Abstract
Background
High-risk programs provide recommendations for surveillance/risk reduction for women at elevated risk for breast cancer development. This study evaluated the impact of high-risk surveillance program participation on clinicopathologic breast cancer features at the time of diagnosis.
Methods
Women followed in the authors’ high-risk program (high-risk cohort [HRC]) with a diagnosis of breast cancer from January 2015 to June 2021 were identified and compared with the general population of women undergoing breast cancer surgery at Memorial Sloan Kettering Cancer Center (MSK; general cohort [GC]) during the same period. Patient and tumor factors were collected. Clinicopathologic features were compared between the two cohorts and in a subset of women with a family history of known BRCA mutation.
Results
The study compared 255 women in the HRC with 9342 women in the GC. The HRC patients were slightly older and more likely to be white and have family history than the GC patients. The HRC patients also were more likely to present with DCIS (41 % vs 23 %; p < 0.001), to have smaller invasive tumors (pT1: 100 % vs 77 %; p < 0.001), and to be pN0 (95 % vs 81 %; p < 0.001). The HRC patients had more invasive triple-negative tumors (p = 0.01) and underwent less axillary surgery (p < 0.001), systemic therapy (p < 0.001), and radiotherapy (p = 0.002). Among those with a known BRCA mutation, significantly more women in the HRC underwent screening mammography (75 % vs 40 %; p < 0.001) or magnetic resonance imaging (MRI: 82 % vs 9.9 %; p < 0.001) in the 12 months before diagnosis.
Conclusions
Women followed in a high-risk screening program have disease diagnosed at an earlier stage and therefore require less-intensive breast cancer treatment than women presenting to a cancer center at the time of diagnosis. Identification of high-risk women and implementation of increased surveillance protocols are vital to improving outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Breast cancer remains the most common non-skin cancer diagnosis among women in the United States, with more than 280,000 new cases of invasive and 50,000 cases of non-invasive breast cancer diagnosed annually.1 Unfortunately, more than 40,000 women still die of this disease each year.2
Given the prevalence of this disease, appropriate risk assessment, education on risk reduction, screening, and early detection are vital components of women’s health. Although an average-risk woman in the United States has a 12 % lifetime risk for the development of breast cancer, several factors modify this baseline risk profile, including hereditary breast cancer syndromes, family history, personal history of high-risk breast lesions, prior chest wall radiation at a young age, endogenous hormone exposure, breast density, and lifestyle factors.3,4,5,6,7,8,9 Increased attention is being paid to identification of these risk factors among unaffected women to allow tailored recommendations for screening and risk reduction, to reduce cancer incidence and increase rates of early detection.
The number of women in the United States at increased risk for breast cancer who could benefit from appropriate counseling and screening is high. For example, it is estimated that there are nearly 1 million unaffected, unidentified BRCA mutation carriers in the United States, and that 13 % of women between 30 and 50 years of age are at elevated risk secondary to a family history of breast cancer. This equates to more than 5 million women in the United States.10
High-risk screening with both mammogram and magnetic resonance imaging (MRI) is recommended for women with a genetic predisposition or strong family history of breast cancer given the ability of MRI to detect earlier-stage disease and reduce interval cancer development in the highest-risk cohorts.11 However, despite strong evidence, appropriate high-risk screening is dramatically underutilized in community and primary care settings.12,13 High-risk programs aim to address this issue by providing personalized risk assessment and education, implementing standard-of-care screening, counseling on risk-reducing options, and offering participation in research for the high-risk community.
The Memorial Sloan Kettering Cancer Center (MSK) (New York, NY, USA) high-risk program, known as the RISE (Risk Assessment, Imaging, Surveillance and Education) program, provides more than 5000 annual high-risk visits for women with a family history of breast cancer, known genetic predisposition, personal history of breast atypia or lobular carcinoma in situ (LCIS), and history of chest-wall radiation before the age of 30 years. During their visits, women are provided with education on breast cancer risk, undergo routine exams, are scheduled for appropriate breast screening, and are counseled on options for risk reduction, including chemoprevention, surgery when appropriate, and lifestyle modifications.
This study assesses the impact of participation in a high-risk breast cancer surveillance program on the stage and clinicopathologic features of breast cancers at the time of diagnosis by comparing tumor and treatment factors among women followed in our high-risk program with those of women who presented to MSK for care at the time of breast cancer diagnosis.
Methods
Upon MSK institutional review board approval, women followed in the high-risk RISE program from January 2015 to June 2021 with a subsequent breast cancer diagnosis (high-risk cohort) were identified from our institutional RISE program database. Risk factors, including family history of breast cancer, history of breast atypia, history of LCIS, presence of a BRCA deleterious mutation or other breast cancer genetic syndrome, and receipt of prior chest wall radiation therapy were collected. As a comparison group, patients treated for non-metastatic breast cancer at MSK (general cohort) who presented to the center at the time of diagnosis during the same time frame were identified from an institutional database, and data on family history of breast cancer and BRCA mutation status were abstracted.
Patient, tumor, and treatment factors were obtained for the women in both cohorts, including age at diagnosis, race, body mass index (BMI), tumor histology, T stage, N stage, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, nuclear grade, receipt of breast and axillary surgery, receipt of systemic therapy, and receipt of adjuvant radiation therapy. Details on screening mammography and screening MRI were obtained for the subset of patients with a known BRCA deleterious mutation in both the high-risk and general cohorts.
Comparisons of clinicopathologic features were performed among the entire high-risk and general cohorts, as well as among the subset of patients with a family history of breast cancer and those with a deleterious BRCA mutation. These analyses excluded 5 women in the high-risk cohort and 68 women in the general cohort with stage IV disease diagnosed at presentation.
Demographic and clinicopathologic characteristics were compared between the high-risk and general cohorts using Fisher’s exact test or the chi-square test for categorical variables, and the t test or Wilcoxon rank-sum test for continuous variables. Additionally, separate subgroup analyses were performed for individuals with a family history of breast cancer or BRCA mutations. A subset analysis was performed to assess differences in pT stage among those with invasive disease only, excluding patients treated with neoadjuvant systemic therapy given the impact of neoadjuvant therapy on pathologic T stage.
All statistical analyses were performed using R 4.2.14 Type 1 error rates for all comparisons were set to an alpha of 0.05.
Results
RISE Population
Of 3542 women followed in the high-risk MSK RISE program between January 2015 and June 2021, 255 (7.2 %) experienced breast cancer. Clinical features and risk factors for this group are outlined in Table 1. The median age at cancer diagnosis was 57 years.
Most of the women (81 %) had a family history of breast cancer, whereas 36 % had a personal history of breast atypia, 3 % had a history of LCIS, 28 % had a known BRCA1 or BRCA2 deleterious mutation, and nearly half (49 %) of the patients who experienced breast cancer had a combination of risk factors present. Of the 255 women in this cohort, 9 (4 %) had a history of chemoprevention use before the diagnosis of breast cancer.
Comparison of MSK RISE Cohort Versus MSK General Cohort
The MSK general cancer cohort during this same period included 9342 women with a median age at diagnosis of 55 years. The women in the high-risk (MSK RISE) cohort were more likely to be white (88 % vs 77 %; p < 0.001) and to have a family history of breast cancer (81 % vs 54 %; p < 0.001) than the MSK general cohort. The patients in the high-risk cohort were more likely to present with ductal carcinoma in situ (DCIS) than with invasive cancer (41 % vs 23 %; p < 0.001) and more likely to be node-negative than the patients in the general cohort (95 % vs 81 %; p < 0.001).
Among the patients with an invasive breast cancer, those in the high-risk cohort had smaller diagnosed tumors than those in the general cohort (pT1: 100 % vs 71 %; p < 0.001). A similar result was found in the comparison of patients with invasive disease, excluding those who had received NAC (pT1 in the MSK RISE cohort: 100 % vs 77 %; p < 0.001). In addition, the patients followed in the high-risk program with invasive cancer were more likely to have triple-negative disease (17 % vs 10 %; p = 0.01) and well-differentiated tumors (24 % vs 14 %; p = 0.002) while less likely to have HER2-overexpressing tumors (16 % vs 4 %; p < 0.001).
From a treatment standpoint, 63 % of the women in both cohorts underwent lumpectomy. However, the rates of bilateral mastectomy were higher in the high-risk population (25 % vs 7 %; p < 0.001). Fewer women in the high-risk cohort required axillary lymph node dissection (ALND) (2 % vs 12 %; p < 0.001), chemotherapy (18 % vs 39 %; p < 0.001), or any form of systemic therapy or radiation, including postmastectomy radiation therapy (all p < 0.05; Table 2).
Comparison of Women with a Family History of Breast Cancer
In the comparison of the 205 women from the high-risk cohort and the 3654 women from the general cohort with a family history of breast cancer, similar results were observed (Table 3). The patients followed in the high-risk program with a family history of breast cancer were slightly older at diagnosis than those in the general cohort (57 vs 54 years; p = 0.01) and more likely to be white (90 % vs 82 %; p = 0.02). The women with a family history of breast cancer in the high-risk cohort were more likely to present with DCIS than those with an invasive histology (38 % vs 15 %; p < 0.001) and significantly more likely to be pN0 (95 % vs 79 %; p < 0.001) than those in the general cohort. Similarly, among the women with invasive breast cancer, the patients in the high-risk cohort were more likely to have pT1 than those with pT2-4 tumors (100 % vs 71 %; p < 0.001), well-differentiated tumors (25 % vs 13 %; p < 0.001), or HER2-negative tumors (95 % vs 84 %; p < 0.001). Among the women with a family history of breast cancer, those followed in the high-risk program had higher rates of bilateral mastectomy and were less likely to undergo ALND, chemotherapy, any systemic therapy, or radiation therapy (all p < 0.001).
Comparison of Women with BRCA Mutations
The high-risk cohort had 71 women with a known deleterious BRCA mutation compared with 232 women in the general cohort. In both groups, slightly more than half of the women with a deleterious BRCA mutation had a BRCA1 mutation (54 % and 53 % in the high-risk and general cohorts, respectively). A comparison of these cohorts is shown in Table 4, with similar patterns identified. The women with a BRCA mutation in the high-risk cohort presented at a slightly older age than those in the general cohort (49 vs 45 years; p = 0.008) and were more likely to present with DCIS (31 % vs 8.6 %; p < 0.001). Among the women with invasive tumors, those in the RISE high-risk cohort had smaller invasive tumors (pT1 100 % vs 64 %; p < 0.001). There were no differences in invasive tumor receptor profile, with 45 % and 42 % of the patients in the high-risk and general cohorts, respectively, having triple-negative disease.
The women followed in the high-risk program had higher rates of bilateral mastectomy (65 % vs 13 %; p < 0.001) and were less likely to receive chemotherapy, endocrine therapy, or postmastectomy radiation therapy (all p < 0.001). Compared with the general cohort, significantly more women in the high-risk cohort with a BRCA mutation had undergone a screening mammogram (75 % vs 40 %; p < 0.001) or a screening MRI (82 % vs 9.9 %; p < 0.001) in the 12 months before their cancer diagnosis.
Discussion
This comparison of cancer and treatment characteristics between women followed in a high-risk program and those who presented to a cancer center at the time of diagnosis showed that among a population of breast cancer patients, those followed in the high-risk program were more likely to present with earlier-stage disease, including higher rates of DCIS, pT1 tumors, and node-negative disease. Despite presenting with more triple-negative tumors, the women followed in the high-risk program had earlier-stage disease diagnosed, and therefore were less likely to require ALND, systemic therapy, or radiation therapy—all of which are treatment components that may confer potential significant toxicity.
Among the cohort of women with a known BRCA mutation for whom imaging details were available, a significant difference in the use of both screening mammography and MRI was noted in the 12 months before diagnosis for the women in the high-risk cohort versus those in the general cohort. Receipt of increased surveillance in the RISE cohort appears to be associated with identification of earlier-stage disease, including more DCIS and node-negative tumors at diagnosis.
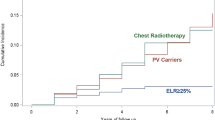
Women with a deleterious BRCA germline mutation have a significantly greater risk of breast cancer development than the general population, with a cumulative lifetime risk of approximately 70 %.15 Given the substantial breast cancer risk in this population, enhanced screening is important to improve early detection.
Multiple studies have reported improved sensitivity for early disease detection with MRI versus mammography among women with a genetic predisposition or strong family history of breast cancer.16 Warner et al.17 performed a prospective surveillance study with and without breast MRI among 1275 women with a deleterious BRCA mutation. The women screened with MRI had a higher incidence of DCIS or stage I breast cancer and a lower incidence of stages II to IV disease than the non-MRI group. The adjusted hazard ratio for the diagnosis of advanced-stage (II–IV) disease associated with MRI screening was 0.3 (95 % confidence interval [CI], 0.12–0.72; p = 0.008). The guidelines from the American Cancer Society and American College of Radiology recommend breast MRI screening as an adjunct to mammographic screening for women with a BRCA mutation or other high-risk breast cancer genetic syndrome, those with a lifetime risk of breast cancer greater than 20 % as defined by a risk model largely dependent on family history, and those who received radiation to the chest between the ages of 10 and 30 years.16
Despite the known benefits of high-risk screening among select high-risk populations, many women in the community are not undergoing appropriate MRI screening. In a survey of primary care providers, a minority (31 %) correctly defined women at high risk for breast cancer, and only 12 % recommended screening MRI for high-risk women.12 A cross-sectional study by Miles et al.13 of more than 422,000 women undergoing screening mammography assessed availability and utilization of MRI screening among high-risk women, defined as those with more than a 20 % lifetime risk. The authors reported that 44 % of the high-risk women underwent screening at a facility with on-site MRI availability, but that only 6.6 % of the high-risk women obtained a screening breast MRI within a 2-year window from their screening mammogram.
Notably, many of the women in the general cohort with a BRCA mutation likely did not undergo genetic testing until the time of breast cancer diagnosis, and therefore may not have been recommended to undergo high-risk screening before diagnosis. Identification of women appropriate for genetic testing when unaffected can affect outcomes if high-risk screening and prevention recommendations are implemented for those found to have deleterious mutations. A study by our group reported that 65 % of women presenting to the RISE high-risk program qualified for genetic evaluation, although 40 % had not undergone prior genetic testing, highlighting another important element of a high-risk program.
Studies report that understanding one’s breast cancer risk is associated with adherence to appropriate screening. Young et al.18 reported on perceived cancer risk among both black and white women with elevated breast cancer risk and found that women who overestimated or accurately estimated risk were more likely to utilize MRI screening. Education on risk as well as on appropriate breast imaging recommendations among high-risk women is an essential component of a high-risk program given the impact of risk estimation on adherence to screening recommendations.18,19
The ultimate goals of a high-risk program are early detection and prevention. Women within the RISE high-risk program are counseled on the options for risk reduction, including chemoprevention, surgical risk reduction, and lifestyle modifications. In this report, we describe earlier detection of cancers among women followed in a dedicated high-risk program compared with the cohort of women presenting to a cancer center at the time of diagnosis. This finding is likely multifactorial, related to improved adherence to high-risk screening recommendations, regular clinical follow-up, and education on risk-reducing strategies. These data support the implementation of high-risk programs or other mechanisms to ensure primary care provider awareness to improve identification of unaffected gene carriers, provide individualized risk assessment, and counsel women on appropriate high-risk screening recommendations, which will in turn have an impact on clinical outcomes among women at increased risk for breast cancer development. Given the racial differences in the cohorts, it also is imperative that these initiatives target individuals of all racial and ethnic backgrounds equally to minimize disparities in care that may affect subsequent breast cancer outcomes.
The strengths of our study include its robust clinicopathologic information on patients in both its high-risk and general cohorts, with data on risk factors including both family history and BRCA status, which allowed for a direct comparison between these groups.
The limitations of our study include those inherent to a retrospective study. It is possible that the MSK general cohort contained cancers perceived to be higher risk due to referral bias, falsely elevating the benefit of the high-risk screening program. Conversely, some women in the MSK general cohort may have been followed in a high-risk program outside the RISE program and then transferred to MSK for cancer care. Furthermore, 40 % of the women in the RISE high-risk cohort had a personal history of a high-risk breast lesion, including atypia or LCIS, and, unfortunately, a history of atypical hyperplasia or LCIS was not available from the general MSK population for comparison. Atypical hyperplasia and LCIS are associated with an increase in breast cancer development, with an absolute risk of approximately 1% to 12 % per year, respectively.6,20
The current guidelines do not support the routine use of MRI screening among patients with high-risk breast lesions,16 although controversy exists regarding appropriate screening for patients with a personal history of high-risk lesions. There is ongoing effort to identify the optimal screening for women who have these benign breast lesions, with data evaluating the role of contrast-enhanced spectral mammography (CESM) emerging.21 The current imaging algorithm in the RISE program includes annual mammogram and MRI screening for individuals who meet standard criteria for MRI screening, including known moderate- or high-risk pathogenic variant, lifetime risk greater than 20 % secondary to family history, and prior chest wall radiation before the age of 30 years.
For individuals with moderate risk secondary to high-risk breast lesions, including atypical hyperplasia or lobular carcinoma in situ, we recommend annual mammography with consideration of contrast-enhanced spectral mammography, an imaging method not universally available. Routine use of MRI in this population is reserved for those with additional risk factors present that would warrant increased screening, such as a strong family history of breast cancer.
Importantly, our study population timeframe encompassed 2016 through June 2021—the last 1.5 years of which overlapped with the COVID-19 pandemic, which likely had an impact on breast screening availability and adherence. In addition, a higher proportion of the women followed in the RISE program were white compared with the MSK general cohort. Racial and ethnic differences in the cohorts also could have contributed to the differences observed in the stage and biology of the breast cancers identified.
Conclusions
Women followed in a high-risk program are more likely to undergo guideline-concordant high-risk screening, to have earlier-stage disease diagnosed, and to require less-intensive treatment for breast cancer than women presenting to a cancer center at the time of diagnosis. Women with a history of a breast cancer genetic syndrome, a strong family history of breast cancer, a personal history of a high-risk breast lesion, or a history of chest wall radiation at a young age benefit from evaluation and follow-up evaluation in a high-risk screening program. More work is needed to identify and correct disparities in access to high-risk screening programs, and to ensure that all individuals at elevated risk are able to benefit from these strategies.
References
American Cancer Society. Cancer Facts and Figures 2022. Atlanta: American Cancer Society; 2022.
U.S. Cancer Statistics Working Group. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999–2018). U.S. Department of Health and Human Services Centers for Disease Control and Prevention and National Cancer Institute. www.cdc.gov/cancer/dataviz. Accessed 17 June 2024.
Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity, and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–89.
Easton DF, Pharoah PDP, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast cancer risk. N Engl J Med. 2015;372:2243–57.
Thorat MA, Balasubramanian R. Breast cancer prevention in high-risk women. Best Pract Res Clin Obstet Gynaecol. 2020;65:18–31.
King TA, Pilewskie M, Muhsen S, et al. Lobular carcinoma in situ: a 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol. 2015;33:3945–52.
Menes TS, Kerlikowske K, Lange J, Jaffer S, Rosenberg R, Miglioretti DL. Subsequent breast cancer risk following diagnosis of atypical ductal hyperplasia on needle biopsy. JAMA Oncol. 2017;3:36–41.
Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–75.
Sankila R, Garwicz S, Olsen JH, et al. Risk of subsequent malignant neoplasms among 1641 Hodgkin’s disease patients diagnosed in childhood and adolescence: a population-based cohort study in the five Nordic countries. Association of the Nordic Cancer Registries and the Nordic Society of Pediatric Hematology and Oncology. J Clin Oncol. 1996;14:1442–6.
Drohan B, Roche CA, Cusack JC, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19:1732–7.
Pilewskie M, Zabor EC, Gilbert E, et al. Differences between screen-detected and interval breast cancers among BRCA mutation carriers. Breast Cancer Res Treat. 2019;175:141–8.
Amornsiripanitch N, Ameri SM, Goldberg RJ. Primary care providers underutilize breast screening MRI for high-risk women. Curr Prob Diagnostic Radiol. 2021;50:489–94.
Miles R, Wan F, Onega TL, et al. Underutilization of supplemental magnetic resonance imaging screening among patients at high breast cancer risk. J Women’s Health. 2018;27:748–54.
Hornik K, the R Core Team. The R FAQ. 2024. https://CRAN.R-project.org/doc/manuals/R-FAQ.html.
Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16.
Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89.
Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–25.
Young JML, Postula KJV, Duquette D, Gutierrez-Kapheim M, Pan V, Katapodi MC. Accuracy of perceived breast cancer risk in black and white women with an elevated risk. Ethn Dis. 2022;32:81–90.
Katapodi MC, Dodd MJ, Lee KA, Facione NC. Underestimation of breast cancer risk: influence on screening behavior. Oncol Nurs Forum. 2009;36:306–14.
Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast: risk assessment and management options. N Engl J Med. 2015;372:78–89.
Hogan MP, Amir T, Sevilimedu V, Sung J, Morris EA, Jochelson MS. Contrast-enhanced digital mammography screening for intermediate-risk women with a history of lobular neoplasia. AJR Am J Roentgenol. 2021;216:1486–91.
Acknowledgments
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant P30CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pilewskie, M., Eroglu, I., Sevilimedu, V. et al. Participation in a High-Risk Program Is Associated with a Diagnosis of Earlier-Stage Disease Among Women at Increased Risk for Breast Cancer Development. Ann Surg Oncol 31, 6764–6773 (2024). https://doi.org/10.1245/s10434-024-15633-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15633-x