood-brain barrier disruption in patients with mild Alzheimer’s disease with an implantable ultrasound device. Alzheimers Res Ther 2022;14:1–13. doi:https://doi.org/10.1186/S13195-022-00981-1/FIGURES/5
Meng Y, Goubran M, Rabin JS, et al. Blood-brain barrier opening of the default mode network in Alzheimer’s disease with magnetic resonance-guided focused ultrasound. Brain 2023;146. doi:https://doi.org/10.1093/BRAIN/AWAC459
D’Haese PF, Ranjan M, Song A, et al. β-Amyloid Plaque Reduction in the Hippocampus After Focused Ultrasound-Induced Blood-Brain Barrier Opening in Alzheimer’s Disease. Front Hum Neurosci 2020;14. doi:https://doi.org/10.3389/FNHUM.2020.593672
Mehta RI, Carpenter JS, Mehta RI, et al. Blood-brain barrier opening with MRI-guided focused ultrasound elicits meningeal venous permeability in humans with early Alzheimer disease. Radiology 2021;298:654–62. doi:https://doi.org/10.1148/radiol.2021200643
Meng Y, MacIntosh BJ, Shirzadi Z, et al. Resting state functional connectivity changes after MR-guided focused ultrasound mediated blood-brain barrier opening in patients with Alzheimer’s disease. Neuroimage 2019;200:275–80. doi:https://doi.org/10.1016/J.NEUROIMAGE.2019.06.060
Meng Y, Abrahao A, Heyn CC, et al. Glymphatics Visualization after Focused Ultrasound-Induced Blood–Brain Barrier Opening in Humans. Ann Neurol 2019;86:975–80. doi:https://doi.org/10.1002/ANA.25604
Gandhi K, Barzegar-Fallah A, Banstola A, et al. Ultrasound-Mediated Blood-Brain Barrier Disruption for Drug Delivery: A Systematic Review of Protocols, Efficacy, and Safety Outcomes from Preclinical and Clinical Studies. Pharmaceutics 2022;14. doi:https://doi.org/10.3390/PHARMACEUTICS14040833
Mehta RI, Carpenter JS, Mehta RI, et al. Blood-Brain Barrier Opening with MRI-guided Focused Ultrasound Elicits Meningeal Venous Permeability in Humans with Early Alzheimer Disease. Radiology 2021;298:654. doi:https://doi.org/10.1148/RADIOL.2021200643
Kovacsa ZI, Kima S, Jikariaa N, et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A 2017;114:E75–84. doi:https://doi.org/10.1073/PNAS.1614777114
Pelekanos M, Leinenga G, Odabaee M, et al. Establishing sheep as an experimental species to validate ultrasound-mediated blood-brain barrier opening for potential therapeutic interventions. Theranostics 2018;8:2583. doi:https://doi.org/10.7150/THNO.22852
Horodyckid C, Canney M, Vignot A, et al. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J Neurosurg 2017;126:1351–61. doi:https://doi.org/10.3171/2016.3.JNS151635
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023 Jan 5;388(1):9–21. doi: https://doi.org/10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.
Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain - Implications for Alzheimer disease. Nat Rev Neurol 2015;11:457–70. doi:https://doi.org/10.1038/nrneurol.2015.119
Jordão JF, Thévenot E, Markham-Coultes K, et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 2013;248:16–29. doi:https://doi.org/10.1016/J.EXPNEUROL.2013.05.008
Leinenga G, Koh WK, Götz J. A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease. Alzheimers Res Ther 2021;13:1–14. doi:https://doi.org/10.1186/S13195-021-00809-4/FIGURES/4
Dubey S, Heinen S, Krantic S, et al. Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc Natl Acad Sci U S A 2020;117:32691–700. doi:https://doi.org/10.1073/PNAS.1908658117
Bathini P, Sun T, Schenk M, et al. Acute Effects of Focused Ultrasound-Induced Blood-Brain Barrier Opening on Anti-Pyroglu3 Abeta Antibody Delivery and Immune Responses. Biomolecules 2022;12. doi:https://doi.org/10.3390/BIOM12070951
Rezai AR, D’Haese PF, Finomore V, Carpenter J, Ranjan M, Wilhelmsen K, Mehta RI, Wang P, Najib U, Vieira Ligo Teixeira C, Arsiwala T, Tarabishy A, Tirumalai P, Claassen DO, Hodder S, Haut MW. Ultrasound Blood-Brain Barrier Opening and Aducanumab in Alzheimer’s Disease. N Engl J Med. 2024 Jan 4;390(1):55–62. doi: https://doi.org/10.1056/NEJMoa2308719. PMID: 38169490.
Dubey S, Heinen S, Krantic S, et al. Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2020;117(51):32691–32700. doi:https://doi.org/10.1073/pnas.1908658117
Pacia CP, Yuan J, Yue Y, et al. Focused Ultrasound-mediated Liquid Biopsy in a Tauopathy Mouse Model. Radiology. 2023;307(2):e220869. doi:https://doi.org/10.1148/radiol.220869
Zhou M, Fu X, Ma B, Chen Z, Cheng Y, Liu L, Kan S, Zhao X, Feng S, Jiang Z, Zhu R. Effects of low-intensity ultrasound opening the blood-brain barrier on Alzheimer’s disease-a mini review. Front Neurol. 2023 Nov 1;14:1274642. doi: https://doi.org/10.3389/fneur.2023.1274642. PMID: 38020620; PMCID: PMC10646525.
Barakos, J., Purcell, D., Suhy, J. et al. Detection and Management of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Anti-Amyloid Beta Therapy. J Prev Alzheimers Dis 9, 211–220 (2022). https://doi.org/10.14283/jpad.2022.21
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: N.L. and S.E.B. have received an honorarium from the Focused Ultrasound Foundation for serving on an expert steering committee on focused ultrasound in Alzheimer’s Disease. M.M. reports grant funding from the Ontario Brain Institute, the Canadian Institutes of Health Research, Woman’s Brain Health Initiative, Brain Canada, Weston Brain Institute and Washington University, outside of this submitted work. M.M. has received personal fees for serving on a Scientific Advisory Committee for Ionis Pharmaceuticals, Alector Pharmaceuticals, Wave Life Sciences, and Biogen Canada, outside of this submitted work. M.M. has received royalties from Henry Stewart Talks, outside of this submitted work. M.M. is a clinical trial site investigator for Roche, and Alector Pharmaceuticals, outside of this submitted work. A.P.,T.W.,Y.M. and I.A. have no competing interests to disclose.
Rights and permissions
About this article
Cite this article
Patwardhan, A., Wilkinson, T., Meng, Y. et al. Safety, Efficacy and Clinical Applications of Focused Ultrasound-Mediated Blood Brain Barrier Opening in Alzheimer’s Disease: A Systematic Review. J Prev Alzheimers Dis 11, 975–982 (2024). https://doi.org/10.14283/jpad.2024.85
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2024.85