Abstract
Background
Invasive mucinous adenocarcinoma (IMA) is distinct from non-mucinous adenocarcinoma, but studies on recurrent IMA are scarce. Thus, this study aimed to evaluate the recurrence patterns of IMA and the role of pulmonary local therapy (LT) in resectable pulmonary recurrence of IMA.
Methods
The study reviewed 403 patients with surgically resected IMA between 1998 and 2018. The recurrence patterns were categorized as solitary pulmonary recurrence (SPR), multiple pulmonary recurrence (MPR), and extra-pulmonary recurrence (EPR). The clinicopathologic characteristics, overall survival (OS), and post-recurrence survival (PRS) were analyzed according to the recurrence pattern and LT administration.
Results
Recurrences were found in 91 (22.6%) patients, including 18 patients with SPR, 37 patients with MPR, and 36 patients with EPR. Compared with the MPR and EPR groups, the SPR group had a longer disease-free interval (32.5 vs. 9.6 vs. 10.1 months, respectively; p < 0.01) and a better OS (5-year OS: 88.5%, 41.5%, and 22.9%, respectively; p < 0.01). In case of resectable pulmonary recurrence, pulmonary LT was administered to 15 patients with SPR and 3 patients with MPR. These patients showed a better 5-year PRS than the other patients with pulmonary recurrence (86.3% vs. 30.4%; p < 0.01). Notably, long-term survival was observed for one patient with MPR undergoing LT and two patients with SPR undergoing a second LT for a second pulmonary recurrence.
Conclusions
In this series, the patients with recurrent IMA showed different prognoses according to the recurrence pattern. The patients with pulmonary recurrence of IMA undergoing LT showed a favorable prognosis, suggesting the potential role of LT for resectable pulmonary recurrence of IMA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Invasive mucinous adenocarcinoma (IMA) of the lung is a distinct subtype of lung adenocarcinoma comprising up to 5% of all lung adenocarcinomas.1,2,3 It is defined histologically by columnar cells with intracytoplasmic mucin as well as a lepidic growth pattern4 and characterized genetically by frequent KRAS mutations.5,6 Compared with non-mucinous adenocarcinomas, IMAs have a tendency for intrapulmonary progression, and extrathoracic metastasis is rare.6,7,8 This distinct pattern of progression indicates that the tumor spread of IMA might be mediated by aerogenous spread.9,10
Recently, Yang et al.11 showed that nearly all separate IMA lesions involving contralateral lobes, either synchronous or metachronous, are clonally related, suggesting intrapulmonary spread. In addition, these authors found that a subset of patients can show extremely protracted intrapulmonary progression after primary tumor resection.
Despite the distinct characteristics of IMA, the recurrence pattern in patients undergoing curative-intent lung resection for IMA and the survival outcomes according to this pattern remain largely unexplored. In addition, while current guidelines recommend local therapy (LT) for local recurrence of non-small cell lung cancer (NSCLC) and LT is increasingly being adopted to treat oligorecurrences of various malignancies,12,13 the role of LT in resectable pulmonary recurrence of IMA has not been examined.
Therefore, in this study analyzing a large cohort of surgically resected IMA, we aimed to stratify patients with IMA by recurrence patterns and delineate their clinicopathologic characteristics and survival outcomes. Furthermore, we examined the potential role of LT in case of pulmonary recurrence of IMA.
Methods
Study Population
We reviewed the Registry for Thoracic Cancer Surgery at the Samsung Medical Center (Seoul, Korea), which includes all patients who have undergone surgery for lung cancer since 1995. The study identified 525 patients who underwent curative-intent lung resection for IMA between July 1998 and December 2018. From these 525 patients, the study excluded 122 who had a cancer history (n = 67), double primary cancer (n = 38), a history of preoperative chemotherapy (n = 16), or incomplete resection during surgery (n = 1). Finally, 403 patients were included in this study (Fig. 1). The institutional review board (IRB) approved this retrospective study, and informed consent for the use of patients’ medical data was waived (IRB no. 2022-10-126; 14 November 2022).
Preoperative Staging, Surgery, and Pathologic Evaluation
For diagnostic evaluation, chest computed tomography (CT) was used as the primary diagnostic method, and positron emission tomography was widely used after 2003. Additionally, for pathologic mediastinal lymph node evaluation, endo-bronchial ultrasound largely replaced the conventional mediastinoscopy, starting from 2009.
All pulmonary resections were performed by thoracic surgeons at the Samsung Medical Center. The operative procedures included wedge resection, segmentectomy, lobectomy, and pneumonectomy. The surgical extent was selected by considering the size and location of the tumor with a multidisciplinary approach and patients’ cardiopulmonary function. Systematic mediastinal lymph node dissection was performed for patients undergoing lobectomy or segmentectomy.
For wedge resections, lobe-specific selective lymph node dissection was performed, targeting specific levels based on the affected lobe as follows: levels 4 and 7 for the right upper and middle lobes, levels 7 and 9 for the right lower lobe, levels 5, 6, and 7 for the left upper lobe, and levels 7 and 9 for the left lower lobe. For cases in which lymph node enlargement or suspected lymph node metastasis was observed during the procedure, frozen section biopsies were performed. Subsequently, systematic lymph node dissection was performed in cases confirmed to be positive for malignancy.
Pathologists at the center examined all postoperative histologic specimens according to the fourth edition of the WHO Classification of Lung Tumors.14 The pathologic stage was determined per the seventh edition of the American Joint Committee on Cancer staging manual. According to previous studies, we defined high-grade IMAs as tumors with ≥10% micro-papillary or solid pattern.15,16,17 Lymphovascular invasion (LVI) and visceral pleural invasion (VPI) also were examined.
Follow-Up Evaluation After Initial Surgery
All the patients in our study underwent postoperative chest CT surveillance for a span of 5 years. During the initial 2-year period after surgery, they underwent chest CT examinations every 3 months, then at 6-month intervals for the ensuing 3 years. Depending on patients’ symptomatic presentations and clinical judgment during outpatient visits, additional diagnostic procedures, including brain CT scans, brain magnetic resonance imaging, and other relevant techniques, were used.
Definition of Recurrence and Recurrence Pattern Groups
Recurrences were determined by imaging techniques with or without pathologic confirmation. Radiologic recurrence was defined as solid nodules, part-solid nodules, or consolidative lesions. Because IMA can mimic inflammatory lesions, it was compared with the prior image, and the recurrences were determined at the physician’s discretion when they grew during the follow-up period.
Based on the unique characteristics of IMA, with aerogenous spread9,10 and distinct post-recurrence survival (PRS) of patients with NSCLC according to single or multiple recurred lesions,18 the recurrence patterns were categorized as follows: solitary pulmonary recurrence (SPR), multiple pulmonary recurrence (MPR), and extra-pulmonary recurrence (EPR). Recurrence limited to the lung parenchyma was categorized as pulmonary recurrence (SPR/MPR). Recurrence was categorized as MPR when multiple lesions were detected on CT at the time of the initial recurrence. Recurrence in locations other than the lung parenchyma (i.e., pleura, pericardium, extrathoracic organ, and lymph nodes) was categorized as EPR. When pathologic confirmation of the recurrent lesion was performed, diagnosis of the same IMA histology was considered as recurrence based on a previous report.11
Local Therapy
Local therapy was defined as local radical treatment, including surgery and radiation therapy (RT), and pulmonary LT included surgical lung resection and RT in the lung parenchyma. All patients with pulmonary recurrences were candidates for pulmonary LT. Considering the predicted postoperative pulmonary function, cardiac function, and other comorbidities of patients, LT was administered for the resectable pulmonary lesion in the lung parenchyma.
Statistical Analysis
Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Continuous variables were compared using the Wilcoxon rank-sum test. Overall survival (OS) and post-recurrence survival (PRS) were analyzed using Kaplan–Meier survival curves. The differences among the survival curves were tested for statistical significance using the two-tailed log-rank test. Cox proportional hazards regression analysis was performed to analyze effects of risk factors for OS (death) and disease-free survival (DFS, recurrence, or death).
To analyze the independent effect of each risk factor, multivariable analysis was performed using all the risk factors as independent variables that satisfied a p value lower than 0.1 in the univariable analysis. Sex was excluded in the multivariable analysis due to multicollinearity. The data of two patients who underwent pneumonectomy were excluded in the analysis because these data did not show any type of event.
All p values lower than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 21.0 (IBM Corp, Armonk, NY, USA) and R 4.0.0 (Vienna, Austria; http://www.R-project.org/).
Results
Patient Characteristics
Recurrence was identified in 91 (22.6%) patients, with SPR occurring in in 18 patients, MPR in 37 patients, and EPR in 36 patients. The clinicopathologic characteristics of the three groups are summarized in Table 1. The SPR group had a lower rate of LVI and a longer median disease-free interval (DFI) (32.5 months) than the MPR group (9.6 months) or EPR group (10.1 months). Sublobar resection was undertaken in 36 cases (Table 1), with recurrence in five cases (1 MPR and 4 EPR cases). Recurrence in the residual lung was found in one case involving a patient who had undergone superior segmentectomy of the right lower lobe.
Overall Survival
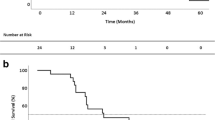
The median follow-up time was 78.4 months for the SPR group, 38 months for the MPR group, and 27.8 months for the EPR group, The 5-year OS was 88.5% for the SPR group, 41.5% for the MPR group, and 22.9% for the EPR group (Fig. 2). The 5-year OS differed significantly among the subgroups (SPR vs. MPR [p < 0.001], SPR vs. EPR [p < 0.001], MPR vs. EPR [p = 0.02]). The SPR and non-recurrence groups did not differ significantly in terms of OS (p = 0.425).
Post-recurrence Survival
The baseline characteristics of recurrent patients according to the treatment method were analyzed. The 5-year PRS was 74.1% for the patients undergoing LT only, 22.6%, for those who had systemic therapy (±LT), and 0% for those who had no treatment (Fig. 3A). In case of pulmonary recurrences, the patients undergoing pulmonary LT showed a higher PRS than the other patients (86.3% vs. 30.4%; Fig. 3B).
Multivariate Cox Regression Analysis for OS and DFS
In the multivariate analysis, age, shorter OS and DFS were associated with current smoker status, stage, high-grade histology, and LVI (Table S1). In the multivariate analysis performed on the patients who had recurrence, a shorter OS was associated with age, current smoker status, recurrence pattern, and DFI (Table S2).
Analyses of Recurrent Cases Treated With LT
Among the 18 patients with SPR, 15 received pulmonary LT, and 3 did not receive LT because of poor pulmonary function, refusal of further treatment, and follow-up loss, respectively (Table 2). The LTs consisted of wedge resection (n = 11), segmentectomy (n = 2), or radiotherapy (n = 2). Among the patients with SPR who received LT (n = 15), 11 patients were alive without further recurrent disease. As shown in Fig. 4A, the majority of the SPR patients controlled with LT (n = 11) exhibited low-grade histology without LVI (Table S3). The median DFI of these patients was 34.7 months.
Four patients (SPR cases 2, 5, 7, and 10; Table 2) had a second pulmonary recurrence after LT. Two patients (SPR cases 2 and 10) had pulmonary recurrence after DFIs of 35.0 and 32.5 months, respectively, and underwent LT (wedge resection). After the LT, they had the second pulmonary after DFIs of 26.9 and 23.5 months, respectively. Both patients were treated with a second pulmonary LT and were alive without disease 86 and 42.9 months, respectively, after the second LT. Two patients with SPR (SPR cases 5 and 7) who had pulmonary recurrence after DFIs of 29.7 and 10.5 months, respectively, exhibited a second recurrence after LT following DFIs of 20.2 and 11.7 months, respectively. Both patients died due to disease progression after 87.4 and 33.2 months of PRS, respectively.
Three patients in the MPR group received LT (Table 2). All three patients had a second pulmonary recurrence after DFIs of 27.4, 17.7, and 15.6 months, respectively. In one case with intermediate DFI (27.4 months), recurrent lesions in two contralateral lung sites were detected and treated with LT (MPR case 1; Fig. 4B). Both primary and recurrent tumors displayed low-grade histology with no LVI (Table S3). After 24.5 months, the patients experienced a second recurrence. No further treatment was delivered, and the patients were alive with an increased disease burden at the last follow-up visit (155.6 months after the initial surgical treatment). In one case with a DFI of 17.7 months (MPR case 2; Fig. 4C), recurrent lesions involving the contralateral lung were treated with LT. The tumors exhibited a micropapillary pattern and LVI, and the patient had recurrence 7.3 months after LT and died of disease 13.1 months after the second recurrence.
Discussion
This study examined the recurrence patterns of IMA using a large cohort and demonstrated that IMA patients with SPR had highly favorable OS compared with those who had MPR or EPR. Furthermore, the patients undergoing pulmonary LT for recurrence showed a better PRS than those without LT. Although most of the patients with LT had SPR, a subset of patients with MPR showed long-term survival after LT, suggesting the potential role of LT in resectable pulmonary recurrence of IMA.
Overall, 60.5% of the patients showed recurrences limited to the lung parenchyma (SPR or MPR), consistent with previous studies showing that the majority of IMAs exhibit recurrences limited to the lung.6,7,8 In contrast, approximately 40% of the patients showed EPR, indicating a varied disease progression pattern observed in IMA cases.
The clinicopathologic analyses in this study showed that the SPR group had a longer DFI. These results are line with those of NSCLCs, demonstrating that patients with oligorecurrences exhibit a longer DFI.19 Notably, the SPR group showed a highly favorable OS, with a 5-year OS of 88.5%. This survival outcome is a distinct feature different from that of conventional NSCLC patients with a single recurrence18,20 and supports a recent observation in which a subset of IMAs presented peculiarly indolent biology with extremely prolonged DFI and long-term survival after LT.11 This indolent biology of IMA suggests the need for extending the postoperative surveillance period beyond 5 years. Compared with the SPR group, the MPR and EPR groups had a shorter DFI and a poorer OS. In addition, they were associated with aggressive histologic features including a micropapillary pattern and LVI. Because previous studies showed that histologic patterns and LVI are important prognostic factors,16,17 the MPR and EPR groups may represent a more aggressive subset among IMAs. Multivariate Cox regression analysis in this study also showed that recurrence pattern, high-grade histology, and LVI were prognostic factors (Tables S1 and S2).
Our study showed that patients who had pulmonary LT exhibited excellent survival. Among 15 patients who had SPR treated with LT, 11 had no further recurrent disease. Even for two patients with a second recurrence, long-term survival was observed after the second LT. The DFI was approximately 3 years for the patients who were successfully controlled by LT. Among three patients with MPR who received LT, one patient with a relatively short DFI (17.7 months) went through disease progression and died of cancer shortly after LT. Histologic examination of these tumors disclosed a micropapillary pattern and LVI. In contrast, another patient with MPR had progressive disease after LT, but the disease progressed slowly, and the patient is alive at this writing 106.7 months after LT. For this patient, the DFI was relatively prolonged (27.4 months), and both the primary and recurrent tumors showed no aggressive histologic features. Collectively, these findings suggest the potential benefit of LT for patients with recurrent IMA and show that both the clinical course and pathologic features can be helpful in predicting the recurrent IMA.
The distinct biology of IMA is schematically summarized in Fig. 5. Initially, IMAs with the most aggressive biology present as unresectable disease or recur shortly after surgical resection via both systematic (hematogenous or lymphatic) and aerogenous spread. Among these patients, those exhibiting early single pulmonary recurrence could be seen as the “tip of the iceberg.” For these patients, short-term CT follow-up periods could show the broader scope of the disease, much like detection of an iceberg's submerged portion, and help to avoid unneeded local treatments. However, IMAs with moderately aggressive biology may present with SPR (or rarely, MPR) after initial surgical resection, with an intermediate DFI (suggested as 3 years in this report). Among these tumors, a subset may be controlled by LT. Finally, for the least aggressive tumors, SPR may appear with a long latency and can be treated by LT.11 We speculate that the latter two scenarios may represent “genomically metastatic but surgically curable disease” as referenced by Goto.21 However, this condition in IMAs may better be described as pulmonary “implantation” of IMA because they may differ from the oligometastatic state.11 Because effective systemic regimens for IMAs are lacking,7 we surmise that timely performance of LT for recurrent disease can contribute to better disease control and prolonged OS in a subset of recurrent IMAs.
This study had several limitations. First, the study was a single-institution, retrospective study with potential bias. Due to the limited number of cases with recurrence, our findings need further validation based on a large multicenter cohort.
Second, during the study period of 20 years, advancements in pre- and postoperative management occurred. These advancements could potentially have influenced patients’ recurrence and survival outcomes. However, we were unable to include these temporal factors in the analysis.
Third, comprehensive genomic profiling data (i.e., next-generation sequencing), which may aid in differentiating recurrences from second primary tumors, are lacking.11,22 Although most of the previously reported metachronous IMAs were clonal,11,23 the possibility of having separate primary IMAs, although low, cannot be completely ruled out. This potential confounder could have influenced our study's outcomes. For future studies, incorporating comprehensive genomic profiling data will be essential.
In summary, our study emphasizes that IMAs show distinct patterns of intrapulmonary progression. Patients with SPR who have a longer DFI and less aggressive histology could potentially benefit from LT. Further studies involving multiple institutions are warranted to confirm our findings.
Abbreviations
- IMA:
-
Invasive mucinous adenocarcinoma
- KRAS:
-
Kirsten rat sarcoma virus
- LT:
-
Local therapy
- NSCLC:
-
Non-small cell lung cancer
- LVI:
-
Lymphovascular invasion
- VPI:
-
Visceral pleural invasion
- CT:
-
Computed tomography
- PRS:
-
Post-recurrence survival
- SPR:
-
Solitary pulmonary recurrence
- MPR:
-
Multiple pulmonary recurrence
- EPR:
-
Extra-pulmonary recurrence
- RT:
-
Radiation therapy
- OS:
-
Overall survival
- DFI:
-
Disease-free interval
References
Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64.
Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–46.
Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Borczuk AC. WHO classification of tumours: thoracic tumours. Lyon, France: International Agency for Research on Cancer; 2021.
Chang JC, Offin M, Falcon C, et al. Comprehensive molecular and clinicopathologic analysis of 200 pulmonary invasive mucinous adenocarcinomas identifies distinct characteristics of molecular subtypes. Clin Cancer Res. 2021;27:4066–76.
Shim HS, Kenudson M, Zheng Z, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2015;10:1156–62.
Cha YJ, Kim HR, Lee HJ, Cho BC, Shim HS. Clinical course of stage IV invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2016;102:82–8.
Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6:508–12.
Duruisseaux M, Antoine M, Rabbe N, et al. The impact of intracytoplasmic mucin in lung adenocarcinoma with pneumonic radiological presentation. Lung Cancer. 2014;83:334–40.
Wislez M, Massiani MA, Milleron B, et al. Clinical characteristics of pneumonic-type adenocarcinoma of the lung. Chest. 2003;123:1868–77.
Yang SR, Chang JC, Leduc C, et al. Invasive mucinous adenocarcinomas with spatially separate lung lesions: analysis of clonal relationship by comparative molecular profiling. J Thorac Oncol. 2021;16:1188–99.
Jasper K, Stiles B, McDonald F, Palma DA. Practical management of oligometastatic non-small cell lung cancer. J Clin Oncol. 2022;40(6):635–41.
Isbell JM, Li BT, Gomez DR. The emerging role of local therapy in oligometastatic non-small cell lung cancer. J Thorac Cardiovasc Surg. 2022;163:819–25.
Travis WD. The 2015 WHO classification of lung tumors. Pathologe. 2014;35(Suppl 2):188.
Gow C-H, Hsieh M-S, Liu Y-N, Lee Y-H, Shih J-Y. Clinicopathological features and survival outcomes of primary pulmonary invasive mucinous adenocarcinoma. Cancers. 2021;13:4103.
Hwang S, Han J, Choi M, Ahn MJ, Choi YS. Size of non-lepidic invasive pattern predicts recurrence in pulmonary mucinous adenocarcinoma: morphologic analysis of 188 resected cases with reappraisal of invasion criteria. J Pathol Transl Med. 2017;51:56–68.
Chang WC, Zhang YZ, Lim E, Nicholson AG. Prognostic impact of histopathologic features in pulmonary invasive mucinous adenocarcinomas. Am J Clin Pathol. 2020;154:88–102.
Sonoda D, Matsuura Y, Kondo Y, et al. A reasonable definition of oligo-recurrence in non-small cell lung cancer. Clin Lung Cancer. 2022;23:82–90.
Hishida T, Yoshida J, Aokage K, Nagai K, Tsuboi M. Postoperative oligo-recurrence of non-small cell lung cancer: clinical features and survival. Eur J Cardio-Thorac Surg. 2016;49:847–53.
Han SJ, Cho S, Yum S, Kim K, Jheon S. Surgical treatment of pulmonary oligorecurrence after curative resection for non-small cell lung cancer. Interactive CardioVasc Thorac Surg. 2020;30:18–23.
Goto T. Genomically metastatic, but surgically curable? J Thorac Oncol. 2022;17:e49-50.
Chang JC, Alex D, Bott M, et al. Comprehensive next-generation sequencing unambiguously distinguishes separate primary lung carcinomas from intrapulmonary metastases: comparison with standard histopathologic approach. Clin Cancer Res. 2019;25:7113–25.
Kim M, Hwang J, Kim KA, et al. Genomic characteristics of invasive mucinous adenocarcinoma of the lung with multiple pulmonary sites of involvement. Mod Pathol. 2022;35:202–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare no potential conflict of interest. There is no source of any financial or material support..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, D.W., Hwang, S., Hong, T.H. et al. Distinct Recurrence Pattern and Survival Outcomes of Invasive Mucinous Adenocarcinoma of the Lung: The Potential Role of Local Therapy in Intrapulmonary Spread. Ann Surg Oncol 31, 201–212 (2024). https://doi.org/10.1245/s10434-023-14373-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14373-8