Abstract
Purpose
For operable triple-negative breast cancer (TNBC) treated with neoadjuvant chemotherapy (NAC), clinical prognostication and postoperative decision-making relies exclusively on whether a pathologic complete response (pCR) is achieved or not. We evaluated whether extent of disease at presentation further influenced overall survival (OS) among patients with pCR or with residual disease (RD) following NAC.
Methods
Patients with stage I–III TNBC who underwent NAC were identified from the National Cancer Database from 2010 to 2019. Overall survival was assessed by disease extent using the Kaplan–Meier method and Cox proportional hazards regression for univariate and multivariable analysis.
Results
A total of 35,598 patients met inclusion criteria, and 11,967 achieved pCR. Ten-year OS was 88.5% and varied by cT and cN category at presentation. Best 10-year OS was seen in patients with cT1-2, cN0 (90.9%) and was worst in those with cT3-4, cN2-3 disease (72.0%).
A total of 23,631 patients had RD. Ten-year OS was 60.1% and varied by cT and cN category at presentation. Best 10-year OS was seen in patients with cT1-2, cN0 (73.0%) and was worst in those with cT3-4, cN2-3 disease (36.3%).
Notably, OS was significantly poorer for patients with cT3-4, cN2-3 disease at diagnosis and pCR versus those with cT1-2 cN0 and RD (aHR 1.30, 95% confidence interval 1.03–1.63, p = 0.03).
Conclusions
Among patients with TNBC, extent of disease at presentation was prognostic for OS independently of response to NAC. Patients with advanced stage at presentation had poorer OS even in the context of pCR. Further investigation is needed to evaluate whether additional adjuvant therapy strategies should be considered for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Breast cancer remains the most commonly diagnosed malignancy in women outside of non-melanoma skin cancers.1 For 2024, the American Cancer Society estimates 310,720 new cases of female breast cancer with an estimated 42,250 deaths.2 The treatment of breast cancer is generally multidisciplinary in nature with the use of multiple modalities of treatment, which can include surgery, chemotherapy, radiation, and endocrine therapy, all of which impact outcomes.3 Treatment recommendations are based on the stage of disease at presentation and the receptor status of the cancer. Breast cancers that lack the expression of the estrogen receptor (ER), progesterone receptor (PR), and do not over-express the human epidermal growth factor receptor 2 (HER2) are considered triple-negative breast cancers (TNBC). Triple-negative breast cancers represent approximately 15% of diagnosed breast cancers and tend to be more aggressive than hormone-responsive breast cancers with higher risks of early recurrence and poorer overall survival.4,5,6
Over the past decade, the standard treatment of TNBC has shifted from upfront surgery to increased use of neoadjuvant chemotherapy (NAC) followed by operation, especially in patients with larger tumors or node-positive disease.7 Complete eradication of disease following NAC, resulting in no residual invasive disease in the breast or in the lymph nodes, is considered a pathologic complete response (pCR). A pCR has been correlated with a decreased risk of recurrence and better survival than residual disease (RD).8,9 This has been demonstrated specifically in TNBC with patients who achieve a pCR.10 The 2021 American Society of Clinical Oncology (ASCO) guidelines recommend NAC for TNBC in patients who are clinically node-positive and/or have T1c disease or larger. Neoadjuvant chemotherapy also was recommended for patients in whom the finding of RD would guide recommendations for adjuvant therapy.11 The benefits of NAC include de-escalation of surgical intervention with a higher rate of breast conservation and a lower rate of axillary lymph node dissection.12,13,14 However, most importantly, tumor response to NAC provides valuable prognostic information that subsequently guides adjuvant treatment recommendations, which impact long-term patient outcomes.15,16
Patients achieving a pCR are not recommended to receive escalation of adjuvant systemic therapy, as their outcomes have been favorable in most published studies.8,17,18 However, patients with RD following NAC for TNBC are recommended to receive additional systemic therapy, with addition of capecitabine or olaparib (the latter for patients with germline BRCA mutations), either with or without pembrolizumab, because these agents have been shown to improve survival outcomes in patients with RD after NAC.15,16,19 Furthermore, clinical trials evaluating novel therapeutic agents in the adjuvant setting largely focus their eligibility criteria on patients with RD after NAC.
Traditionally, the overall stage at presentation (tumor size and nodal status) has been proven to impact overall survival with more extensive disease at presentation leading to poorer overall survival (OS).20,21 However, in the era of NAC, clinicians and researchers generally prioritize response to chemotherapy to estimate prognosis, with traditional anatomical stage of disease at presentation thought to be less impactful.8,9,17,18 However, recent studies have suggested that in patients with HER2+ disease, the stage at presentation may continue to impact outcomes within patients achieving a pCR based on the disease stage at presentation.22 The goal of this study was to evaluate the impact of the extent of disease at presentation on the OS in patients treated with NAC for TNBC.
Methods
Patient Cohort and Study Design
The National Cancer Database (NCDB) 2020 Participant User File was used to identify the cohort of patients with stage I-III TNBC diagnosed from 2010 to 2019. The National Cancer Database is a clinical oncology database sourced from hospital registry data. Data are collected from more than 1,500 Commission on Cancer (CoC) accredited facilities and captures greater than 70% of newly diagnosed cancer cases nationally.23 The Mayo Clinic Institutional Review Board deemed analysis of the deidentified NCDB file as exempt from review.
Patients with stage I–III TNBC treated between 2010 and 2019 with NAC followed by operation were included in our study. ER and PR status were recorded as negative if <1% of cells stained positive. HER2 status was recorded from the overall summary of results, including immunohistochemistry, fluorescent in situ hybridization, and chromogenic in situ hybridization when performed, and defined as negative if 0 or 1+ by immunohistochemistry or not amplified on fluorescent in situ hybridization. We defined NAC as chemotherapy initiated within 30 to 365 days before first surgery. Patients with distant metastasis (cM1) at diagnosis were excluded, as were patients with previous history of cancer and those with no treatment at the reporting facility. Patients were classified as having multicentric disease if they had a site code of C50.9 and as unicentric disease if they had a site code of C50.0-C50.8. Clinical stage was defined by using the 7th edition of the American Joint Committee on Cancer (AJCC) breast cancer staging system; cases diagnosed and coded under other AJCC editions were restaged to the 7th edition using the individual clinical T (cT) and clinical N (cN) components to derive a consistent TNM stage version across the study cohort. Patients were categorized based on response to chemotherapy using pathologic T and N categories from surgery into either pCR (defined as ypT0/Tis, ypN0) versus any residual invasive disease (RD).
The primary outcome was OS at 5 years and 10 years for patients with a pCR at the time of operation based on extent of disease at presentation. A secondary analysis evaluated OS in patients with RD at the time of operation as categorized by extent of disease at presentation for comparison purposes.
Statistical Analysis
Overall survival was estimated by using the Kaplan–Meier method and reported with 95% confidence intervals (CI). Univariate associations of factors with OS were assessed by using Cox proportional hazards regression and reported using hazard ratios (HR). Multivariable analysis was performed similarly and reported with adjusted hazard ratios (aHR). All clinically relevant variables available in the NCDB were assessed on univariate analysis and were included in the multivariable model without use of variable selection procedures. Adjustment variables include age, race, ethnicity, Charlson-Deyo comorbidity score, grade, histology, multicentricity, cT and cN category, type of breast surgery (breast-conserving surgery or mastectomy), and receipt of adjuvant radiation therapy. Using the adjusted effect sizes for each cT and cN category, we combined groups with similar effect sizes to create six cT/cN combination categories representing disease extent at presentation to allow estimation of OS within relevant clinical stage strata for patients with pCR. The same clinical stage strata were also assessed in patients with RD as a comparison. Multivariable Cox proportional hazard regression in the entire cohort of TNBC treated with NAC (combining the pCR and RD subgroups) also was used to estimate adjusted effects (aHR) comparing between specific groups conditional on response (pCR or RD) and cT/cN category. Analysis was performed by using SAS (Version 9.4, SAS Institute Inc., Cary, NC) and R software (Version 4.3.1, www.r-project.org). P < 0.05 was considered statistically significant.
Results
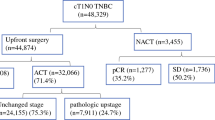
We identified 35,598 patients with stage I-III TNBC treated with NAC followed by surgery who met study inclusion criteria. Of these, 11,967 (33.6%) had a pCR and 23,631 (66.4%) had RD at the time of operation. The median follow-up among those alive at last follow-up was 4.3 years for patients with pCR and 4.5 years for patients with RD.
Among patients with pCR, the median age was 51 (interquartile range 43–59). Most were white (73.8%) and not of Hispanic ethnicity (90.1%), and most (87.9%) had a Charlson-Deyo comorbidity score of 0. Most tumors were grade 3 (89.3%) and invasive ductal carcinoma histology (96.6%). Clinical T category at presentation was cT1 in 23.7%, cT2 in 59.2%, cT3 in 11.9%, and cT4 in 5.1%, with 6.5% having multicentric disease. Nodal status at presentation was cN0 in 65.9%, cN1 in 26.4%, cN2 in 4.1%, and cN3 in 3.5%. Using AJCC 7th edition, 46.2% of tumors were stage IIA, 19.9% stage IIB, and 17.8% stage I disease. The remaining 16.1% had stage III disease. After NAC, 49.1% underwent breast conserving surgery and 50.9% underwent mastectomy (Table 1).
On univariate analysis of the patients treated with NAC achieving pCR, factors associated with worse OS included age (HR 1.40 for every 10-year increase in age, 95% CI 1.31–1.49, p < 0.001), a comorbidity score of 1 or 2+ versus 0 (HR 1.38, 95% CI 1.11–1.72, p = 0.004, and HR 2.87, 95% CI 2.04–4.03, p < 0.001, respectively), histologies other than invasive ductal carcinoma histology (HR 2.60, 95% CI 2.01–3.37, p < 0.001), multicentric disease (HR 1.65, 95% CI 1.3–2.09, p < 0.001), higher clinical T category at presentation [cT3 associated with HR 1.79 (95% CI 1.4–2.3, p < 0.001), and cT4 HR 3.98 (95% CI 3.09–5.13, p < 0.001), each vs. cT1], nodal metastases at presentation [cN1 associated with HR 1.77 (95% CI 1.50–2.08, p < 0.001), cN2 HR 2.96 (95% CI 2.27–3.85, p < 0.001), and cN3 HR 4.43 (95% CI 3.45–5.69, p < 0.001), each vs. cN0]. Hispanic ethnicity (HR 0.73, 95% CI 0.54–0.97, p = 0.03) was associated with improved OS (Table 2).
All variables were included in a multivariable model. Both cT and cN categories had statistically significant associations (p < 0.001) with OS on multivariable analysis adjusting for demographics, treatment, and other clinical variables. However, there were no significant differences seen between cT0, cT1, and cT2 categories. Therefore, patients with cT3 (HR 1.6 vs. cT1, p < 0.001) or cT4 (HR 2.2 vs. cT1, p < 0.001) were combined because of smaller sample sizes and each being different than cT1 and cT2. Clinical nodal categories were grouped into cN0, cN1, and cN2/cN3 by using similar rationale. Hazard ratios from the multivariable model were used to create cT/cN groupings that captured the combined effect of the two variables while preserving adequate group sizes for estimation. Thus, subsequent analysis was performed by using the following categories: cT1-2/cN0, cT0-2/cN1, cT0-2/cN2-3, cT3-4/cN0, cT3-4/cN1, cT3-4/cN2-3 (Supplemental Table 1).
A multivariable Cox proportional hazards regression model using the extent of disease categorized as above, adjusted for demographic, treatment, and other clinical variables is reported in Fig. 1. Factors associated with poorer OS on multivariable analysis included increasing patient age (aHR = 1.36 per 10-year increase, p < 0.001), comorbidity score of 2+ (aHR = 2.28 vs. score of 0, p < 0.001), histologies other than invasive ductal carcinoma (aHR=1.87, p < 0.001), and omission of radiation (aHR = 2.35 with breast-conserving surgery, p < 0.001). Hispanic ethnicity was associated with better OS (aHR = 0.73, p = 0.04).
The extent of disease at presentation remained significantly associated with OS. Among patients with pCR, 5- and 10-year OS were 92.7% and 88.5%, respectively, but decreased with increasing T and N category at presentation. The best OS was observed in patients with cT1-2, cN0 disease (5- and 10-year OS of 95.4% and 90.9%, respectively), followed by patients with cT0-2, cN1 disease (5- and 10-year OS of 92.5% and 88.6%, respectively). The worst OS rates were seen in patients with cT3-4, cN2-3 disease with 5- and 10-year OS of 78.1% and 72.0%, respectively (Table 3; Fig. 2A).
In this cohort, 23,631 patients had residual disease following NAC. The 5- and 10-year OS estimates were 68.1% and 60.1%, respectively. Overall survival varied by presenting cT category and cN status in a similar pattern as seen in patients with pCR. In patients with residual disease, 5- and 10-year overall survival was highest for cT1-2, cN0 disease (80.5% and 73.0%, respectively), followed by cT0-2, cN1 disease (66.7% and 59.2%, respectively). The poorest 5- and 10-year OS was seen in patients with cT3-4, cN2-3 disease who had RD at the time of operation (40.0% and 36.3% respectively) (Table 3; Fig. 2B).
Notably, OS was significantly poorer for patients with pCR who presented with cT3-4, cN2-3 disease compared with patients with residual disease who presented as cT1-2, cN0 on univariate analysis (HR 1.28, 95% CI 1.01–1.61, p = 0.04); Supplemental Fig. 1), as well as on multivariable analysis adjusted for age, race, ethnicity, comorbidity score, multicentricity, grade, and histology (aHR 1.30, 95% CI 1.03–1.63, p = 0.03).
Discussion
This study suggests that the extent of disease at presentation remains relevant and prognostic for patients with TNBC achieving pCR after NAC. Despite achieving pCR, patients with more advanced stages of disease at presentation had poorer OS at 5 and 10 years after surgery compared to those with less extensive disease. Remarkably, those with cT3-T4, cN2-3 disease not only had the poorest OS of all patients with a pCR, but also had worse OS than patients with RD with less extensive disease (cT1-2, cN0) at presentation. Thus, the extent of disease at presentation has the potential to further stratify patient outcome among patients with a pCR. Furthermore, in patients with RD at the time of operation, poorer OS was associated with more advanced stage at presentation in a comparable manner as those with a pCR, with the poorest OS once again seen in patients with cT3-4, cN2-3 disease.
The association between pCR and long-term outcomes has been extensively demonstrated. The findings from NSABP-18 demonstrated that patients with a pCR following NAC had improved disease-free survival, recurrence-free survival, and distant disease-free survival compared with patients with RD following NAC.24 The Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) Analysis and subsequent publications evaluating outcomes after NAC showed improved outcomes for patients with pCR, particularly in patients with triple-negative or HER2-positive breast cancer.8,25,26 The favorable EFS and OS rates observed among patients with pCR have led to clinical practice guidelines recommending no escalation of systemic therapy among these patients.27
Given the poorer outcomes seen in patients with RD after NAC, clinical trials have focused on systemic therapy escalation strategies for these patients. The CREATE-X trial randomly assigned patients with HER2-negative breast cancer and RD after NAC to receive adjuvant capecitabine versus no further adjuvant therapy. At 5 years, patients in the capecitabine arm had a significant improvement in DFS and OS, with the most significant findings seen in patients with TNBC.15 Following the publication of these findings, administration of 6 months of adjuvant capecitabine became the standard of care for patients with RD at the time of operation after NAC for TNBC.27,28 Similarly, the OlympiA trial randomly assigned patients with HER2-negative breast cancer and RD after NAC (or high-risk features if surgery pursued upfront) to receive adjuvant olaparib versus placebo. Administration of olaparib was associated with improved invasive DFS and OS, which led to the FDA approval of adjuvant olaparib in this context.16
While for patients treated with upfront surgery, pathologic stage is the primary information used for prognostication and adjuvant treatment recommendations, in patients with TNBC the extent of disease at presentation is used to guide the decision to administer NAC (and to choose a neoadjuvant regimen), whereas pathologic response is predominantly used to prognosticate and guide adjuvant treatment. However, the data presented suggest that the extent of disease at presentation may impact outcomes beyond the prognostic information obtained with response to NAC, information that could have clinical implications. Our findings are in alignment with previous work, such as the development of the NeoBioScore, which assesses prognosis based on clinical stage before treatment, the pathologic stage following treatment, the receptor status, and the grade of the tumor.29,30
A recent study evaluated outcomes for patients with HER2+ breast cancer according to response to NAC. Findings indicated that the extent of disease at presentation influenced outcomes of patients achieving a pCR. While confirming that pCR is prognostic for EFS and OS, it was shown that tumor size and nodal status at diagnosis were each independent risk factors for EFS in patients with HER2+ breast cancer who achieved a pCR. Interestingly, patients with a pCR with T1-2/N+ disease at presentation did not have a significant difference in EFS compared with patients with RD with negative nodes at presentation. It was suggested that the utilization of tumor response alone to guide adjuvant treatment should be questioned, given the impact of extent of disease at presentation on outcomes in patients with a pCR.22 These findings in HER2+ breast cancer align with our findings in TNBC.
Of interest, in our cohort, the OS of the group of patients with cT3-T4, cN2-3 disease who achieved a pCR was statistically worse than the OS of patients who presented with cT1-T2, cN0 disease and had RD at the time of operation. Despite this, in current clinical practice, the former group of patients would not be considered for escalation of systemic therapy after surgery, whereas the latter group would. These results, if confirmed in further studies, would suggest that patients with high clinical stages at presentation should be considered for prospective clinical trials evaluating escalation of therapy regardless of tumor response to NAC.
This study has several limitations. It is retrospective and, as such, subject to all the inherent biases of retrospective studies. The clinical management of TNBC has significantly changed since the inclusion period of this dataset. Currently, patients with TNBC are generally treated with neoadjuvant chemoimmunotherapy following the KEYNOTE-522 trial regimen, published in 2020.19 Furthermore, patients with RD will generally receive not only pembrolizumab, but also adjuvant capecitabine, based on the CREATE-X trial results published in 2017,15 only two years before the completion of this patient cohort. The impact of these advances on practice patterns cannot be fully evaluated by this cohort as most predated these changes in practice, limiting our ability to assess the effect of presenting stage on OS in patients treated with current systemic therapy approaches. Data needed to calculate the Residual Cancer Burden (RCB) is not available in NCDB. Residual Cancer Burden has prognostic implications with higher RCB scores being associated with worse survival.17,18,31,32,33 As such, we are unable to evaluate whether the higher stage at presentation was associated with higher RCB after NAC, which in turn may be a driver of worse outcomes in patients with more extensive disease at presentation among patients with RD. TNBC is a heterogenous disease, and NCDB does not include information on subtypes of TNBC and biology of the residual disease, which also impact prognosis and are unable to be accounted for in this study.4 Detailed information regarding the treatments received before operation is not available in the NCDB, nor is there information regarding whether a systemic therapy regimen was completed or terminated early because of side effects or poor tolerance. Furthermore, recurrence data are not available in NCDB, so RFS or EFS cannot be evaluated in this study. As such, OS events may include nonbreast cancer death events that may further confound the results.
Conclusions
Our study shows that, among patients with TNBC treated with NAC, the extent of disease at presentation further stratifies OS, both in the context of a pCR and RD. Furthermore, patients with larger, node-positive tumors at presentation who achieve a pCR had inferior OS than patients with smaller, node-negative disease who had RD. If these findings are validated in other datasets, particularly in datasets amenable to interrogation of recurrence events, future investigation of adjuvant systemic therapy escalation strategies for patients with advanced disease stage at presentation who achieve a pCR may be warranted. These patients are currently excluded from ongoing clinical trials of adjuvant therapy escalation under the assumption that achieving pCR equates to a favorable prognosis.
References
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. https://doi.org/10.1001/jama.2018.19323.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. https://doi.org/10.3322/caac.21820. (Epub 2024 Jan 17).
McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57(Suppl 1):9S-16S. https://doi.org/10.2967/jnumed.115.157834.
Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389(10087):2430–42. https://doi.org/10.1016/S0140-6736(16)32454-0. (Epub 2016 Dec 7).
Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer–current status and future directions. Ann Oncol. 2009;20(12):1913–27. https://doi.org/10.1093/annonc/mdp492. (Epub 2009 Nov 9).
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. https://doi.org/10.1158/1078-0432.CCR-06-3045.
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018;25(8):2241–8. https://doi.org/10.1245/s10434-018-6531-5. (Epub 2018 May 21).
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. https://doi.org/10.1016/S0140-6736(13)62422-8.
von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804. https://doi.org/10.1200/JCO.2011.38.8595. (Epub 2012 Apr 16).
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. https://doi.org/10.1200/JCO.2007.14.4147.
Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–505. https://doi.org/10.1200/JCO.20.03399. (Epub 2021 Jan 28).
Leon-Ferre RA, Hieken TJ, Boughey JC. The Landmark Series: Neoadjuvant chemotherapy for triple-negative and HER2-positive breast cancer. Ann Surg Oncol. 2021;28(4):2111–9. https://doi.org/10.1245/s10434-020-09480-9. (Epub 2021 Jan 23).
Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, Boccardo F, Boddington C, Bogaerts J, Bonadonna G, Bradley R. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. https://doi.org/10.1016/S1470-2045(17)30777-5.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61. https://doi.org/10.1001/jama.2013.278932.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59. https://doi.org/10.1056/NEJMoa1612645.
Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–405. https://doi.org/10.1056/NEJMoa2105215. (Epub 2021 Jun 3).
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22. https://doi.org/10.1200/JCO.2007.10.6823. (Epub 2007 Sep 4).
Yau C, Osdoit M, van der Noordaa M, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–60. https://doi.org/10.1016/S1470-2045(21)00589-1. (Epub 2021 Dec 11).
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/NEJMoa1910549.
Chavez-MacGregor M, Mittendorf EA, Clarke CA, Lichtensztajn DY, Hunt KK, Giordano SH. Incorporating tumor characteristics to the American joint committee on cancer breast cancer staging system. Oncologist. 2017;22(11):1292–300. https://doi.org/10.1634/theoncologist.2017-0116. (Epub 2017 Jun 7).
Edge SB, Compton CC. The American joint committee on cancer: 7th the edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. https://doi.org/10.1245/s10434-010-0985-4.
van Mackelenbergh MT, Loibl S, Untch M, et al. Pathologic complete response and individual patient prognosis after neoadjuvant chemotherapy plus anti-human epidermal growth factor receptor 2 therapy of human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2023;41(16):2998–3008. https://doi.org/10.1200/JCO.22.02241. (Epub 2023 Apr 19).
Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–8. https://doi.org/10.1001/jamaoncol.2016.6905.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 2023;41(10):1795–808. https://doi.org/10.1200/JCO.22.02571.
Spring L, Greenup R, Niemierko A, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw. 2017;15(10):1216–23. https://doi.org/10.6004/jnccn.2017.0158.
Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838–48. https://doi.org/10.1158/1078-0432.CCR-19-3492. (Epub 2020 Feb 11).
National Comprehensive Cancer Network. Breast Cancer. Adjuvant systemic therapy after preoperative systemic therapy BINV-16 (Version 5.2023). http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 24 Jan (2024).
Denduluri N, Chavez-MacGregor M, Telli ML, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2018;36(23):2433–43. https://doi.org/10.1200/JCO.2018.78.8604. (Epub 2018 May 22).
Mittendorf EA, Vila J, Tucker SL, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2(7):929–36. https://doi.org/10.1001/jamaoncol.2015.6478.
Bergquist JR, Murphy BL, Storlie CB, et al. Incorporation of treatment response, tumor grade and receptor status improves staging quality in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2017;24(12):3510–7. https://doi.org/10.1245/s10434-017-6010-4. (Epub 2017 Aug 21 PMID: 28828583).
Peintinger F, Sinn B, Hatzis C, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol. 2015;28(7):913–20. https://doi.org/10.1038/modpathol.2015.53. (Epub 2015 May 1).
Campbell JI, Yau C, Krass P, et al. Comparison of residual cancer burden, American joint committee on cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2017;165(1):181–91. https://doi.org/10.1007/s10549-017-4303-8. (Epub 2017 Jun 2).
Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–60. https://doi.org/10.1200/JCO.2015.63.1010. (Epub 2017 Jan 30).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
No disclosures relevant to this manuscript. Dr. Boughey receives research support paid to her institution from Eli Lilly and SymBioSis and is on a DSMB for CairnsSurgical. She has received honoraria for speaking for PER, PeerView, EndoMag and contributed a chapter to UpToDate. Roberto A. Leon-Ferre reports consulting fees paid to Mayo Clinic from Gilead Sciences, AstraZeneca, and Lyell Immunopharma, and fees for CME activities from MJH Life Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented as an oral presentation at the Society for Surgical Oncology 2024 meeting on March 20–23, 2024.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carroll, J.F., Hoskin, T.L., Leon-Ferre, R.A. et al. Impact of Presenting Stage on Overall Survival in Patients Treated with Neoadjuvant Chemotherapy for Triple Negative Breast Cancer. Ann Surg Oncol 31, 5132–5140 (2024). https://doi.org/10.1245/s10434-024-15583-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15583-4