Abstract
Background
Subjective cognitive decline (SCD) as an early pathological manifestation of brain aging has become more prevalent among older adults.
Objectives
We aimed to investigate the associations of subjective cognitive decline (SCD) with the combined risk of cognitive impairment and dementia.
Design
We performed a systematic review and meta-analysis via searching Embase, PubMed and Cochrane electronic databases from January 1 st 1970 to June 4th, 2020.
Setting
Prospective cohort studies
Participants
Healthy individuals were recruited from community, clinics and population.
Measurements
Healthy individuals with SCD were classified into exposure groups, while those without were considered as the reference group. Adjusted relative risks (RR) were estimated in a random-effects model. Both primary and subgroup analyses were conducted.
Results
Of 28,895 identified studies, 21 studies containing 22 cohorts were eligible for inclusion in the meta-analysis. SCD increased the risk of subsequent cognitive disorders (RR=2.12, 95% confidence intervals [CI] =1.75–2.58, I2=87%, P<0.01). To be specific, SCD conferred a 2.29-fold excess risk for cognitive impairment (RR=2.29, 95% CI=1.66–3.17, I2=83%, P<0.01) and a 2.16-fold excess risk for dementia (RR=2.16, 95% CI=1.63–2.86, I2=81%, P<0.01). In subgroup analyses, participants with SCD in the subgroup of 65–75 years old, long-education (>15 years) subgroup and subgroup of clinics showed a higher risk of developing objective cognitive disorders.
Conclusions
SCD is associated with an increased combined risk of cognitive impairment and incident dementia and should be considered a risk factor for objective cognitive disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Longer life expectancy has led to the growth of the older population, and older adults might account for nearly 16% of the world’s population by 2050 (1). Disorders of aging, especially neurodegenerative changes, which eventually result in dementia, has become an increasing concern, in recent years (2). With a lack of curative treatments for cognitive impairment and dementia, many studies have focused on identifying risk factors at the prodromal and preclinical stages of Alzheimer’s disease (AD) (3). As an early pathological manifestation of brain aging, subjective cognitive decline (SCD), has become a research hotspot (4).
An international working group called the Subjective Cognitive Decline Initiative (SCD-I) focusing on advances in related research has been established (5). SCD could be defined as a self-experienced persistent decline in cognitive capacity in comparison with a previously normal status, which is unrelated to an acute event. Moreover, normal age-, sex- and education-adjusted performance on standardized cognitive tests is used to classify mild cognitive impairment (MCI) (6, 7). SCD has several alternative names, including subjective cognitive complaints (SCC) (8, 9), subjective memory decline (SMD) (10) and subjective memory complaints (SMC) (11). A previous systematic analysis provided evidence for the prognostic validity of memory complaints to predict the risk for subsequent dementia and cognitive impairment (12), while it might ignore the baseline cognitive status of included individuals. Besides, healthy controls without memory complaints should be taken into the consideration as the reference group to ensure the preciseness of analysis. Therefore, we conducted this meta-analysis in healthy population with more strict inclusion criteria. Our aim was to explore the association of SCD with the combined risk of cognitive impairment and dementia in longitudinal studies.
Methods
Search Strategy
This meta-analysis was conducted following the guidelines of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) (13) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14). PubMed, Embase and Cochrane databases were searched with the same strategy ‘(subjective memory decline OR concern* OR complaint* OR SCD OR SMC OR SMD OR SCC) AND (risk OR association) AND (dementia OR cogniti* OR alzheimer* OR MCI OR mild cognitive impairment)’ from January 1st, 1970 to June 4, 2020. Conference abstracts and unpublished studies were also reviewed. Additional studies were identified by screening related reviews and reference lists of studies. If full texts were unavailable, we contacted corresponding authors.
Study Selection
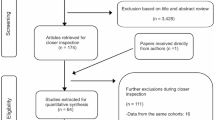
The study selection process was described in Fig.1. There were 28,895 studies from three databases, after deleting duplicates in the EndNote. Studies which met the following criteria were eligible: (1) studies investigating the association of subjective memory complaints with cognitive impairment or dementia (all-cause dementia [ACD] or vascular dementia [VaD] or AD); (2) prospective longitudinal studies with a follow-up of at least 6 months; (3) studies including cognitively normal participants at baseline who were divided into an exposure group with subjective cognitive concerns (assessed by various questionnaires) and a reference group without complaints; (4) studies using recognized diagnostic criteria for objective cognitive performance (including cognitive impairment or dementia) as an end point of the study, such as the criteria made by National Institute on Aging-Alzheimer’s Association (NIA-AA). We did not place language restrictions upon the eligibility criteria of included studies. Randomized clinical trials were excluded, as therapies, psychological suggestions and interventions provided may influence the associations of subjective memory complaints with cognitive impairment and incident dementia. Moreover, people with psychoactive medication use, neurological disease (e.g. Parkinson’s disease, epilepsy, and multiple sclerosis), history of brain lesion (e.g. infection and infarction), head trauma or other systematic diseases of sufficient severity to adversely affect cognition were also excluded. First, after screening the titles and abstracts, we excluded the articles unconcerned with our topic, and only included topic related ones (n=165) for further selection. We then read full texts of those potential eligible articles, searched bibliographies of relevant reviews or meta-analyses, and finally selected 21 articles based on the criteria mentioned above.
Data Extraction
We extracted authors, year of publication, study period, country, language, sample size, inclusion or exclusion criteria, source of participants, age, numbers of male and female individuals, education, follow-up time, methods of diagnosis, count data, unadjusted and adjusted estimates of odds ratio (OR), relative risk (RR), hazard risk (HR) and their 95% confidence intervals (CI) for cognitive impairment or incident dementia. As for we encountered some studies from same cohorts, we chose the study with the largest number of included participants at the baseline. Among effective values reported in the studies, we chose the maximally adjusted estimates. If effective values were not available directly, we used RR calculated by the ratio comparing the of incident rates of cognitive impairment or dementia between exposed and reference groups. Information was first extracted by one investigator, and then checked independently by another two authors. Discrepancies were resolved by discussion. When the data we required were not available in the article, we contacted the corresponding authors for original information.
Quality Assessment
The Newcastle-Ottawa Scale (NOS) has been used to assess the quality of published non-randomized studies in meta-analyses (15, 16). The NOS contains eight items which can be categorized into three dimensions (selection, comparability and outcome). A star system is employed to allow a semi-quantitative assessment of study quality, with a maximum of one star for each item except the comparability item which allows the assignment of two stars (17). The highest quality studies could be awarded a maximum of nine stars.
Statistical Analysis
We mainly analyzed the pooled RR, showing whether individuals with SCD at baseline were more likely than those without to develop cognitive impairment and dementia during follow-up in our study. Given that ORs tend to overestimate the effect sizes compared with RRs/HRs particularly when the incidence is not low, we transformed ORs into RRs using the following algorithm:(18)
RRadjusted = ORadjusted /[(1 − P0) + (P0 × ORadjusted]
P0 indicates the incidence of endpoint (dementia or cognitive decline) in the non-exposed group of the cohort. When P0 is not available, the incidence rate of total sample was used as a proxy.(18) HR, compared with RR, additionally considering the factor of time, might be approximately equal to RR at a point in time. Effective values across studies were combined to provide overall estimates and their 95% CIs using random-effects DerSimonian-Laird models (19). Participants with cognitive disorders were additionally stratified into cognitive impairment and dementia groups. Further subgroup analyses (stratified by different age, sex, year of education, follow-up time and source) were also conducted to investigate whether other factors would change the results using the same models. When calculating RR and 95%CI in subgroup analyses, participants without SCD in each subgroup were considered as the reference group. Each subgroup of basic characteristics might include at least three studies to ensure the reliability of subgroup analyses.
Heterogeneity between studies was assessed by I2 test statistics for each analysis. An I2 of less than 25% is considered as no statistical heterogeneity, 25% to 50% as low statistical heterogeneity, 50% to 75% as medium statistical heterogeneity, and more than 75% as high statistical heterogeneity (20). Meta-regression analyses (n≥10) were also conducted with robust variance estimation, assessing the potentially important covariates that might exert a substantial impact on between-study heterogeneity. Sensitivity analyses were additionally carried out to explore the source of heterogeneity by excluding one study at a time.
We also evaluated the potential publication bias with funnel plots for the outcomes, the symmetry of which was detected by Egger’s test. Egger’s test, also known as linear regression method, uses standard normal deviate and precision of included studies to establish regression equation (21). Moreover, if statistically significant publication bias was detected, the trim-and fill method was used to adjust for bias. A two-tailed P values <0.05 is considered as statistically significant. Statistical analyses were conducted in R (R programming).
Results
Basic characteristics of included studies
A total of 21 studies (22–42) were selected for our metaanalyses (Fig.1). In a study conducted by Snitz et.al (36), both the individuals from communities and clinics were divided into SCD and non-SCD groups. We consider this study as two independent cohorts to include in our meta-analysis. Therefore, 22 cohorts were ultimately included in our meta-analyses. The basic characteristics of included studies are presented in Table 1. A total of 47,805 individuals from the 22 studies were included at baseline. The median of the mean age was 72.70 years old (ranging from 62.8 to 83.31 years old), except one study (25) which did not report its mean age. The female proportion of included studies ranged from 46.46% to 100% and the average years of education was more than 9 years. The average length of follow-up across studies ranged from 2 to 18 years (mean: 5.4; standard deviation [SD]: 3.6), showing an obvious difference among studies, which might contribute to higher heterogeneity. Effective values are also presented in Table 1, ranging from 0.20 to 70.10.
Methods for assessing SCD (eg. “Do you feel like your memory is becoming worse?”) and criteria of diagnosing cognitive impairment or dementia, like NIA-AA, are presented in eTable 1 and eTable 2, respectively (Supplementary materials). Bias assessment based on the Newcastle-Ottawa Scale is provided in Supplementary eTable 3. All the included studies were of high quality, as they all got 7 or more than 7 stars (a maximum of 9 stars) (43).
Results of primary analyses
In the primary analyses, SCD showed an increased risk of developing subsequent cognitive impairment or dementia in Fig.2 (RR=2.12, 95%CI=1.75–2.58, 12=87%, P<0.01). Among the 22 cohorts included in our study, 11 cohorts with cognitive impairment as the outcome showed that 1,481 out of the total 8,346 individuals progressed into cognitive impairment at the last follow-up visit, demonstrating a significant association between SCD and cognitive impairment (RR=2.29, 95%CI=1.66–3.17, I2=83%, P<0.01) (Fig.3). And among the participants with SCD, the risk of developing dementia (RR=2.16, 95%CI=1.63–2.86, I2=81%, P<0.01) was similar to that of developing cognitive impairment (Fig.3). Individuals in four studies (24, 26, 29, 34) progressed to either cognitive impairment or dementia. Moreover, two (24, 34) of the four studies showed separate incidence rates of cognitive impairment and dementia. The other two studies showed incidence rate ratios of mixed cognitive disorders, which made it difficult for us to get the numbers of individuals who progressed to different types of cognitive disorders.
Results of subgroup analyses
For further analysis, all included studies were stratified into subgroups based on their demographic characteristics, including age, female proportion, years of education, follow-up time and source of participants (Supplementary eTable 4). We observed that SCD conferred an excess risk of subsequent cognitive impairment in the individuals aged 65–75 years old (RR=2.29, 95%CI=1.83–2.88, I2=87%, P<0.01) (Supplementary eFig 1). SCD showed similar risks for cognitive disorders in the two subgroups stratified by female proportion (Female>50%: RR=2.18, 95%CI=1.26–3.75, 12=75%, P<0.01; Female≤50%: RR=2.11, 95%CI=1.69–2.64, I2=89%, P<0.01) (Supplementary eFig.2). There was a trend for well-educated individuals (>15 years) to be more strongly influenced by SCD (RR=3.71, 95%CI=2.10–6.56, I2=79%, P<0.01) (Supplementary eFig.3). In the subgroup with longer follow-up, individuals with SCD had a nearly doubled risk of progression to cognitive disorders (cognitive impairment and dementia) compared to those without (RR=1.98, 95%CI=1.61–2.44, I2=89%, P<0.01) (Supplementary eFig.4). In the subgroup of different settings, individuals with SCD showed approximately twice higher risks for cognitive disorders in community (RR=2.08, 95%CI=1.58–2.75, I2=88%, P<0.01) and population (mixed settings) groups (RR=1.93, 95%CI=1.37–2.72, I2=89%, P<0.01), as well as a four times higher risk for cognitive disorders in clinics (RR=4.25, 95%CI=1.08–16.77, I2=85%, P<0.01), compared with those without SCD (Supplementary eFig.5). The influence of SCD on the risks of cognitive disorders in various subgroups were summarized in eFig.6 (Supplementary materials).
When we further divided cognitive disorders into cognitive impairment and dementia, subgroup analyses were also conducted and the results were shown in eTable 5 and eTable 6 (Supplementary materials). In the subgroup analyses of the 11 cohorts focused on cognitive impairment, individuals with SCD had a higher risk of subsequent cognitive impairment in the subgroup of female proportion>50% (RR=2.64, 95%CI=1.61–4.33, I2=87%, P<0.01) (eFig.7) and in the subgroup of > 15 years of education (RR=2.64, 95%CI=1.61–4.33, I2=87%, P<0.01) (eFig.8). Additionally, the influence of SCD on cognitive impairment showed nearly no marked difference between individuals with and without the APOE ε4 allelic gene (APOE ε4+, RR=1.67, 95%CI=1.07-2.61, I2=58%, P=0.07; APOE ε4-, RR=1.89, 95%CI=1.17–3.03, I2=85%, P<0.01) (eFig.9). Individuals with SCD also showed higher risks of cognitive impairment in subgroup of 65–75 years old (RR=2.69, 95%CI=1.79–4.04, I2=83%, P<0.01), subgroup of shorter follow-up (RR=3.49, 95%CI=2.14–5.69, I2=0%, P<0.01) and subgroup of individuals from clinics (RR=8.06, 95%CI=1.68–38.67, I2=66%, P=0.09). Results on the influence of SCD on the progression into cognitive impairment were summarized in eFig.10 (Supplementary materials).
In the subgroup analysis of the cohorts which progressed into dementia, SCD individuals in the subgroup of short follow-up time showed a higher incidence rate of dementia (RR=3.40, 95%CI=1.46–7.89, I2=34%, P=0.22) (eFig.11). Moreover, when we classified dementia into AD and Non-AD groups, SCD showed a significant association with AD (RR=2.39, 95%CI=1.00–5.74, I2=76%, P<0.01), while it had a non-significant association with non-AD dementia (RR=1.37, 95%CI=0.93–2.03, I2=0%, P=0.73) (eFig.12). Results in subgroups of 75–85 years old (RR=1.75, 95%CI=1.43–2.14, I2=0%, P=0.93), female proportion more than 50% (RR=2.10, 95%CI=1.44–3.05, I2=85%, P<0.01) and individuals from clinics (RR=1.77, 95%CI=1.41–2.22, I2=0%, P=0.62) might need further investigation, since they were limited by the numbers of included studies in the subgroups. Results on the influence of SCD on the progression into dementia were summarized in Supplementary eFig.13.
Meta-Regression Analysis, Sensitivity Analysis and Publication Bias
Based on the results of meta-regression analysis (Supplementary eTable 7), the influence of the covariates on heterogeneity, such as participant’s mean age (p=0.163; 95%CI, −0.100238 to 0.0181451; τ2=0.1979), female proportion (p=0.271; 95%CI, −3.221842 to 0.9562178; τ2=0.2041), years of education (p=0.329; 95%CI, −0.068722 to 0.1892374; τ2=0.3314) and length of follow-up (p=0.231; 95%CI, −0.0968754 to 0.0248457; τ2=0.1917) were not statistically significant, as the two-tailed P values were all greater than 0.05, ranging from 0.072 to 0.928.
The sensitivity analysis showed two studies (36, 38) significantly influenced the heterogeneity (Supplementary eFig.14). When each of the studies was excluded separately, the heterogeneities still remained at 84%. The funnel plot showed relatively bilateral symmetry and the p value was 0.4557 (Supplementary eFig.15), indicating no publication bias.
Discussion
SCD was associated with a higher risk of subsequent cognitive disorders, which increased that SCD would possibly elevate the risk. Individuals with SCD showed higher risks of subsequent cognitive impairment and dementia both of which were more than two-fold compared with those without. When data were stratified by their basic characteristics, participants with SCD in subgroup of 65–75 years old, subgroup of female proportion more than 50%, long education subgroup, short follow-up subgroup and subgroup of individuals from clinics had higher risks of objective cognitive disorders. SCD participants showed significant higher risk of developing cognitive impairment compared to non-SCD participants, but there was nearly no marked difference in the rate of progression to cognitive impairment between individuals with/without the APOE ε4 allelic gene in SCD participants. Moreover, SCD participants also showed a significantly higher risk of developing AD dementia rather than non-AD dementia compared with those without SCD.
Biological alternations induced by SCD could occur before objective cognitive decline, such as gray matter volume reduction (44). Individuals with SCD have also been reported to have larger white matter hyperintensity (WMH) volumes, hippocampal atrophy (45) and increased β-amyloid (Aβ) deposition (46, 47), which are typical characteristics of AD. Furthermore, some studies illustrated that SCD was a subjective symptom reflecting anxiety or depression about senility and health rather than neurodegenerative causes (48, 49) and was just a risk factor rather than a mechanism underlying preclinical AD or other neurodegenerative dementias (6), as many participants with SCD might not develop subsequent cognitive impairment or even dementia (50). Hence, SCD was more likely to be a risk factor for cognitive disorders. Previous studies also suggested that cognitively unimpaired individuals with SCD were at a significantly increased risk of future objective cognitive disorders and clinical progression to symptomatic disease stages (12, 36, 51) which was in accordance with our results that SCD conferred excess risks of subsequent cognitive impairment and dementia. Furthermore, individuals with SCD were considered as high-risk individuals and they need necessary interventions during stages at which objective cognitive impairment remains clinically unapparent.(52)
What was more, Wang et.al found that age modified the association between SCD and future cognitive disorders, with HR decreasing from 6.0 at age 70 to 1.6 at age 80 (42). Though previous studies have proven that older elderly are more likely to develop cognitive disorders than younger elderly (12, 35, 53, 54), older elderly may have a casual attitude towards their cognitive conditions. Older elderly are less likely to worry about themselves, so subjective complaints from younger elderly are likely to be more predictive than those from older elderly. Therefore, this might explain our result that the influence of SCD was more obvious in the subgroup of older elderly. In the subgroup analysis by female proportion, individuals with SCD showed a nearly 2.5 times risk of developing cognitive impairment than those without in the subgroup of female proportion more than 50%, which was consistent with the previous conclusion that women were prone to cognitive impairment (27). A previous study reported that education affected the process of memory decline (55). Well-educated people usually seem knowledgeable, and more concerned about their health, suggesting their self-reported of SCD is more accurate. For this possible reason, longer education may contribute to an increased risk of progression from SCD to cognitive disorders, which was in accordance with the results of our subgroup analysis stratified by education including the one of all 22 studies with the outcome of cognitive disorders and the one of the 11 studies with the outcome of cognitive impairment.
Individuals with SCD in the subgroup of follow up>3years showed lower risks of developing cognitive disorders, especially dementia, compared with the subgroup of not more than 3 years, which might be explained by the increased drop-out rate or increased mortality of participants during longer follow-up. Several studies (5, 36) clearly showed that settings might affect the influence of SCD. In our study, SCD showed the strongest association with cognitive disorders in individuals chosen from clinics, as people might be classified explicitly and diagnosed in clinical settings, using available and easily measurable criteria and standard definitions of cognitive impairment or/and dementia. Moreover, previous studies also illustrated that patients in clinics were more likely to experience the first sign or the preclinical stage of a neurodegenerative disease (47, 56). A study found a significant effect of APOEε4 on memory (57). And our result suggested that SCD was a risk factor for cognitive impairment independent of the APOEε4 gene, which was likely to be limited by insufficient samples. Additionally, some individuals with SCD showed gray matter volume reduction (44) and greater similarity to an AD gray matter pattern (58) compared with subjects without SCD, which was consistent with our subgroup analyses.
There was considerable heterogeneity, which might be due to the different characteristics of individuals. Therefore, we conducted specific analyses, such as subgroup analyses based on different characteristics of studies and sensitivity analysis to find out cohorts which were significantly different from others. Apart from basic characteristics, measurements of SCD have also been reported to influence the risk of developing cognitive impairment (51). Cohorts included in our study used different assessments of SCD, which might be one of the factors leading to a bit higher heterogeneity. Recruiting larger samples, comparing important characteristics of participants, unifying the assessment of SCD and searching for methods to lower drop-out rates are necessary in future well-designed longitudinal studies.
The primary strength of our meta-analysis lies in the unity in design of studies (prospective longitudinal studies). The prospective longitudinal study minimized the potential influence of recall and selection bias, which might be inevitable in retrospective design. Besides, our retrieval was comprehensive, since we screened the three databases involving almost all available assays. Also, our search term contained, as more as possible, expressions of the same meaning we focus on (including SCD and dementia), and used “OR” as conjunctions for expressions of the same meanings, which could expand our retrieval range. Furthermore, our studies had independent blind assessments or reliable diagnostic criteria of outcomes (cognitive impairment and dementia), which were reflected in the Newcastle-Ottawa Scale questionnaire (Supplementary eTable 3). Studies included are all of high quality (Newcastle-Ottawa Scale ≥ 6 stars) (43), without having publication bias. Overall, the result that SCD increases the risk of subsequent cognitive impairment and dementia is reliable.
Limitations
There are some limitations in our meta-analysis. First, various questionnaires had different criteria for identifying SCD, which might contribute to a lack of uniformity in diagnosis of SCD. In addition, due to the association of patients and informants, the accuracy of SCD detection could be easily influenced by informants’ expectations of being normal. Second, during the follow-up, as time went on, more and more participants dropped out. Those who are lost to follow-up usually tend to be older, sicker, and have lower socioeconomic status, which might lead to attrition bias. Finally, the reliability of our subgroup analyses might be oppugned owing to our insufficient studies in certain subgroups and the possibility of type I error. Future studies are required to reduce these limitations and make more reliable inferences.
Conclusions
In conclusion, SCD is associated with an increased risk of objective cognitive disorders, including cognitive impairment and incident dementia. Individuals with SCD in subgroup of 65–75 years old, subgroup of female proportion more than 50%, longer education subgroup and subgroup of individuals from clinics showed higher risks of cognitive disorders. SCD deserve more attention, as it could serve as a potential target for early intervention trials in cognitive disorders.
References
Kowal P, Goodkind, D., He, W. An Aging World: 2015, International Population Reports. U.S. Government Printing Office, Washington DC. http://www.census.gov/library/publications/2016/demo/P95-16-1html. 2016.
Oliveira AV, Vilaça R, Santos CN, Costa V, Menezes R. Exploring the power of yeast to model aging and age-related neurodegenerative disorders. Biogerontology. 2017;18(1):3–34.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers & Dementia. 2011;7(3):280–92.
Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers & Dementia. 2014;10(1):76–83.
Jessen F, Amariglio RE, Van BM, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers & Dementia the Journal of the Alzheimers Association. 2014;10(6):844–52.
Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2017;13(3):296–311.
Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. The Lancet Neurology. 2020;19(3):271–8.
Gatchel JR, Marshall GA, Burnham S, Kirn D, Jonas V, Rentz DM, et al. Examining relationships among subjective cognitive concerns and positive and negative afffect in cognitvely normal older adults using aweekly, internet-based method: A pilot study. American Journal of Geriatric Psychiatry. 2018;26(3 Supplement 1):S95–S6.
Lojo-Seoane C, Pereiro AX, Campos-Magdaleno M, Mallo SC, Facal D, Juncos-Rabadan O. RELATIONSHIP BETWEEN SUBJECTIVE COGNITIVE COMPLAINTS (SCCS), DEPRESSION AND COGNITIVE PERFORMANCE. Alzheimer’s and Dementia. 2018;14(7 Supplement):P1187–P8.
Haley AP, Hoth KF, Gunstad J, Paul RH, Jefferson AL, Tate DF, et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiatry. 2009;17(11):976–85.
Miyagawa T, Iwata A. Subjective memory complaints (SMC). Nihon rinsho Japanese journal of clinical medicine. 2016;74(3):451–4.
Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta psychiatrica Scandinavica. 2014;130(6):439–51.
Stroup DF, Berlin JA, Morton SC, Olkin I,., Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. 2008.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. BMJ. 2015;349(1):g7647–g.
Guofeng C, Chen Y, Rong W, Ruiyu L, Kunzheng W. Patients with metabolic syndrome have a greater rate of complications after arthroplasty: A systematic review and meta-analysis. Bone & joint research. 2020;9(3):120–9.
Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, et al.. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5.
Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. Bmj. 2014;1:f7450.
Dersimonian RL, N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj British Medical Journal. 2011;343(7829):889–93.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (London, England). 2018;391(10128):1357–66.
Ávila-Villanueva M, Maestú F, Fernández-Blázquez MA. Internal Consistency Over Time of Subjective Cognitive Decline: Drawing Preclinical Alzheimer’s Disease Trajectories. J Alzheimers Dis. 2018;66(1):173–83.
Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, Kamp GJ, Van, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57(12):2217–22.
Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2014;22(12):1642–51.
Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. The American journal of psychiatry. 1999;156(4):531–7.
Gifford KA, Liu D, Lu Z, Tripodis Y, Cantwell NG, Palmisano J, et al. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimers Dement. 2014;10(3):319–27.
Heser K, Kleineidam L, Wiese B, Oey A, Roehr S, Pabst A, et al. Subjective Cognitive Decline May Be a Stronger Predictor of Incident Dementia in Women than in Men. Journal of Alzheimer’s Disease. 2019.
Howieson DB, Mattek N, Dodge HH, Erten-Lyons D, Zitzelberger T, Kaye JA. Memory Complaints in Older Adults: Prognostic Value and Stability in Reporting over Time. SAGE Open Med. 2015;3.
Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K. Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology. 2015;85(21):1852–8.
Mol ME, van Boxtel MP, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht Aging Study. International journal of geriatric psychiatry. 2006;21(5):432–41.
Muller-Gerards D, Weimar C, Abramowski J, Tebrugge S, Jokisch M, Dragano N, et al. Subjective cognitive decline, APOE epsilon4, and incident mild cognitive impairment in men and women. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring. 2019;1:221–30.
Nunes T, Fragata I, Ribeiro F, Palma T, Maroco J, Cannas J, et al. The outcome of elderly patients with cognitive complaints but normal neuropsychological tests. J Alzheimers Dis. 2010;19(1):137–45.
Qi XM, Gu L, Tang HD, Chen SD, Ma JF. Association of Source of Memory Complaints and Increased Risk of Cognitive Impairment and Cognitive Decline: A Community-Based Study. Chinese medical journal. 2018;131(8):894–8.
Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s and Dementia. 2010;6(1):11–24.
Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2018;15(3):465–76.
Snitz BE, Wang T, Cloonan YK, Jacobsen E, Chang CH, Hughes TF, et al. Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimers Dement. 2018;14(6):734–42.
St John P, Montgomery P. Are cognitively intact seniors with subjective memory loss more likely to develop dementia? International journal of geriatric psychiatry. 2002;17(9):814–20.
Tomata Y, Sugiyama K, Kaiho Y, Sugawara Y, Hozawa A, Tsuji I. Predictive ability of a simple subjective memory complaints scale for incident dementia: Evaluation of Japan’s national checklist, the “Kihon Checklist”. Geriatrics & gerontology international. 2017;17(9):1300–5.
Tsutsumimoto K, Makizako H, Doi T, Hotta R, Nakakubo S, Makino K, et al. Subjective Memory Complaints are Associated with Incident Dementia in Cognitively Intact Older People, but Not in Those with Cognitive Impairment: A 24-Month Prospective Cohort Study. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2017;25(6):607–16.
van Wanrooij LL, Richard E, Jongstra S, Moll van Charante EP, van Gool WA. Associations of Subjective Memory Complaints and Simple Memory Task Scores With Future Dementia in the Primary Care Setting. Annals of family medicine. 2019;17(5):412–8.
Verdelho A, Madureira S, Moleiro C, Santos CO, Ferro JM, Erkinjuntti T, et al. Self-perceived memory complaints predict progression to Alzheimer disease. The LADIS study. J Alzheimers Dis. 2011;27(3):491–8.
Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, et al. Subjective memory deterioration and future dementia in people aged 65 and older. Journal of the American Geriatrics Society. 2004;52(12):2045–51.
Fralick M, Sy E, Hassan A, Burke MJ, Mostofsky E, Karsies T. Association of Concussion With the Risk of Suicide: A Systematic Review and Meta-Analysis. JAMA Neurology. 2018.
Wirth M, Bejanin A, La Joie R, Arenaza-Urquijo EM, Gonneaud J, Landeau B, et al. Regional patterns of gray matter volume, hypometabolism, and beta-amyloid in groups at risk of Alzheimer’s disease. Neurobiology of aging. 2018;1:140–51.
van Rooden S, van den Berg-Huysmans AA, Croll PH, Labadie G, Hayes JM, Viviano R, et al. Subjective Cognitive Decline Is Associated with Greater White Matter Hyperintensity Volume. J Alzheimers Dis. 2018;66(3):1283–94.
Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–6.
Perrotin A, La Joie R, de La Sayette V, Barré L, Mézenge F, Mutlu J, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2017;13(5):550–60.
Hill NL, Mogle J, Wion R, Munoz E, DePasquale N, Yevchak AM, et al. Subjective Cognitive Impairment and Affective Symptoms: A Systematic Review. Gerontologist. 2016;56(6):e109–e27.
Pedro MC, Mercedes M-P, Ramón L-H, Borja MR. Subjective memory complaints in elderly: relationship with health status, multimorbidity, medications, and use of services in a population-based study. International psychogeriatrics. 2016;28(11):1903–16.
Kryscio RJ, Abner EL, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, et al. Self-Reported Memory Complaints: A Comparison of Demented and Unimpaired Outcomes. The journal of prevention of Alzheimer’s disease. 2016;3(1):13–9.
van Harten AC, Mielke MM, Swenson-Dravis DM, Hagen CE, Edwards KK, Roberts RO, et al. Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology. 2018;91(4):e300–e12.
Chao RY, Chen TF, Chang YL. Executive Function Predicts the Validity of Subjective Memory Complaints in Older Adults beyond Demographic, Emotional, and Clinical Factors. The journal of prevention of Alzheimer’s disease. 2021;8(2):161–8.
Cedres N, Machado A, Molina Y, Diaz-Galvan P, Hernández-Cabrera JA, Barroso J, et al. Subjective Cognitive Decline Below and Above the Age of 60: A Multivariate Study on Neuroimaging, Cognitive, Clinical, and Demographic Measures. Journal of Alzheimer’s Disease. 2019.
Hao L, Wang X, Zhang L, Xing Y, Guo Q, Hu X, et al. Prevalence, Risk Factors, and Complaints Screening Tool Exploration of Subjective Cognitive Decline in a Large Cohort of the Chinese Population. Journal of Alzheimers Disease. 2017;10(Suppl 1):1–18.
Reid LM, Maclullich AMJ. Subjective Memory Complaints and Cognitive Impairment in Older People. Dementia & Geriatric Cognitive Disorders. 2006;22(5–6):471–85.
Ruiz CA, Rodríguez-Gómez O, Alegre M, Valero S, Boada M. Impact of Recruitment Methods in Subjective Cognitive Decline. Alzheimers & Dementia the Journal of the Alzheimers Association. 2016;12(7):P437–P8.
Tomoyuki N, Shunichiro S, Bolati K, Nobuto S, Tohru O, Heii A, et al. Age-Related Association between Apolipoprotein E ε4 and Cognitive Function in Japanese Patients with Alzheimer’s Disease. Dementia & Geriatric Cognitive Disorders Extra. 2013;3(1):66–73.
Peter J, Scheef L, Abdulkadir A, Boecker H, Heneka M, Wagner M, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2014;10(1).
Acknowledgements
None.
Funding
Funding: This study was supported by grants from the National Key R&D Program of China (2018YFC1314700), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Author information
Authors and Affiliations
Contributions
Author’s Contributions: JTY, QD and LT conceptualized and designed the study. XTW, ZTW, HYH, YQ, MW, XNS and WX conducted the study. XTW, ZTW, HYH, YQ and MW analyzed and extracted data. XTW, ZTW and JTY wrote the first draft of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Standards: None
Electronic supplementary material
42414_2021_128_MOESM1_ESM.docx
Association of subjective cognitive decline with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective longitudinal studies
Rights and permissions
About this article
Cite this article
Wang, XT., Wang, ZT., Hu, HY. et al. Association of Subjective Cognitive Decline with Risk of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of Prospective Longitudinal Studies. J Prev Alzheimers Dis 8, 277–285 (2021). https://doi.org/10.14283/jpad.2021.27
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2021.27