Abstract
In vivo Alzheimer’s disease diagnosis and staging is traditionally based on clinical features. However, the agreement between clinical and pathological Alzheimer’s disease diagnosis, whose diagnosis assessment includes amyloid and Braak histopathological tau staging, is not completely convergent. The development of positron emission tomography (PET) tracers targeting neurofibrillary tangles offers prospects for advancing the staging of Alzheimer’s disease from both biological and clinical perspectives. Recent advances in radiochemistry made it possible to apply the postmortem Braak staging framework to tau-PET images obtained in vivo. Here, our aim is to provide a narrative review of the current literature on the relationship between Alzheimer’s disease clinical features and the PET-based Braak staging framework. Overall, the available studies support the stepwise increase in disease severity following the advance of PET-based Braak stages, with later stages being associated with worse cognitive and clinical symptoms. In line with this, there is a trend for unimpaired cognition, mild cognitive impairment, and Alzheimer’s disease dementia to be compatible with early, intermediate, and late patterns of tau deposition based on PET-based Braak stages. Moreover, neuropsychiatric symptom severity seems to be linked to the extent of tau-PET signal across Braak areas. In sum, this framework seems to correspond well with the clinical progression of Alzheimer’s disease, which is an indication of its potential utility in research and clinical practice, especially for detecting preclinical tau levels in individuals without symptoms. However, further research is needed to improve the generalizability of these findings and to better understand the applications of this staging framework.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In clinical practice, the diagnosis and staging of Alzheimer’s disease (AD) dementia is traditionally based on clinical features. AD’s first widely recognized and implemented diagnostic criteria, proposed in 1984, characterized it as an insidious-onset disease with progressive decline in memory and other cognitive domains with no considerable motor, sensory, or coordination deficits early in its course (1). Over the years, significant progress has been made in understanding the impact of AD pathophysiology in vivo using biomarkers. Researchers have developed several imaging and fluid biomarkers based on AD neuropathological hallmarks, including extracellular amyloid β (Aβ) plaques, neurofibrillary tangles (NFT), and neurodegeneration (2).
Following these advances, the National Institute on Aging and Alzheimer’s Association (NIA-AA) proposed, in 2018, a research diagnostic framework integrating the results of biomarker assessment as part of AD diagnostic (3). This framework shifted the academic definition of AD in living individuals towards a biological conceptualization using the AT(N) system, which summarizes the status of individuals based on biomarker evidence of Aβ (A), tau (T), and neurodegeneration (N) biomarkers (3). This AD reconceptualization became important considering those cases where there is a disagreement between the typical clinical presentation and the pathological definitions of AD: biological abnormalities appear in asymptomatic individuals and typical AD clinical features are manifested by patients without AD neuropathology (4,5). Therefore, an in vivo biological staging system for AD would allow for a better selection of candidates for disease-modifying therapies and the possibility of tracking the progression of neuropathological changes.
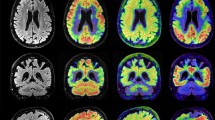
Even though AD staging systems were already well-established in postmortem neuropathology, efforts to translate these to in vivo studies were only recently undertaken due to advancements in the field (6,7) (Figure 1). The development of positron emission tomography (PET) tracers targeting NFT allowed for the translation, in 2016 (8), of the Braak NFT histopathological staging system for its use in vivo. Proposed in 1991, this histopathological classification describes the hierarchical and cumulative tau deposition in the brain into six stages, with the following topographic hallmarks: transentorhinal cortex (Braak I); entorhinal cortex and hippocampus CA1 sector (Braak II); hippocampus (extension of damage), amygdala, and adjacent neocortical areas (Braak III); associative neocortex (initial involvement; Braak IV); associative neocortex (extension of damage), notably in temporal, parietal, and occipital areas (Braak V); primary motor and sensory fields (Braak VI) (6). The Braak neuropathological stages are now integrated into the AD neuropathological diagnostic criteria (9–11). These stages coincide well with clinical manifestations: stages I–II correspond to preclinical AD, III–IV to prodromal dementia, and V–VI to fully installed dementia (6,7).
Since 2016, several research groups have attempted to establish the clinical correlates of the Braak NFT staging framework as assessed with tau-PET, here termed PET-based Braak staging. This allows for the understanding of possible diagnostic and prognostic values of this framework, as well as its applicability in the selection of participants for clinical trials. Thus, our aim with this study is to provide a narrative review of studies assessing the relationship between classical clinical features of AD and the PET-based Braak staging framework, a promising candidate for biological staging system for AD.
Methods
In May 2022, we performed a literature search on PubMed and Scopus combining the terms “Alzheimer” AND “Braak” AND (“positron emission tomography” OR “PET”), without restrictions for language or publication date. We screened the search results for studies assessing the clinical correlates of Braak staging assigned with tau PET. Additionally, relevant reports published after the database search date were identified by experts in a non-systematic fashion.
In-vivo braak staging using tau-PET
PET imaging makes it possible to map tau load across the whole human brain. Meanwhile, the Braak neuropathological staging is based on predefined sections of specific brain areas, limiting the identification of alternative NFT accumulation patterns highlighted in more recent neuropathologic observations (9). Indeed, although several studies show a high topographical correspondence between the tau deposition observed in PET and the Braak histopathological descriptions, Braak non-conformant patterns of accumulation have also been noted (10).
Another advantage of tau-PET as compared to neuropathology is the possibility of tracking changes in tau deposition in a longitudinal fashion, allowing the investigation of the relationship between tau accumulation and clinical performance over time. Neuropathological assessments, in turn, only allow for the establishment of cross-sectional associations with antemortem clinical measures. Furthermore, the Braak histopathological staging employs staining techniques that detect only NFT, one of the neurofibrillary changes underlying AD (11–14). Even though autoradiographic reports show a high affinity of tau-PET tracers to tau aggregates characteristic of AD (i.e., mixed 3-repeat/4-repeat tau isoforms) (15–20), the magnitude of the contribution of the tau species identified by different neuropathologic protocols to the signal detected by tau-PET remains to be elucidated. This should be taken into account when studying the clinical correlates of Braak stages assigned with PET.
Additionally, PET imaging is less sensitive and has a lower resolution than neuropathology, making it difficult to assess small brain regions such as those composing early Braak stages. Off-target binding to the choroid plexus may also compromise the assessment of medial temporal structures with first-generation tau-PET ligands (18F-AV1451 and 18F-THK5351) (16). In part, these limitations have been overcome by second-generation tau-PET ligands (18F-MK6240, 18F-PI2620, and 18F-RO948) with greater sensitivity and reduced off-target binding (21–25). Nonetheless, the impact of the properties of different ligands for Braak staging using tau-PET remains to be elucidated (10).
Relationship with cognitive measures
Schöll et al. (2016) evaluated the relationship of [18F] AV1451 tau-PET uptake in Braak regions of interests (ROIs) with cross-sectional and retrospective longitudinal (mean [SD] of 4.1 [2.2] years) cognitive measures in 33 cognitively unimpaired (CU) older adults (16 Aβ+, 17 Aβ−), employing least squares regressions adjusted for age and sex (8). They reported a significant association between tau-PET standardized uptake value ratio (SUVR) in Braak I/II ROIs and cross-sectional (β = −3.12, p = 0.007), as well as longitudinal (β = −0.06, p = 0.006) episodic memory impairment (8). Additionally, longitudinal but not cross-sectional decline in a global cognitive measure (a composite of episodic memory, working memory, and processing speed scores) was related to higher ligand uptake in all Braak ROIs (I/II: β = −0.013, p = 0.009; III/IV: β = −0.015, p < 0.001; V/VI: β = −0.07, p = 0.007) (8). However, [18F]AV1451 uptake had no association with cross-sectional or longitudinal working memory and processing speed performance (8). Interestingly, the average [18F]AV1451 uptake in all Braak ROIs correlated solely with longitudinal global cognitive worsening but not cross-sectional global cognitive decline or episodic memory (8). This suggests a better performance in the Braak ROI than the global approach in predicting cognitive performance (8). Most of these associations remained significant following the addition of cortical Aβ-PET distribution volume ratio (DVR) to the models, except for the relationship between Braak V/VI ROI and longitudinal global cognitive decline (8).
In 2016, Schwarz et al. assessed 187 participants (14 young CU, 42 Aβ− older CU, 87 with mild cognitive impairment [MCI; 40 Aβ−, 47 Aβ+] and 44 with AD dementia [16 Aβ−, 28 16 Aβ+]) with [18F]AV1451 tau-PET (26). Using ordinal logistic regression models adjusted for age, sex and amyloid status, they observed that the estimated Braak stages were significantly associated with cross-sectional global cognitive impairment as assessed by the Mini-Mental State Examination (MMSE) (R2 = 0.21, p = 0.0005) and the Alzheimer’s Disease Assessment Scale-cognitive component (ADAS-Cog) (R2 = 0.22, p = 0.0007) (26). In another study that followed 107 participants (45 Aβ− CU, 7 Aβ+ CU, 31 Aβ+ MCI, and 24 Aβ+ AD dementia) for approximately 2 years, linear regressions adjusted for age, sex, education, and ApoE ε4 status demonstrated a relationship between the progression of cognitive decline and tau propagation to higher Braak ROIs as indexed by [18F]AV1451 PET (27).
Another cross-sectional study, including Aβ− and Aβ+ participants from two different cohorts, used the Spearman rank test with Bonferroni correction to investigate the correlation of cognition with Braak ROIs SUVR and with individually assigned Braak stage (28). In both samples, increasing SUVR in all Braak ROIs and increasing Braak stage correlated with poorer MMSE scores. Differences between the cohorts were observed regarding verbal recall performance. In sample 2, only Braak I/II SUVR correlated with worse verbal recall, while in sample 1, all Braak ROIs and Braak stages demonstrated significant associations (28). This could be an indication of an increased sensitivity of Braak I/II regions to early clinical manifestations of AD. However, clinical and demographic differences between the samples could also be contributing factors. For instance, sample 2 is smaller and most of its participants presented only amnestic mild cognitive deficits. In contrast, sample 1 included participants with different AD clinical variants, most of them having an early age of symptom onset.
Negative correlations (assessed with Bonferroni-corrected Pearson’s test) between MMSE scores and [18F] THK5351 signal in Braak ROIs (I/II: r = −0.57, p < 0.0001; III/IV: r = −0.59, p < 0.0001; V/VI: r = −0.50, p < 0.0001) were also observed in another cross-sectional study with older Aβ− CU participants and Aβ+ participants in the AD continuum (29). Similarly, tau signal in PET-based Braak stages I/II (r = 0.55, p < 0.0001), III/IV (r = 0.67, p < 0.0001), and V/VI (r = 0.60, p < 0.0001) was correlated with ADAS-Cog scores (15). Tau-PET SUVR also correlated negatively with episodic memory immediate recall in Braak I/II (r = −0.57, p < 0.0001), III/IV (r = −0.56, p < 0.0001), and V/VI (r = −0.56, p < 0.0001)) ROIs as well as with delayed recall in Braak I/II (r = −0.58, p < 0.0001), III/IV (r = −0.51, p < 0.0001), and V/VI (r = −0.49, p < 0.0001) ROIs (29). However, the interpretation of these findings should take into account that [18F]THK5351 presents important off-target binding to monoamine oxidase B, a marker linked to neural degeneration and inflammation (30–32).
Using [18F]MK6240 tau-PET, Pascoal et al. (2020) assessed 30 Aβ− young CU, 138 Aβ− and Aβ+ older CU, 67 Aβ− and Aβ+ MCI, and 54 Aβ+ typical and atypical AD dementia participants in a cross-sectional study (25). They applied ordinal logistic regression and observed that the six-stage PET-Braak model was significantly associated with poorer MMSE scores (R2 = 0.51, p < 0.0001). Braak V–VI stages were invariably linked to cognitive impairment, even though the isolated increased tau-PET signal in Braak I ROIs was also associated with a higher prevalence of cognitive impairment (p < 0.0001) (25). Of note, 2% of the participants of this study showed patterns of NFT deposition in tau-PET that differed from the stereotypical patterns described by Braak & Braak.
Another study reported that [18F]MK6240 uptake was cross-sectionally associated with poorer performance in the MMSE in a sample of 101 participants (33). Across all participants, higher [18F]MK6240 uptake in all Braak ROIs was associated with lower MMSE scores, in linear regression models including age, sex, and education as covariates, even after false discovery rate (FDR) correction (r = −0.59 to −0.47, p < 0.0001) (33). Among Aβ+ participants, higher uptake in all Braak ROIs was also associated to lower MMSE scores, surviving FDR correction (r = −0.63 to −0.36; p =1E-6 to 0.007) (33). In Aβ− participants, the only associations surviving FDR correction were between poorer MMSE scores and Braak I, III and V SUVR (33).
A cross-sectional study published in 2021 investigated the association of MMSE scores with [18F]AV1451 PET SUVR in Braak ROIs with FDR-corrected Pearson correlation adjusted for age and gender (34). Among 32 MCI (13 Aβ+, 19 Aβ−) participants, MMSE scores were significantly correlated with SUVR in areas corresponding to Braak stages I–IV, including the entorhinal cortex, the hippocampus, the amygdala, the parahippocampal gyri, and most of the temporal lobe (34). Interestingly, significant correlations between Braak ROIs SUVR and MMSE scores were not observed in the AD group, which included 20 Aβ+ and 6 Aβ− participants (34). Nonetheless, these results should be interpreted carefully given the limited sample size and the fact that Aβ- cognitively impaired participants were analyzed along with Aβ+ subjects.
Rullmann et al. (2022) employed Pearson’s correlation to investigate the relationship between the DVR signal of [18F]PI2620 tau-PET and MMSE in a study including 26 CU (Aβ status not reported) and 38 Aβ+ AD individuals (35). When correcting for age, a higher DVR signal was associated with worse MMSE scores in all PET-based Braak stages, except stage VI, a finding that was suggested by the authors to be related to floor effects of MMSE scores with the progression of AD (35). No association, however, was found between the assigned PET-based Braak stage and MMSE scores (r = 0.24, p = 0.2). Statistical power issues or the fact that only 13% of the studied sample was classified as PET-based Braak stage VI may partially account for these findings.
In turn, Therriault et al. (2022) assigned an individual PET-based Braak stage to 324 Aβ− and Aβ+ participants based on their [18F]MK6240 tau-PET SUVR (36). In this study, the progression of cognitive decline was crosssectionally modeled by grouping participants according to their assigned Braak stage. Group comparisons were established with ANOVA adjusted for multiple comparisons with the Dunnetfs T3 test (36). Participants were assessed in the following cognitive domains: global cognition, executive function, language, memory, and visuospatial abilities. A significant decline in global cognitive scales was seen in participants at PET-based Braak stages IV-VI when compared to controls at Braak 0 (36). Nearly no variation was observed in MMSE scores between participants classified as PET-based Braak stages 0 to III, with impairments starting at stage IV (36). Memory dysfunction was observed starting at Braak stage II and worsening across groups with higher Braak stages (36). Executive function, language, or visuospatial domains were impaired solely at late PET-based Braak stages. Importantly, all participants at stages V or VI displayed some degree of cognitive impairment (36). It should be noted, however, that these analyses were not adjusted for potential confounders (e.g. age and sex).
Fernández-Arias et al. (2023) compared the verbal memory performance of Aβ− and Aβ+ individuals in the aging and AD continuum grouped according to their individually assigned [18F]MK6240 PET-based Braak stage (37). Group comparisons were performed using the Kruskal-Wallis test and post hoc analyses using the Mann-Whitney U-tests with FDR correction (37). While delayed recall was significantly affected in participants classified as PET-Braak stage II and above, as compared to individuals at stage 0, recognition memory impairment started only at PET-Braak stage IV (37). No adjustments were done for possible confounders.
Overall, these findings support the stepwise decline in cognition following the advance of PET-based Braak stages, with later stages being compatible with worse cognitive performance. Several studies also observed an association between worse cognitive performance and higher tau accumulation in Braak regions. Furthermore, these results indicate that this framework is capable of modeling the sequential decline of different cognitive domains that is characteristic of AD dementia. Supplementary Table 1 depicts the features of these studies.
Relationship with global disease severity measures
King-Robson et al. (2021) also investigated the association between Clinical Dementia Rating sum of boxes (CDR-SB; i.e. the raw sum of its domains’ scores) performance and [18F]AV1451 tau-PET SUVR in Braak ROIs using Pearson’s correlation controlling for age and gender with FDR correction (34). Worse CDR-SB scores correlated significantly with higher SUVR in Braak stages I–IV, in the MCI group (13 Aβ+, 19 Aβ−), and in ROIs reflecting Braak stages III–VI, in the AD group (20 Aβ+, 6 Aβ−) (34).
In Pascoal et al. (2020), ordinal logistic regressions accounting for age and gender showed that PET-based Braak stages were significantly associated with a poorer performance in the Clinical Dementia Rating (CDR; calculated through an algorithm using its domains’ scores) (r = 0.73, p < 0.0001) (25). Similarly, in Therriault et al. (2022), stages 0-II were compatible with the absence of dementia (CDR=0), whereas most individuals at PET-based Braak stages III-IV had a CDR of 0.5, indicating either very mild dementia or MCI (36). All participants at Braak stages V or VI presented a CDR > 0, with the majority of participants at stage VI presenting with a CDR of 1 or 2 (36).
In sum, these findings are in line with the idea that higher Braak stages represent a more severe stage of AD. PET-based Braak staging seems to be able to model the increase in clinical severity across the aging and AD continuum. Supplementary Table 2 summarizes these studies’ characteristics.
Relationship with clinical diagnosis
In Schwarz et al. (2016), 92% of Aβ− CU older participants were classified as being at PET-based Braak stage 0 assigned with [18F]AV1451 PET, with the remaining 8% classified as being at Braak stages I, V, and VI (26). Overall, Aβ+ MCI and Aβ+ AD participants had a significantly higher estimated Braak stage than Aβ−MCI and Aβ− AD participants, respectively (26). While 61% of Aβ+ MCI participants were classified at a higher PET-based Braak stage, 81% of Aβ+ AD participants were estimated to be at stages V or VI (26). Even when controlling for age and sex, ordinal logistic regressions showed a significant association between PET-based Braak staging and the diagnostic group (r = 0.22, p = 0.0002) (26).
In Maass et al. (2017), [18F]AV-1451 SUVR at Braak ROIs showed a stepwise increase across diagnostic groups in two different samples (28). They were higher among Aβ+ participants with AD dementia followed by Aβ+ MCI, Aβ+ CU, Aβ− CU, and young CU individuals (28). Braak ROI SUVR showed adequate to excellent power to discriminate between Aβ+ MCI/AD and Aβ− CU individuals (28). Depending on the sample and on whether partial volume correction was performed, the area under the curve (AUC) values ranged from 0.8–0.92 for Braak I/II, 0.78–0.97 III/IV, and 0.76−0.95 for V/VI (28).
In Pascoal et al. (2020), [18F]MK6240 tau-PET assigned Braak stages showed significant associations with clinical diagnosis (CU, MCI, AD) (r = 0.61, p < 0.0001) in ordinal logistic regressions adjusted for age and gender (25). Furthermore, the combination of PET-based Braak staging and [18F]AZD4694 Aβ-PET neocortical SUVR demonstrated a high accuracy to distinguish Aβ+ AD from CU (AUC = 98%) and MCI (AUC = 86%). These tools also had adequate accuracy to distinguish MCI from CU (AUC = 78%) (25).
In a cross-sectional study published in 2020, Leuzy et al. assessed 257 CU (98 Aβ+, 159 Aβ−), 154 MCI (96 Aβ+, 58 Aβ−), 100 Aβ+ AD subjects, and 102 individuals with non-AD neurodegenerative disorders (42 Aβ+, 60 Aβ−) (38). They observed that [18F]RO948 tau-PET uptake in Braak ROIs could effectively distinguish participants with AD dementia from participants with no cognitive impairment or with non-AD disorders (38). Even though better AUC results were observed when employing Braak I–IV ROI to differentiate AD from CU (AUC = 0.98) and from non-AD (AUC = 0.97) individuals, SUVR in Braak I–II, III–IV and V–VI ROIs also showed high accuracy (38). Lower AUC values were observed when using Braak ROIs to distinguish between MCI and non-AD disorders, but Braak I–IV ROIs still showed the most promising results (AUC = 0.73) (38).
Pascoal et al. (2021) followed 125 participants across the aging and AD continuum (17 Aβ+ AD, 21 Aβ+ MCI, 22 Aβ+ CU, and 65 Aβ− CU) for a mean (SD) of 1.16 (0.33) years and assessed the increase of tau signal in Braak ROIs per diagnostic group (39). Interestingly, Aβ− CU and Aβ+ CU groups demonstrated a trend of SUVR increase limited to ROIs corresponding to Braak I-II and I-III, respectively (39). On the other hand, Aβ+ MCI participants had increases in tau-PET signal particularly in Braak IV–V ROIs and Aβ+ AD participants in Braak V-VI ROIs (39). Another study with a 2-year follow-up found that [18F]AV1451 SUVR in Aβ+ participants with MCI or dementia increased mainly in ROIs corresponding to Braak I-IV, with the maximal increase in Braak I-II and III-IV regions belonging respectively to the MCI and the dementia groups (27). In Aβ− and Aβ+ CU individuals, the maximal SUVR progression was observed in Braak I-II ROIs (27).
Across the literature and following what is observed in neuropathological studies, there is a trend for unimpaired cognition, MCI, and AD dementia to be compatible with lower, intermediate, and higher PET-based Braak stages, respectively. Supplementary Table 3 displays the characteristics of these studies.
Relationship with neuropsychiatrie symptoms (NPS)
In a cross-sectional study, Pichet-Binette et al. (2021) evaluated 115 CU participants (Aβ status not reported) from the Pre-symptomatic Evaluation of Experimental or Novel Treatments for AD (PREVENT-AD) cohort, all of which had a high risk of sporadic AD (40). They investigated the univariate parametric correlations of [18F] AV1451 tau-PET SUVR in ROIs representing Braak stages I, III and IV with the following behavioral features: NPS (apathy, anxiety, depression, stress), cognitive lifestyle factors (lifetime cognitive activity, years of education), and personality traits (agreeableness, conscientiousness, extraversion, neuroticism, openness) (40). Braak I ROIs SUVR was significantly associated to apathy (r = 0.24, p < 0.01), depression (r = 0.23, p < 0.05), lifetime cognitive activity (r = −0.29, p < 0.001), as well as the conscientiousness (r = -0.21, p < 0.05), extraversion (r = −0.22, p < 0.05), neuroticism (r = 0.24, p < 0.01), and openness (r = −0.34, p < 0.001) personality traits. PET-based Braak stages III (r = −0.21, p < 0.05) and IV (r = −0.19, p < 0.05) were related only to lifetime cognitive activity, but the associations did not survive FDR correction (40). Since this study was conducted with highly educated individuals with a family history of AD, caution should be taken when generalizing these findings.
In 99 Aβ+ participants across the AD continuum, Yasuno et al. (2021) employed Spearman’s correlation with Bonferroni correction to evaluate the association between [18F]AV1451 SUVR in Braak ROIs and the presence of neuropsychiatric symptoms (NPS) (41). Briefly, NPS were assessed with the 12-item neuropsychiatric inventory (NPI) severity scale, whose domains were grouped into four composite domains based on a factor analysis conducted by Aalten et al. (42): a) hyperactivity (including agitation, euphoria, disinhibition, irritability, and aberrant motor behavior); b) psychosis (including delusions, hallucinations, and night-time behavior); c) affective (including depression and anxiety); and d) apathy (including apathy and eating abnormalities). The SUVR in Braak I/II ROIs was significantly correlated with both the NPI total score (r = 0.43, p < 0.001) and with the NPI affective score (r = 0.31, p = 0.002) (41). No significant correlations were found between Braak III-IV and V-VI ROIs SUVR and NPS (41). Of note, participants with affective symptoms presented a significantly higher tau signal in Braak I-II ROIs, which was indicated to have a potential role in discriminating the presence of affective symptoms consequent to AD pathology (41).
Another study by Tissot et al. (2021), evaluated Aβ+ and Aβ− individuals in the aging and AD continuum with [18F]MK6240 and observed a significant correlation (as assessed by Spearman’s correlation analysis) between the SUVR at PET-based Braak stages I-II (r = 0.27, p < 0.001), III-IV (r = 0.31, p < 0.001), and V-VI (r = 0.3, p < 0.001) and Neuropsychiatric Inventory Questionnaire (NPI-Q) global severity scores (43). The different results compared to Yasuno et al. (2021) may be explained by the higher sensitivity of [18F]MK6240 as compared to [18F]AV1451, a possible under- or overestimation of NPS severity by the NPI-Q in relation to the NPI, and by the fact that, in Tissot et al. (2021), Aβ− individuals were also included.
Taking these results together, we have an indication of a relationship between PET-based Braak stages with neuropsychiatric symptoms. The results of these studies are summarized in Supplementary Table 4. We can speculate that the global neuropsychiatric severity is linked to tau-PET signal in Braak ROIs, but the correspondence with specific NPS remains to be elucidated, especially considering that their prevalence varies with the progression of AD (44,45). Besides, the onset of NPS was suggested to precede tau accumulation and to be related to Aβ-PET but not with tau-PET in Aβ− CU older adults (46). Thus, even though NPS were shown to be associated with tau accumulation in the aging and AD continuum, the direction of this association remains to be fully elucidated (43).
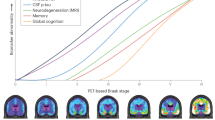
The relationship of different clinical markers of AD with PET-based Braak stages is summarized in Figure 2.
The relationship of different clinical markers of Alzheimer’s disease with PET-based Braak stages
The figure summarizes the findings presented in different studies assessing the clinical correlates of Braak staging assigned with tau positron emission tomography. AD-Alzheimer’s disease; CU - cognitively unimpaired; MCI - mild cognitive impairment; PET - positron emission tomography. Adapted with permission of Macedo et al., 2023 (10)
Limitations
Some limitations in the currently available literature on the issue should be considered. Firstly, most studies do not provide the clinical variants of participants with AD dementia, which is a crucial piece of information for their interpretation, since different AD clinical phenotypes present not only with distinct symptomatology but also with diverse tau propagation profiles (47). Moreover, there is a lack of studies conducted in low, lower-middle, or upper-middle-income countries, hampering the generalizability of the currently available findings. Most of the evidence also comes from studies using [18F] AV1451, a first-generation tau-PET ligand, with other ligands being underrepresented in the literature. For instance, the use of second-generation ligands showing greater sensitivity and less off-target binding is expected to provide more accurate information regarding tau accumulation in earlier Braak stages (25,20–23). Additionally, since the majority of studies focus on global cognition, future studies should investigate further the links with specific cognitive domains (e.g. language or executive function), subtypes of memory (e.g. semantic or spatial memory), neuropsychiatric symptoms (e.g. psychosis, agitation), and performance in activities of daily living. Longer times to follow-up are also needed to have a better picture of the temporal associations of PET-based Braak stages with clinical features of AD.
Conclusions
The PET-based Braak staging framework, a promising biological staging system for AD, seems to correspond well with the clinical severity of the disease. Deterioration in different clinical markers (i.e. cognitive, disease severity, and neuropsychiatric, as well as diagnostic category) show associations with PET-based Braak staging. This is an indication that this framework may be useful in research (e.g. in the selection of participants in clinical trials) and in clinical practice (e.g. as a prognostic tool). However, further research is needed to improve the generalizability of the current findings and to better understand the applicability of this staging framework.
References
McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group* under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–939, doi:https://doi.org/10.1212/WNL.34.7.939.
Williams, D.R. Tauopathies: Classification and Clinical Update on Neurodegenerative Diseases Associated with Microtubule-Associated Protein Tau. Intern. Med. J. 2006, 36, 652–660, doi:https://doi.org/10.1111/j.1445-5994.2006.01153.x.
Jack Jr., C.R.; Bennett, D.A.; Blennow; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 53562, doi:https://doi.org/10.1016/j.jalz.2018.02.018.
Jack, C.R.; Therneau, T.M.; Weigand; et al. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019, 76, 1174, doi:https://doi.org/10.1001/jamaneurol.2019.1971.
Therriault, J.; Pascoal, T.A.; Benedet; et al. Frequency of Biologically-Defined AD in Relation to Age, Sex, APOEε4 and Cognitive Impairment. Neurology 2020, https://doi.org/10.1212/WNL.0000000000011416, doi:https://doi.org/10.1212/WNL.0000000000011416.
Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. (Berl.) 1991, 82, 239–259, doi:https://doi.org/10.1007/BF00308809.
Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer Disease-Associated Neurofibrillary Pathology Using Paraffin Sections and Immunocytochemistry. Acta Neuropathol. (Berl.) 2006, 112, 389–404, doi:https://doi.org/10.1007/s00401-006-0127-z.
Schöll, M.; Lockhart, S.N.; Schonhaut, D.R.; et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 2016, 89, 971–982, doi:https://doi.org/10.1016/j.neuron.2016.01.028.
Murray, M.E.; Graff-Radford, N.R.; Ross, O.A.; Petersen, R.C.; Duara, R.; Dickson, D.W. Neuropathologically Defined Subtypes of Alzheimer’s Disease with Distinct Clinical Characteristics: A Retrospective Study. The Lancet Neurology 2011, 10, 785–796, doi:https://doi.org/10.1016/S1474-4422(11)70156-9.
Macedo, A.C.; Tissot, C.; Therriault, J.; Servaes, S.; Wang, Y.-T.; Fernandez-Arias, J.; Rahmouni, N.; Lussier, F.Z.; Vermeiren, M.; Bezgin, G.; et al. The Use of Tau PET to Stage Alzheimer Disease According to the Braak Staging Framework. J. Nucl. Med. 2023, 64, 1171–1178, doi:https://doi.org/10.2967/jnumed.122.265200.
Dayan, A.D. Quantitative Histological Studies on the Aged Human Brain: I. Senile Plaques and Neurofibrillary Tangles in?Normal? Patients. Acta Neuropathol. (Berl.) 1970, 16, 85–94, doi:https://doi.org/10.1007/BF00687663.
Hubbard, B.M.; Fentonm, G.W.; Anderson, J.M. A Quantitative Histological Study of Early Clinical and Preclinical Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 1990, 16, 111–121, doi:https://doi.org/10.1111/j.1365-2990.1990.tb00940.x.
Kemper TL. Senile Dementia: A Focal Disease in the Temporal Lobe. In; Nandy E, Ed.Senile Dementia: A Biomedical Approach. Elsevier; 1978:105–113.
Wilcock, G.K.; Esiri, M.M. Plaques, Tangles and Dementia. J. Neurol. Sci. 1982, 56, 343–356, doi:https://doi.org/10.1016/0022-510X(82)90155-1.
Lowe, V.J.; Curran, G.; Fang, P.; Liesinger, A.M.; Josephs, K.A.; Parisi, J.E.; Kantarci, K.; Boeve, B.F.; Pandey, M.K.; Bruinsma, T.; et al. An Autoradiographic Evaluation of AV-1451 Tau PET in Dementia. Acta Neuropathol. Commun. 2016, 4, 58, doi:https://doi.org/10.1186/s40478-016-0315-6.
Marquié, M.; Normandin, M.D.; Vanderburg, C.R.; Costantino, I.M.; Bien, E.A.; Rycyna, L.G.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.L.; et al. Validating Novel Tau Positron Emission Tomography Tracer [F-18]-AV-1451 (T807) on Postmortem Brain Tissue: Validation of PET Tracer. Ann. Neurol. 2015, 78, 787–800, doi:https://doi.org/10.1002/ana.24517.
Sander, K.; Lashley, T.; Gami, P.; Gendron, T.; Lythgoe, M.F.; Rohrer, J.D.; Schott, J.M.; Revesz, T.; Fox, N.C.; Årstad, E. Characterization of Tau Positron Emission Tomography Tracer [18 F]AV-1451 Binding to Postmortem Tissue in Alzheimer’s Disease, Primary Tauopathies, and Other Dementias. Alzheimers Dement. 2016, 12, 1116–1124, doi:https://doi.org/10.1016/j.jalz.2016.01.003.
Aguero, C.; Dhaynaut, M.; Normandin, M.D.; Amaral, A.C.; Guehl, N.J.; Neelamegam, R.; Marquie, M.; Johnson, K.A.; El Fakhri, G.; Frosch, M.P.; et al. Autoradiography Validation of Novel Tau PET Tracer [F-18]-MK-6240 on Human Postmortem Brain Tissue. Acta Neuropathol. Commun. 2019, 7, 37, doi:https://doi.org/10.1186/s40478-019-0686-6.
Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging in Neurodegenerative Tauopathies—Still a Challenge. Mol. Psychiatry 2019, 24, 1112–1134, doi:https://doi.org/10.1038/s41380-018-0342-8.
Groot, C.; Villeneuve, S.; Smith, R.; Hansson, O.; Ossenkoppele, R. Tau PET Imaging in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, 20S–26S, doi:https://doi.org/10.2967/jnumed.121.263196.
Wong, D.F.; Comley, R.A.; Kuwabara, H.; Rosenberg, P.B.; Resnick, S.M.; Ostrowitzki, S.; Vozzi, C.; Boess, F.; Oh, E.; Lyketsos, C.G.; et al. Characterization of 3 Novel Tau Radiopharmaceuticals, 11 C-RO-963, 11 C-RO-643, and 18 F-RO-948, in Healthy Controls and in Alzheimer Subjects. J. Nucl. Med. 2018, 59, 1869–1876, doi:https://doi.org/10.2967/jnumed.118.209916.
Kuwabara, H.; Comley, R.A.; Borroni, E.; Honer, M.; Kitmiller, K.; Roberts, J.; Gapasin, L.; Mathur, A.; Klein, G.; Wong, D.F. Evaluation of 18 F-RO-948 PET for Quantitative Assessment of Tau Accumulation in the Human Brain. J. Nucl. Med. 2018, 59, 1877–1884, doi:https://doi.org/10.2967/jnumed.118.214437.
Hostetler, E.D.; Walji, A.M.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Haley, H.; Holahan, M.; et al. Preclinical Characterization of 18 F-MK-6240, a Promising PET Tracer for In Vivo Quantification of Human Neurofibrillary Tangles. J. Nucl. Med. 2016, 57, 1599–1606, doi:https://doi.org/10.2967/jnumed.115.171678.
Pascoal, T.A.; Shin, M.; Kang, M.S.; Chamoun, M.; Chartrand, D.; Mathotaarachchi, S.; Bennacef, I.; Therriault, J.; Ng, K.P.; Hopewell, R.; et al. In Vivo Quantification of Neurofibrillary Tangles with [18F]MK-6240. Alzheimers Res. Ther. 2018, 10, 74, doi:https://doi.org/10.1186/s13195-018-0402-y.
Pascoal, T.A.; Therriault, J.; Benedet, A.L.; Savard, M.; Lussier, F.Z.; Chamoun, M.; Tissot, C.; Qureshi, M.N.I.; Kang, M.S.; Mathotaarachchi, S.; et al. 18F-MK-6240 PET for Early and Late Detection of Neurofibrillary Tangles. Brain 2020, 143, 2818–2830, doi:https://doi.org/10.1093/brain/awaa180.
Schwarz, A.J.; Yu, P.; Miller, B.B.; Shcherbinin, S.; Dickson, J.; Navitsky, M.; Joshi, A.D.; Devous, M.D.; Mintun, M.S. Regional Profiles of the Candidate Tau PET Ligand 18 F-AV-1451 Recapitulate Key Features of Braak Histopathological Stages. Brain 2016, 139, 1539–1550, doi:https://doi.org/10.1093/brain/aww023.
Cho, H.; Choi, J.Y.; Lee, H.S.; Lee, J.H.; Ryu, Y.H.; Lee, M.S.; Jack, C.R.; Lyoo, C.H. Progressive Tau Accumulation in Alzheimer Disease: 2-Year Follow-up Study. J. Nucl. Med. 2019, 60, 1611–1621, doi:https://doi.org/10.2967/jnumed.118.221697.
Maass, A.; Landau, S.; Baker, S.L.; et al. Comparison of Multiple Tau-PET Measures as Biomarkers in Aging and Alzheimer’s Disease. NeuroImage 2017, 157, 448–463, doi:https://doi.org/10.1016/j.neuroimage.2017.05.058.
Nihashi, T.; Sakurai, K.; Kato; et al. Patterns of Distribution of 18F-THK5351 Positron Emission Tomography in Alzheimer’s Disease Continuum. J. Alzheimers Dis. 2022, 85, 223–234, doi:https://doi.org/10.3233/JAD-215024.
Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R.S.; Villemagne, V.L.; Akatsu, H.; Yamamoto, T.; et al. Novel 18 F-Labeled Arylquinoline Derivatives for Noninvasive Imaging of Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2013, 54, 1420–1427, doi:https://doi.org/10.2967/jnumed.112.117341.
Ng, K.P.; Pascoal, T.A.; Mathotaarachchi, S.; Therriault, J.; Kang, M.S.; Shin, M.; Guiot, M.-C.; Guo, Q.; Harada, R.; Comley, R.A.; et al. Monoamine Oxidase B Inhibitor, Selegiline, Reduces 18F-THK5351 Uptake in the Human Brain. Alzheimers Res. Ther. 2017, 9, 25, doi:https://doi.org/10.1186/s13195-017-0253-y.
Harada, R.; Ishiki, A.; Kai, H.; Sato, N.; Furukawa, K.; Furumoto, S.; Tago, T.; Tomita, N.; Watanuki, S.; Hiraoka, K.; et al. Correlations of 18 F-THK5351 PET with Postmortem Burden of Tau and Astrogliosis in Alzheimer Disease. J. Nucl. Med. 2018, 59, 671–674, doi:https://doi.org/10.2967/jnumed.117.197426.
Kreisl, W.C.; Lao, P.J.; Johnson, A.; et al. Patterns of Tau Pathology Identified with 18 F-MK-6240 PET Imaging. Alzheimers Dement. 2022, 18, 272–282, doi:https://doi.org/10.1002/alz.12384.
King-Robson, J.; Wilson, H.; Politis, M. Associations Between Amyloid and Tau Pathology, and Connectome Alterations, in Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 82, 541–560, doi:https://doi.org/10.3233/JAD-201457.
Rullmann, M.; Brendel, M.; Schroeter, M.L..; et al. Multicenter 18F-PI-2620 PET for In Vivo Braak Staging of Tau Pathology in Alzheimer’s Disease. Biomolecules 2022, 12, 458, doi:https://doi.org/10.3390/biom12030458.
Therriault, J.; Pascoal, T.A.; Lussier, F.Z.; et al. Biomarker Modeling of Alzheimer’s Disease Using PET-Based Braak Staging. Nat. Aging 2022, 2, 526–535, doi:https://doi.org/10.1038/s43587-022-00204-0.
Arias, J. F.; Therriault J.; Thomas E.; et al. Verbal Memory Formation across PET-Based Braak Stages of Tau Accumulation in Alzheimer’s Disease. Brain Commun 2023 5, fcad146.
Leuzy, A.; Smith, R.; Ossenkoppele, R.; et al. Diagnostic Performance of RO948 F 18 Tau Positron Emission Tomography in the Differentiation of Alzheimer Disease From Other Neurodegenerative Disorders. JAMA Neurol. 2020, 77, 955, doi:https://doi.org/10.1001/jamaneurol.2020.0989.
Pascoal, T.A.; Benedet, A.L.; Tudorascu, D.L.; et al. Longitudinal 18F-MK-6240 Tau Tangles Accumulation Follows Braak Stages. Brain 2021, 144, 3517–3528, doi:https://doi.org/10.1093/brain/awab248.
Pichet Binette, A.; Vachon-Presseau, É.; Morris, J..; et al. Amyloid and Tau Pathology Associations With Personality Traits, Neuropsychiatric Symptoms, and Cognitive Lifestyle in the Preclinical Phases of Sporadic and Autosomal Dominant Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 776–785, doi:https://doi.org/10.1016/j.biopsych.2020.01.023.
Yasuno, F.; Minami, H.; Hattori, H.; for the Alzheimer’s Disease Neuroimaging Initiative Relationship between Neuropsychiatric Symptoms and Alzheimer’s Disease Pathology: An in Vivo Positron Emission Tomography Study. Int. J. Geriatr. Psychiatry 2021, 36, 598–605, doi:https://doi.org/10.1002/gps.5459.
Aalten, P.; Verhey, F.R.J.; Boziki, M.; et al. Neuropsychiatric Syndromes in Dementia. Dement. Geriatr. Cogn. Disord. 2007, 24, 457–463, doi:https://doi.org/10.1159/000110738.
Tissot, C.; Therriault, J.; Pascoal, T.A.; et al. Association between Regional Tau Pathology and Neuropsychiatric Symptoms in Aging and Dementia Due to Alzheimer’s Disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, doi:https://doi.org/10.1002/trc2.12154.
Lyketsos, C.G.; Steinberg, M.; Tschanz, J.T.; Norton, M.C.; Steffens, D.C.; Breitner, J.C.S. Mental and Behavioral Disturbances in Dementia: Findings From the Cache County Study on Memory in Aging. Am J Psychiatry 2000.
Mega, M.S.; Cummings, J.L.; Fiorello, T.; Gornbein, J. The Spectrum of Behavioral Changes in Alzheimer’s.
Lussier, F.Z.; Pascoal, T.A.; Chamoun, M.; et al. Mild Behavioral Impairment Is Associated with B-amyloid but Not Tau or Neurodegeneration in Cognitively Intact Elderly Individuals. Alzheimers Dement. 2020, 16, 192–199, doi:https://doi.org/10.1002/alz.12007.
Intrinsic Connectivity of the Human Brain Provides Scaffold for Tau Aggregation in Clinical Variants of Alzheimer’s Disease. Sci. Transl. Med. 2022.
Acknowledgement
We thank Macedo et al. (10), as well as the Journal of Nuclear Medicine, for authorizing the adaptation and reproduction of Figure 2.» In this case, we adapted the Figure 1 of the given paper (https://doi.org/10.2967/jnumed.122.265200), which is published under «Immediate Open Access: Creative Commons Attribution 4.0 International License (CC BY) allows users to share and adapt with attribution, excluding materials credited to previous publications.
Funding
Funding: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. This research is supported by the Weston Brain Institute, Canadian Institutes of Health Research (CIHR) (MOP-11-51-31, FRN, 152985, PI:PR-N), the Alzheimer’s Association (NIRG-12- 92090, NIRP-12-259245, PR-N), Fonds de Recherche du Québec - Santé (FRQS; Chercheur Boursier, PR-N and 2020-VICO-279314). P.R-N, SG, and TP are members of the CIHR-CCNA Canadian Consortium of Neurodegeneration in Aging. Canada Foudation for innovation. project 34874. CFI Project 34874 and Colin J. Adair Charitable Foundation.
Author information
Authors and Affiliations
Contributions
Author contributions: Conceptualization and Methodology, ACM and PR-N; Investigation, ACM; Data Curation and Writing - Original Draft Preparation, ACM, DFAD and AOVF; Writing - Review & Editing, ACM, DFAD, AOVF, CT, JT, EA, SS, NR, JFA, Y-TW, AB, ERZ, TAP, SG; Supervision, PR-N.
Corresponding author
Ethics declarations
Conflict of interest: ERZ serves on the scientific advisory board of Next Innovative Therapeutics. SG serves as a scientific advisor for Cerveau and Enigma US. The other authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Macedo, A.C., Durço, D.F.P.A., Tissot, C. et al. Clinical Correlates of the PET-based Braak Staging Framework in Alzheimer’s Disease. J Prev Alzheimers Dis 11, 414–421 (2024). https://doi.org/10.14283/jpad.2024.15
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2024.15