Abstract
Purpose
We aimed to evaluate whether maximal transurethral resection (TUR) affects the oncological outcome of partial cystectomy (PC) performed in patients with muscle-invasive bladder cancer (MIBC), although radical cystectomy (RC) and trimodal therapy (TMT) are regarded as standard treatments for MIBC.
Methods
In this retrospective study, we evaluated the data of 98 patients who underwent PC due to MIBC between January 2006 and December 2018. Of the 98 patients, 71 underwent maximal TUR. We evaluated the recurrence-free survival (PFS), pelvic recurrence-free survival (pPFS), cancer-specific survival (CSS), and overall survival (OS) using the Kaplan–Meier method according to the maximal TUR status. Variables associated with survival were analyzed using Cox regression analyses.
Results
The 5-year PFS (42.5% vs. 20.3%, p = 0.008), pPFS (50.7% vs. 24.1%, p = 0.003), and CSS (74.0% vs. 51.0%, p = 0.016) were also higher in patients who underwent maximal TUR. The multivariable Cox regression analysis showed that maximal TUR was associated with PFS (hazard ratio [HR] = 0.500, p = 0.029), pPFS (HR = 0.353, p = 0.004), and CSS (HR = 0.416, p = 0.027). However, maximal TUR did not affect the OS (HR = 0.618, p = 0.132).
Conclusion
PC resulted in acceptable oncological outcomes in patients with MIBC, while maximal TUR played an important role in improving the oncological outcomes. PC after maximal TUR can be suggested as a treatment option for MIBC patients who are unable to undergo RC and TMT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bladder cancer is one of the most common types of urinary tract cancers, and 550,000 patients worldwide are diagnosed with bladder cancer annually.1 At the time of diagnosis, 70% of primary bladder tumors are non-muscle-invasive bladder cancer (NMIBC), while 30% are muscle-invasive bladder cancer (MIBC).2 Radical cystectomy (RC) with pelvic lymph node dissection (PLND) and urinary diversion is regarded as the gold standard treatment for MIBC according to several guidelines.3,4 Although RC is considered an effective locoregional option for controlling high-risk bladder cancer, 13–67% of patients undergoing RC experience considerable perioperative and postoperative complications.5,6,7 In an effort to decrease the complications and substantial morbidity of open radical cystectomy (ORC), robot-assisted radical cystectomy (RARC) has been increasingly adopted for the treatment of MIBC. Although a recent meta-analysis showed that RARC is associated with a significantly lower major postoperative complication rate compared with ORC, the 90-day major complication rate reached 20%.8 Along with these postoperative complications, the negative effects of RC on urinary, bowel, and sexual function, as well as on body image, can decrease the quality of life.9
To overcome the different problems associated with RC, trimodal therapy (TMT), which involves maximal transurethral resection of the bladder tumor (TURBT), chemotherapy for radiation sensitization, and external beam radiotherapy, is the recommended bladder-preserving approach for patients with MIBC.4 Although no randomized study has compared the oncologic outcomes of RC and TMT performed in patients with MIBC, a previous propensity score analysis reported similar oncologic outcomes between TMT and RC in selective patients.10 In order to preserve bladder function and cure bladder cancer, the indications for TMT should be fully considered; it may be suitable for patients with a solitary tumor, a tumor of < 5 cm in diameter, without or with minimal hydronephrosis, and without concurrent extensive or multifocal carcinoma in situ (CIS).10,11
Partial cystectomy has also been employed as a bladder-preserving approach for MIBC. Partial cystectomy allows complete pathologic staging of the primary tumor with full-thickness excision, removal of the wide portion of the bladder and overlying peritoneum containing the tumor, and PLND.12 Several studies have demonstrated that PC is associated with decreased surgical morbidity.13,14 However, since PC is associated with a higher risk of tumor recurrence and need for secondary therapies, it has been regarded as an inferior treatment option.15,16
During partial cystectomy, the bladder is opened when en bloc resection of a bladder tumor is performed, and urine leakage from the bladder is expected. When residual tumor remains in the bladder before PC is performed, the tumor cells are likely to leak out from this site. This will affect the oncological outcome of patients receiving PC. Hence, we aimed to evaluate the effect of maximal TUR on the prognosis of PC in this study.
Methods
Patient Selection and Characteristics
We reviewed the medical records of 435 patients who underwent partial cystectomy due to bladder tumors between January 2006 and December 2018 at Severance Hospital, Seoul, Korea. The exclusion criteria were for patients with the following characteristics: (1) without urothelial carcinoma; (2) with non-muscle invasive bladder cancer; (3) with multiple tumors or carcinoma in situ (CIS); (4) with distant metastasis; and (5) when clear determination of the status of TURBT was difficult.
We investigated the following data in all patients: sex, age, body mass index, smoking history, tumor size, pathologic tumor stage, surgical margin, adjuvant chemotherapy, and TURBT status. Thereafter, we performed a detailed review of the surgical records and endoscopic image obtained during TURBT. The status of TURBT was evaluated based on the surgical report, pathology report, and endoscopic image. The patients were classified as having a maximal TURBT if the specimen obtained contained a muscle layer as documented in the pathology report and if a maximal resection was performed as indicated in the operative report. If the TURBT status was not determined as documented in the surgical record, endoscopy was carried out after performing TURBT.
The patients were divided into two groups based on TURBT status. Group 1 included 27 patients who did not undergo maximal TURBT, while group 2 included 71 patients who underwent maximal TURBT. These two groups were compared based on the previously mentioned variables to evaluate any significant differences between them.
Follow-Up
The follow-up schedule, including the type of treatment required (adjuvant or salvage chemotherapy), was decided by the attending physician based on the classification of cancer risk. Cystoscopy with urine cytology was performed every 3 months for the first year, every 6 months for the second year, and every 6–12 months thereafter. Imaging studies such as abdominopelvic and chest computed tomography (CT), magnetic resonance imaging of the bladder, bone scan, or positron emission tomography and CT was performed every 3–6 months, as needed, to assess for disease progression. Tumor progression was defined as a diagnosis of MIBC; tumor recurrence in the regional node, pelvic organ, or soft tissue; or distant metastasis.
Statistical Analyses
The patient characteristics were compared between the two groups using the Mann–Whitney U test for continuous data and chi-square test for dichotomous variables. The categorical variables are expressed as frequencies and percentages, while the continuous variables are expressed as medians and interquartile ranges (IQRs). Kaplan–Meier curves and log-rank test were used to depict and compare the progression-free survival (PFS), pelvic progression-free survival (pPFS), cancer-specific survival (CSS), and overall survival (OS). Then, multivariable Cox regression models were constructed to determine the variables associated with the oncologic outcomes. All tests were two sided, and a p value of < 0.05 was considered significant. The statistical analyses were performed using STATA® version 15.1 (StataCorp LLC, College Station, TX, USA).
Good Clinical Practice Protocols
The study was approved by the institutional review board of the Yongin Severance Hospital. (2022-0063-001). This study was performed in accordance with the applicable laws and regulations, good clinical practices, and ethical principles described in the Declaration of Helsinki. Written informed consent was obtained from all patients.
Results
Among 98 patients, 71 underwent maximal TUR. No difference was found in the clinicopathologic characteristics between the two groups, except for the residual tumor size (Table 1).
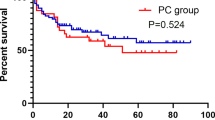
Figure 1 shows the Kaplan–Meir curves of PFS, pPFS, CSS, and OS. With a median follow-up duration of 40.5 months (IQR 20.75–72.25), the 5-year PFS, pPFS, CSS, and OS rates were 36.6%, 43.7%, 66.7%, and 54.3%, respectively. The 5-year PFS rate was higher in group 2 compared with that in group 1 (42.5% vs. 20.3%, p = 0.008). The 5-year pPFS (50.7% vs. 24.1%, p = 0.003) and CSS (74.0% vs. 51.0%, p = 0.016) were also higher in group 2 compared with that in group 1. However, no significant difference was observed in the OS between group 1 and group 2 (58.6% vs. 43.1%, p = 0.126).
Multivariable Cox regression analysis showed that maximal TUR (hazard ratio [HR] = 0.500, 95% confidence interval [CI] 0.0.268–0.933, p = 0.029) was associated with PFS with LN involvement (HR = 2.557, 95% CI 1.204–5.431, p = 0.029) (Table 2). Maximal TUR (HR = 0.353, 95% CI 0.176–0.710, p = 0.004) was related with the reduced risk of pelvic progression. Female gender (HR = 2.215, 95% CI 1.079–4.547, p = 0.030) and LN involvement (HR = 3.171, 95% CI 1.345–7.479, p = 0.008) were associated with pPFS (Table 3). The factors affecting CSS were maximal TUR (HR = 0.416, 95% CI 0.192–0.904, p = 0.027) and LN invasion (HR = 2.892, 95% CI 1.128–7.416, p = 0.027) (Table 4). However, maximal TUR was not associated with OS (HR = 0.618, 95% CI 0.330–1.157, p = 0.132). Older age (HR = 1.047, 95% CI 1.016–1.080, p = 0.003) and LN invasion (HR = 2.921, 95% CI 1.346–6.340, p = 0.007) were associated with worse OS (Table 5).
In patients who achieved pT0, the 5-year PFS, pPFS, CSS, and OS were 73.5%, 78.4%, 92.9%, and 79.6%, respectively. In logistic regression analysis, only pT stage was found to be a predictive factor for pT0 (odds ratio = 0.037, 95% CI 0.005–0.301, p = 0.002, Table 6).
Discussion
Partial cystectomy has been used as a treatment for MIBC for several years. RC and TMT have been recently recommended for the treatment of MIBC, while partial cystectomy alone is no longer recommended. Radiotherapy as treatment for MIBC was suggested during outpatient clinic visits and was carried out every day for 50 days.3,4 Previous studies related to TMT have reported that the treatment completion rate of radiotherapy was > 80%.17,18 However, various challenges were encountered when radiotherapy was implemented in a real clinical setting. Patients living in areas with poor access to hospitals find it difficult to visit the hospital every day. In addition, patients with comorbidities who were not indicated for RC may have found it difficult to visit the hospital every day due to limited mobility. Although patients in whom RC and TMT were difficult to perform have been identified, the role of PC in some MIBC patients remains unknown.
Hence, we reviewed the previous studies regarding the oncologic outcomes of PC. Previously, several studies on partial cystectomy reported a 5-year RFS rate of 39–69%, a CSS rate of 76–84%, and an OS rate of 57–79%.16,19,20,21 However, all of these studies were conducted among patients with NMIBC. Unfortunately, only a few studies have evaluated the oncologic outcome of partial cystectomy in patients with MIBC. Among the few studies, Chung et al.22 reported outcomes of PC in patients with urothelial carcinoma, and they demonstrated a 5-year PFS rate of 49% in MIBC patients. Kassouf et al.12 reported 5-year RFS, CSS and OS rates of 39%, 87%, and 67%, respectively. Meanwhile, our study reports 5-year PFS, pPFS, CSS, and OS rates of 36.6%, 43.7%, 66.7%, and 54.3%, respectively. Our results showed relatively worse oncological outcomes compared with those of previous studies. Moreover, the proportion of patients with T3 and lymph node metastasis in our study is higher than that in previous studies. Therefore, the oncologic outcome was slightly worse because the patients included in our study had a higher bladder cancer stage.
Previous studies have shown that the most important factor affecting the prognosis of bladder cancer is the tumor stage. Several studies indicated that T stage and N stage were important prognostic factors for MIBC.23,24 However, the number of studies that have analyzed the factors that can affect the oncological outcomes of PC is limited. A recent study established a prognostic model for MIBC using CSS data obtained from the Surveillance, Epidemiology, and End Results database.25 In this study, Zhan et al. reported that the factors associated with CSS were age, TNM stage, tumor size, and number of harvested LNs. As is generally known, the presence of LN invasion was also found to be associated with the oncologic outcomes in our study. However, T stage was associated with survival only in the univariate analysis of pelvic recurrence, while other studies reported that this factor did not have a significant effect on the oncologic outcome. Moreover, maximal TUR played an important role in the PFS, pPFS, and CSS. Previously published studies have reported the importance of maximal TUR. James et al.26 reported that maximal TUR improved the prognosis in patients who underwent RC after receiving neoadjuvant chemotherapy (NAC). In the group that underwent maximal TUR, more patients showed a complete response after NAC and reported significantly higher OS and CSS. The researchers viewed maximal TUR before NAC as a type of cytoreductive surgery and thought that NAC had a favorable effect as the primary tumor burden was reduced. However, since PC was performed without NAC in our study, another factor may explain why maximal TUR improved the survival rate. As mentioned in the Introduction, urine leakage occurs as the bladder opens, and the risk of seeding increases if a residual tumor exists. Accordingly, the oncologic outcome might be worse in patients who did not undergo maximal TUR.
Another unusual finding in our results is the fairly high proportion of patients who did not receive PLND. Although not shown in the Results Section, all clinical stage N1–3 patients underwent PLND, and the younger the patient, the higher the rate of receiving PLND. It seems that a significant number of patients did not undergo PC with a definitely curative aim because they were not suitable for both RC and RT. We believe that a study on the oncological results in patients who underwent both PC and PLND is also necessary.
Several factors were not analyzed but had a possible influence on the patients’ prognoses. First, we could not evaluate the effect of surgical margin on the oncologic outcomes. When performing PC, a margin of at least 1 cm should be secured, and resection should be performed.27 In our results, because the number of patients with positive margins was not relatively large, the margins could not be included as variables in the Cox proportional hazard analysis. And prostatic urethra biopsy was not performed in all patients. Prostatic urethra biopsy was performed only when there was evidence of gross tumor invasion. Therefore, in practice, the possibility that residual tumor remains after even maximal TUR cannot be completely ruled out. During the follow-up period, progression of prostate was observed in one patient each from group 1 and group 2. Another important factor is NAC. According to several guidelines, NAC is recommended in MIBC, and several studies reported that NAC improved the survival of patients with MIBC.28,29 However, our study did not evaluate the effect of NAC on the survival rates. NAC was not covered by health insurance during the period when the MIBC patients included in our study underwent PC. Therefore, none of the patients received chemotherapy prior to surgery. Bazzi et al.30 reported 5-year RFS and OS rates of 28% and 63% in 36 patients who underwent PC after NAC. Recently, a study on the role of NAC, LND, and treatment delay in patients who underwent PC, using the National Cancer Database, was reported. Lenis et al.31 reported that adequate LND improved the OS, but NAC was not associated with OS. This may be because patients who received PC were generally older and received NAC less frequently, making it difficult to perform an accurate evaluation. Based on these studies, NAC seemed to improve the therapeutic effect of PC for MIBC. Second-look TUR may be helpful in performing maximal TUR. It has been reported that residual cancer remains in 20–30% of cases when second-look TUR is performed, even in high-grade T1 bladder cancer.32,33 In MIBC, there is a very high possibility that residual tumor will remain after the initial TURBT. Performing second-look TUR will contribute to maximally reducing residual tumor, which may also improve the oncological outcome of PC.
Another limitation is that our results might be sensitive to selection bias owing to the retrospective and non-randomized nature of this study. For example, due to different surgeon preferences, narrow-banding imaging (NBI) or blue light cystoscopy were not used in all patients to confirm CIS or tumor. Because NBI or blue light improves the overall CIS detection rate,34 patients who did not use it are more likely to have undetected CIS or tumor. And this can adversely affect oncological outcomes. Lastly, the number of patients included in this study was relatively small, and the follow-up duration was short. Nevertheless, our study involved more patients than previous studies and suggested that maximal TUR performed before PC could improve the oncological outcome of PC.
Conclusion
PC can achieve acceptable oncological outcomes in patients with MIBC. Moreover, maximal TUR plays an important role in improving the PC results. Among the MIBC patients encountered in real clinical practice, some experienced difficulties receiving RC or TMT; hence, PC after maximal TUR may be one of the treatment options for these patients.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018;68(6):394–424.
Ham WS, Park JS, Jang WS, Choi YD, Kim J. Prognostic Value of Prostate Volume in Non-muscle Invasive Bladder Cancer. Sci Rep. 2021;11(1):18784.
National Comprehensive Cancer Network. Bladder cancer (Version 2.2022). Accessed 5 June 2022. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol. 2021;79(1):82–104.
Svatek RS, Fisher MB, Matin SF, et al. Risk Factor Analysis in a Contemporary Cystectomy Cohort Using Standardized Reporting Methodology and Adverse Event Criteria. J Urol. 2010;183(3):929–34.
Hirobe M, Tanaka T, Shindo T, et al. Complications Within 90 Days After Radical Cystectomy for Bladder Cancer: Results of a Multicenter Prospective Study in Japan. Int J Clin Oncol. 2018;23(4):734–41.
Maibom SL, Røder MA, Poulsen AM, et al. Morbidity and Days Alive and Out of Hospital Within 90 Days Following Radical Cystectomy for Bladder Cancer. Eur Urol Open Sci. 2021;28:1–8.
Zhou N, Tian F, Feng Y, et al. Perioperative Outcomes of Intracorporeal Robot-Assisted radical Cystectomy Versus Open Radical Cystectomy: A Systematic Review and Meta-Analysis of Comparative Studies. Int J Surg. 2021;94:106137.
Choi H, Park JY, Bae JH, Tae BS. Health-Related Quality of Life After Radical Cystectomy. Transl Androl Urol. 2020;9(6):2997–3006.
Kulkarni GS, Hermanns T, Wei Y, et al. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. J Clin Oncol. 2017;35(20):2299–305.
Kimura T, Ishikawa H, Kojima T, et al. Bladder Preservation Therapy for Muscle Invasive Bladder Cancer: The Past, Present and Future. Jpn J Clin Oncol. 2020;50(10):1097–107.
Kassouf W, Swanson D, Kamat AM, et al. Partial Cystectomy for Muscle Invasive Urothelial Carcinoma of the Bladder: a Contemporary Review of the M. D. Anderson Cancer Center Experience. J Urol. 2006;175(6):2058–62.
Chang SS, Cookson MS, Baumgartner RG, Wells N, Smith JA Jr. Analysis of Early Complications After Radical Cystectomy: Results of a Collaborative Care Pathway. J Urol. 2002;167(5):2012–6.
Chung R, Moran GW, Wang C, McKiernan JM, Anderson CB. Partial Cystectomy: Review of a Single Center Experience from 2004 to 2019. Urol Oncol. 2022. https://doi.org/10.1016/j.urolonc.2022.09.003.
Stein JP, Lieskovsky G, Cote R, et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J Clin Oncol. 2001;19(3):666–75.
Capitanio U, Isbarn H, Shariat SF, et al. Partial Cystectomy does not Undermine Cancer Control in Appropriately Selected Patients with Urothelial Carcinoma of the Bladder: A Population-Based Matched Analysist. Urology. 2009;74(4):858–64.
Michaelson MD, Hu C, Pham HT, et al. A Phase 1/2 Trial of a Combination of Paclitaxel and Trastuzumab With Daily Irradiation or Paclitaxel Alone With Daily Irradiation After Transurethral Surgery for Noncystectomy Candidates With Muscle-Invasive Bladder Cancer (Trial NRG Oncology RTOG 0524). Int J Radiat Oncol Biol Phys. 2017;97(5):995–1001.
Coen JJ, Zhang P, Saylor PJ, et al. Bladder Preservation With Twice-a-Day Radiation Plus Fluorouracil/Cisplatin or Once Daily Radiation Plus Gemcitabine for Muscle-Invasive Bladder Cancer: NRG/RTOG 0712-A Randomized Phase II Trial. J Clin Oncol. 2019;37(1):44–51.
Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al. Partial Cystectomy: A Contemporary review of the Memorial Sloan–Kettering Cancer Center Experience and Recommendations for Patient Selection. J Urol. 2004;172(3):878–81.
Smaldone MC, Jacobs BL, Smaldone AM, Hrebinko RL Jr. Long-Term Results of Selective Partial Cystectomy for Invasive Urothelial Bladder carcinoma. Urology. 2008;72(3):613–6.
Golombos DM, O’Malley P, Lewicki P, Stone BV, Scherr DS. Robot-Assisted Partial Cystectomy: Perioperative Outcomes and Early Oncological Efficacy. BJU Int. 2017;119(1):128–34.
Chung R, Moran GW, Wang C, McKiernan JM, Anderson CB. Partial Cystectomy: Review of a Single Center Experience from 2004 to 2019. Urol Oncol. 2022;40(12):538.e1-e5.
Hussein AA, Elsayed AS, Aldhaam NA, et al. Ten-Year Oncologic Outcomes Following Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol. 2019;202(5):927–35.
Ham WS, Rha KH, Han WK, et al. Oncologic Outcomes of Intracorporeal vs Extracorporeal Urinary Diversion After Robot-Assisted Radical Cystectomy: A Multi-Institutional Korean Study. J Endourol. 2021;35(10):1490–7.
Zhan X, Chen T, Jiang M, et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Muscle-Invasive Bladder Cancer Patients after Partial Cystectomy. J Oncol. 2022;2022:2665711.
James AC, Lee FC, Izard JP, et al. Role of Maximal Endoscopic Resection Before Cystectomy for Invasive Urothelial Bladder Cancer. Clin Genitourin Cancer. 2014;12(4):287–91.
Knoedler JJ, Boorjian SA, Kim SP, et al. Does Partial Cystectomy Compromise Oncologic Outcomes for Patients with Bladder Cancer Compared to Radical Cystectomy? A matched case-control analysis. J Urol. 2012;188(4):1115–9.
Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant Chemotherapy Plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N Engl J Med. 2003;349(9):859–66.
Pfister C, Gravis G, Fléchon A, et al. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J Clin Oncol. 2022;40(18):2013–22.
Bazzi WM, Kopp RP, Donahue TF, et al. Partial Cystectomy after Neoadjuvant Chemotherapy: Memorial Sloan Kettering Cancer Center Contemporary Experience. Int Sch Res Notices. 2014;2014:702653.
Lenis AT, Fero KE, Ojeaburu L, et al. The Role of Neoadjuvant Chemotherapy, Lymph Node Dissection, and Treatment Delay in Patients with Muscle-Invasive Bladder Cancer Undergoing Partial Cystectomy. Urol Oncol. 2021;39(8):496.e17-e24.
Ferro M, Di Lorenzo G, Buonerba C, et al. Predictors of Residual T1 High Grade on Re-Transurethral Resection in a Large Multi-Institutional Cohort of Patients with Primary T1 High-Grade/Grade 3 Bladder Cancer. J Cancer. 2018;9(22):4250–4.
Ferro M, Vartolomei MD, Cantiello F, et al. High-Grade T1 on Re-Transurethral Resection after Initial High-Grade T1 Confers Worse Oncological Outcomes: Results of a Multi-Institutional Study. Urol Int. 2018;101(1):7–15.
Kumarasegaram V, Drejer D, Jensen JB. Detection Rate of Carcinoma In Situ During TURBT Following Shift from Photodynamic Diagnosis to Narrow Band Imaging in a Single University Hospital. Urology. 2022;161:83–6.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea [Grant Number: HI17C1095], the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) [Grant Number: 2019R1A2C1002863 and 2022R1A2C2003831], and a faculty research grant from Yonsei University College of Medicine (6-2021-0106).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ham, W.S., Park, J.S., Jang, W.S. et al. Role of Maximal Transurethral Resection Preceding Partial Cystectomy for Muscle-Invasive Bladder Cancer. Ann Surg Oncol 31, 1384–1392 (2024). https://doi.org/10.1245/s10434-023-14449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14449-5