Abstract
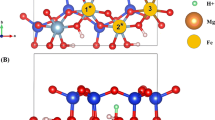
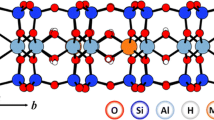
A detailed structural characterization of organo-clays is a key in understanding their properties. In this work, mono-, di-, tri-, and tetra-butylammonium (nBA; n = 1–4) cations intercalated in the layered clay mineral montmorillonite (Mnt) have been studied for the first time by combining a theoretical approach based on density functional theory (DFT) and infrared spectroscopy. The DFT calculations revealed the detailed structure and position of nBA cations in the interlayer space. A relation between the basal spacing (d001 parameter) and the cation size and structure was found, and explained with respect to the structure, composition, and size of the organic cations. Hydrogen bonds between -NHx/-CH3/-CH2 groups of the nBA cations and oxygen atoms of the basal planes of the Mnt layers were found to be an important factor for the arrangement and energetic stabilization of cations in the interlayer space. The N–H…O hydrogen bonds are stronger than C–H…O hydrogen bonds and the stabilization decreases with decreased number of bands. Analysis of DFT-calculated vibrational modes helped in understanding a problematic region of the experimental infrared spectra (4000–3000 cm-1), in which assignment of all vibrational modes unambiguously was not possible because of a significant overlap of broad bands.
Similar content being viewed by others
References
Aggarwal, V., Chien, Y.Y., and Teppen, B.J. (2007) Molecular simulations to estimate thermodynamics for adsorption of polar organic solutes to montmorillonite. European Journal of Soil Science, 58, 945–957.
Balan, E., Lazzeri, M., Delattre, S., Meheut, M., Refson, K., and Winkler, B. (2007) Anharmonicity of inner-OH stretching modes in hydrous phyllosilicates: assessment from first-principles frozen-phonon calculations. Physics and Chemistry of Minerals, 34, 621–625.
Berghout, A., Tunega, D., and Zaoui, A. (2010) Density functional theory (DFT) study of the hydration steps of Na+/Mg2+/Ca2+/Sr2+/Ba2+-exchanged montmorillonites. Clays and Clay Minerals, 58, 174–187.
Bhattacharyya, K.G. and Sen Gupta, S. (2006) Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu(II) from aqueous solution. Separation and Purification Technology, 50, 388–397.
Bhattacharyya, K.G. and Sen Gupta, S. (2009) Calcined tetrabutylammonium kaolinite and montmorillonite and adsorption of Fe(II), Co(II) and Ni(II) from solution. Applied Clay Science, 46, 216–221.
Blochl, P.E. (1994) Projector augmented-wave method. Physical Review B, 50, 17953–17979.
Breen, C., Watson, R., Madejová, J., Komadel, P., and Klapyta, Z. (1997) Acid-activated organoclays: Preparation, characterization and catalytic activity of acid-treated tetraalkylammonium-exchanged smectites. Langmuir, 13, 6473–6479.
Chun, Y., Sheng, G.Y., and Boyd, S.A. (2003) Sorptive characteristics of tetraalkylammonium-exchanged smectite clays. Clays and Clay Minerals, 51, 415–420.
Czimerova, A., Ceklovsky, A., and Bujdak, J. (2009) Interaction of montmorillonite with phenothiazine dyes and pyronin in aqueous dispersions: A visible spectroscopy study. Central European Journal of Chemistry, 7, 343–353.
de Paiva, L.B., Morales, A.R., and Valenzuela Diaz, F.R. (2008) Organoclays: Properties, preparation and applications. Applied Clay Science, 42, 8–24.
Desiraju, G.R. and Steiner, T. (2006) The Weak Hydrogen Bond in Structural Chemistry and Biology 2nd Edition. Oxford University Press, Oxford, UK.
Frost, R.L., Zhou, Q., He, H., and Xi, Y. (2008) An infrared study of adsorption of para-nitrophenol on mono-, di- and tri-alkyl surfactant intercalated organoclays. Spectrochimica Acta Part A — Molecular and Biomolecular Spectroscopy, 69, 239–244.
Fu, Y.-T. and Heinz, H. (2010a) Cleavage energy of alkylammonium-modified montmorillonite and relation to exfoliation in nanocomposites: Influence of cation density, head group structure, and chain length. Chemistry of Materials, 22, 1595–1605.
Fu, Y.-T. and Heinz, H. (2010b) Structure and cleavage energy of surfactant-modified clay minerals: Influence of CEC, head group and chain length. Philosophical Magazine, 90, 2415–2424.
Ghiaci, M., Kalbasi, R.J., and Sedaghat, M.E. (2003) A kinetic study of 2-ethyl-1-hexanol oxidation by dichromate using clay-supported 1-butyl 4-aza-1-azonia bicyclo 2.2.2 octane chloride as the phase-transfer catalyst. Organic Process Research & Development, 7, 936–938.
Guegan, R. (2010) Intercalation of a nonionic surfactant (C10E3) bilayer into a Na-montmorillonite clay. Langmuir, 26, 19175–19180.
Guegan, R. (2013) Self-assembly of a non-ionic surfactant onto a clay mineral for the preparation of hybrid layered materials. Soft Matter, 9, 10913–10920.
Hafner, J. (2003) Vibrational spectroscopy using ab initio density-functional techniques. Journal of Molecular Structure, 651-653, 3–17.
He, H., Frost, R.L., Xi, Y.F., and Zhu, J.X. (2004) Raman spectroscopic study of organo-montmorillonites. Journal of Raman Spectroscopy, 35, 316–323.
He, H., Ma, L., Zhu, J., Frost, R.L., Theng, B.K.G., and Bergaya, F. (2014) Synthesis of organoclays: A critical review and some unresolved issues. Applied Clay Science, 100, 22–28.
Heinz, H., Koerner, H., Anderson, K.L., Vaia, R.A., and Farmer, B.L. (2005) Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chemistry of Materials, 17, 5658–5669.
Heinz, H., Vaia, R.A., Krishnamoorti, R., and Farmer, B.L. (2007) Self-assembly of alkylammonium chains on montmorillonite: Effect of chain length, head group structure, and cation exchange capacity. Chemistry of Materials, 19, 59–68.
Jankovic, L., Kronek, J., Madejova, J., and Hronsky, V. (2015) (9,10-Dihydroxyoctadecyl)ammonium: A structurally unique class of clay intercalable surfactants. European Journal of Inorganic Chemistry, 2841–2850.
Jeffrey, G.A. (1997) An Introduction to Hydrogen Bonding. Oxford University Press: New York.
Klebow, B. and Meleshyn, A. (2012) Monte Carlo Study of the Adsorption and Aggregation of Alkyltrimethylammonium Chloride on the Montmorillonite-Water Interface. Langmuir, 28, 13274–13283.
Kresse, G. and Furthmuller, J. (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B, 54, 11169–11186.
Kresse, G. and Hafner, J. (1993) Ab-initio molecular-dynamics for open-shell transition-metals. Physical Review B, 48, 13115–13118.
Kresse, G. and Joubert, D. (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 59, 1758–1775.
Kukkadapu, R.K. and Boyd, S.A. (1995) Tetramethylphosphonium-smectite and tetramethylammonium-smectite as adsorbents of aromatic and chlorinated hydrocarbons — effect of water on adsorption efficiency. Clays and Clay Minerals, 43, 318–323.
Lagaly, G., Ogawa, M., and Dékány, I. (2006) Chapter 7.3 Clay Mineral Organic Interactions. 1, 309–377.
Lawrence, M.A.M., Kukkadapu, R.K., and Boyd, S.A. (1998) Adsorption of phenol and chlorinated phenols from aqueous solution by tetramethylammonium- and tetramethylphosphonium-exchanged montmorillonite. Applied Clay Science, 13, 13–20.
Lee, J.F., Mortland, M.M., Chiou, C.T., Kile, D.E., and Boyd, S.A. (1990) Adsorption of benzene, toluene, and xylene by 2 tetramethylammonium-smectites having different charge-densities. Clays and Clay Minerals, 38, 113–120.
Madejová, J., Palkova, H., and Komadel, P. (2010) IR spectroscopy of clay minerals and clay nanocomposites. Pp. 22–71 in: Spectroscopic Properties of Inorganic and Organometallic Compounds: Techniques, Materials and Applications, Volume, 41 (J. Yarwood, R. Douthwaite, and S.B. Duckett, editors). The Royal Society of Chemistry, Cambridge, UK.
Madejová, J., Pálková, H., and Jankovič, L’. (2012) Degradation of surfactant-modified montmorillonites in HCl. Materials Chemistry and Physics, 134, 768–776.
Pálková, H., Jankovič, L’., Zimowska, M., and Madejová, J. (2011) Alterations of the surface and morphology of tetraalkyl-ammonium modified montmorillonites upon acid treatment. Journal of Colloid and Interface Science, 363, 213–222.
Palkova, H., Hronsky, V., Jankovič, L’., and Madejová, J. (2013) The effect of acid treatment on the structure and surface acidity of tetraalkylammonium-montmorillonites. Journal of Colloid and Interface Science, 395, 166–175.
Perdew, J.P., Burke, K., and Wang, Y. (1996) Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Physical Review B, 54, 16533–16539.
Raussell-Colom, J.A. and Serratosa, J.M. (1987) Reactions of clays with organic substances. Pp. 371–422 in: Chemistry of Clays and Clay Minerals (A.C.D. Newman, editor). Longman Scientific and Technical, Essex, UK.
Ruiz-Hitzky, E. and Van Meerbeek, A. (2006) Clay mineral- and organoclay-polymer nanocomposite. Pp. 583–621 in: Handbook of Clay Science, 1 (F. Bergaya, B.K.G. Theng, and G. Lagaly, editors). Developments in Clay Science. Elsevier.
Sen Gupta, S. and Bhattacharyya, K.G. (2005) Interaction of metal ions with clays: I. A case study with Pb(II). Applied Clay Science, 30, 199–208.
Sen Gupta, S. and Bhattacharyya, K.G. (2006) Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium derivatives. Journal of Hazardous Materials, 128, 247–257.
Seyidoğlu, T. and Yilmazer, U. (2013) Modification and characterization of bentonite with quaternary ammonium and phosphonium salts and its use in polypropylene nanocomposites. Journal of Thermoplastic Composite Materials, 28, 86–110.
Scholtzova, E. and Smrcok, L. (2009) Hydrogen bonding and vibrational spectra in kaolinite-dimethylsulfoxide and -dimethylselenoxide intercalates — a solid-state computational study. Clays and Clay Minerals, 57, 54–71.
Scholtzova, E., Benco, L., and Tunega, D. (2008) A model study of dickite intercalated with formamide and N-methylformamide. Physics and Chemistry of Minerals, 35, 299–309.
Scholtzová, E., Tunega, D., Madejová, J., Pálková, H., and Komadel, P. (2013) Theoretical and experimental study of montmorillonite intercalated with tetramethylammonium cation. Vibrational Spectroscopy, 66, 123–131.
Scholtzová, E., Madejová, J., and Tunega, D. (2014) Structural properties of montmorillonite intercalated with tetraalkylammonium cations — Computational and experimental study. Vibrational Spectroscopy, 74, 120–126.
Steiner, T. (2002) The hydrogen bond in the solid state. Angewandte Chemie (International ed. in English), 41, 49–76.
Stevens, J.J. and Anderson, S.J. (1996) Orientation of trimethylphenylammonium (TMPA) on Wyoming montmorillonite: Implications for sorption of aromatic compounds. Clays and Clay Minerals, 44, 132–141.
Sun, L.L., Tanskanen, J.T., Hirvi, J.T., Kasa, S., Schatz, T., and Pakkanen, T.A. (2015) Molecular dynamics study of montmorillonite crystalline swelling: Roles of interlayer cation species and water content. Chemical Physics, 455, 23–31.
Szczerba, M., Klapyta, Z., and Kalinichev, A. (2014) Ethylene glycol intercalation in smectites. Molecular dynamics simulation studies. Applied Clay Science, 91-92, 87–97.
Teppen, B.J., Yu, C.H., Miller, D.M., and Schafer, L. (1998) Molecular dynamics simulations of sorption of organic compounds at the clay mineral aqueous solution interface. Journal of Computational Chemistry, 19, 144–153.
Theng, B.K.G. (1974) The Chemistry of Clay-Organic Reactions. Adam Hilger, London.
Tributh, H. and Lagaly, G. (1986) Aufbereitung und Identifizierung von Boden- und Lagerstättentonen. GIT Labor-Fachzeitschrift, 30, 524–529.
Tsipurski, S.I. and Drits, V.A. (1984) The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites studied by oblique-texture electron diffraction. Clay Minerals, 19, 177–193.
Vaia, R.A., Teukolsky, R.K., and Giannelis, E.P. (1994) Interlayer structure and molecular environment of alkylammonium layered silicates. Chemistry of Materials, 6, 1017–1022.
Wibowo, T.Y., Abdullah, A.Z., and Zakaria, R. (2010) Organomontmorillonites as catalysts for selective synthesis of glycerol monolaurate. Applied Clay Science, 50, 280–281.
Yariv, S. (2001) IR spectroscopy and Thermo-IR spectroscopy in the study of the fine structure of organo-clay complexes. Pp. 345–462 in: Organo-Clay Complexes and Interactions. (S. Yariv and H. Cross). Marcel Dekker, Inc, New York.
Zhu, J.X., He, H.P., Zhu, L.Z., Wen, X.Y., and Deng, F. (2005) Characterization of organic phases in the interlayer of montmorillonite using FTIR and C-13 NMR. Journal of Colloid and Interface Science, 286, 239–244.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholtzová, E., Madejová, J., Jankovič, L. et al. Structural and Spectroscopic Characterization of Montmorillonite Intercalated with N-Butylammonium Cations (N = 1-4) — Modeling and Experimental Study. Clays Clay Miner. 64, 401–412 (2016). https://doi.org/10.1346/CCMN.2016.0640404
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2016.0640404