Abstract
Background
Identification of risk factors facilitates the prevention of breast cancer-related lymphedema (BCRL). Several published systematic reviews have already addressed the risk factors for BCRL. This study aimed to systematically identify potential risk factors for BCRL and evaluate the quality of evidence.
Methods
The study followed methodologic guidance from the Joanna Briggs Institute, and the Cochrane Handbook. The following electronic databases were systematically searched from inception to 15 November 2022: PubMed, Embase, CINAHL, Web of Science, Scopus, CNKI, SinoMed, Wanfang, JBI Database, Cochrane Database, ProQuest, and PROSPERO. Two authors independently screened studies, extracted data, and assessed methodologic quality using AMSTAR2, risk of bias using ROBIS, and evidence quality using GRADE. The study evaluated overlap, assessed the small-study effect, and calculated the I2 statistic and Egger’s P value as needed.
Results
The study included 14 publications comprising 10 meta-analyses and 4 systematic reviews. The authors identified 39 factors and 30 unique meta-analyses. In the study, 13 innate personal trait-related risk factors, such as higher body mass index (BMI) and axillary lymph nodes dissection, showed statistically significant associations with BCRL incidence. Breast reconstruction was found to be a protective factor. The methodologic quality was low or critically low. The majority of the systematic reviews and/or meta-analyses were rated as having a high risk of bias. Evidence quality was low for 22 associations and moderate for 8 associations.
Conclusions
The currently identified risk factors for BCRL all are innate personal trait-related factors. Future well-designed studies and robust meta-analyses are needed to explore potential associations between behavioral-, interpersonal-, and environmental-related factors and BCRL, as well as the role of genetic variations and pathophysiologic factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As reported in this study, breast cancer-related lymphedema (BCRL) affected approximately one in five women treated for breast cancer.1 Chronic, progressive, and uncurable, BCRL is caused by an abnormal accumulation of protein-rich lymph fluid in the interstitial spaces due to disruption of the lymphatic system, which manifests as swelling of limb, hand, breast, or chest wall.2
Patients with BCRL experience decreased quality of life accompanied with discomfort symptoms (e.g., swelling, numbness, pain), functional limitations, body image disturbance, sexuality problems, economic burden, and other related psychosocial problems.2,3,4
The contribution of axillary surgery and radiation of regional lymph to the development of BCRL is widely acknowledged.5 An increasing amount of research evidence has demonstrated that the etiology of BCRL is multifaceted and influenced by both unmodifiable factors (e.g., treatment regimens and lymphatic system recovery capacity) and potentially modifiable factors (e.g., body mass index [BMI] and subclinical edema).3,6 Risk factors are characteristics, traits, or exposures that increase an individual’s possibility of experiencing a condition.7 Identification of risk factors, especially modifiable risk factors, offers novel insights into the prevention of BCRL.
In the last two decades, numerous studies have been conducted to investigate potential risk factors associated with the development of BCRL, with a primary focus on sociodemographic, disease, and treatment-related factors. However, the traditionally studied risk factors can provide only a partial explanation for the development of BCRL.
During the past few years, several hypotheses have been proposed to explain the pathogenesis of BCRL. Among these, the lymphatic-failure hypothesis, the hemodynamic hypothesis, and the interstitial hypothesis have received the most attention.8 Despite these efforts, the pathogenesis of BCRL remains incompletely understood. Recent research has indicated that the pathogenesis of secondary lymphedema may involve pathophysiologic factors such as vascular endothelial growth factor C (VEGF-C), Monocyte chemoattractant protein-1 (MCP-1), cluster of differentiation 4+ (CD4+) cells, and genetic predispositions including genetic variations in interleukin (IL), including IL4, IL6, and the like.9,10
Some researchers have evaluated and consolidated the existing evidence on individual or multiple categories of risk factors for BCRL.11,12 Readers, including health care professionals, researchers, and knowledgeable patients, may find it challenging to comprehend information from these systematic reviews (SRs) and/or meta-analyses (MAs), which sometimes present conflicting results. For example, regarding whether older age contributes to the risk of BCRL, one systematic review suggested that age alone did not significantly increase the risk,13 whereas another systematic review concluded that older age was associated with the increase of BCRL incidence.14
Despite numerous systematic reviews on the risk factors for BCRL, a comprehensive and concise research summary applicable to clinical practice still is lacking. An umbrella review, which aims to synthesize the results of SRs/MAs on a certain topic and inform evidence-based clinical practice, would be the most appropriate approach to achieve this goal. Therefore, this umbrella review sought to comprehensively identify, appraise, and synthesize the results of published SRs/MAs that examine the risk factors associated with the development of BCRL and to provide an understandable and comprehensive review that can inform evidence-based clinical practice.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations were followed (Supplemental File 1).15 This umbrella review was conducted under the guidance of the Joanna Briggs Institute Manual for Evidence Synthesis of Umbrella Reviews16 and the Cochrane Handbook for systematic reviews,17 as well as other methodologic articles.18 The protocol had been registered in PROSPERO (CRD42022375710) and published online.19
Information Sources and Search Strategy
The following electronic databases were systematically searched from inception to 15 November 2022: PubMed, Embase, CINAHL, Web of Science, Scopus, CNKI, SinoMed, the Wanfang database, the Joanna Briggs Institute (JBI, Adelaide, Australia) Database of Systematic Reviews and Implementation Reports, the Cochrane Database of Systematic Reviews, the PROSPERO register, and ProQuest Dissertations. Medical subject headings (MeSH) terms and keywords were used in combination. The search terms and detailed search strategies are shown in Supplementary File STable 3. We also hand-searched the reference lists of the included articles for additional studies.
Study Selection and Eligibility
All records were managed by Endnote X9 (Clarivate Analytics, Philadelphia, PA). After de-duplication, two independent authors (A.S., J.B.) screened all the titles and abstracts. Any records identified as potentially eligible by at least one author were retrieved for full-text reading. All discrepancies were discussed and resolved by consensus.
The eligibility criteria based on PECOs (Population, Exposure, Control, Outcomes, Study design) statement were as follows:20 population (SRs/MAs investigating risk factors for BCRL among adult breast cancer survivors [age >18 years] with a history of breast cancer surgery, exposure (SRs/MAs reporting at least one clearly defined risk factor), outcomes (breast cancer-related limb lymphedema used as one of the primary outcomes with definite diagnostic criteria, e.g., relative volume change or relative arm volume increase [RAVI] ≥ 200 mL or 10%),3 and study design (consideration of only SRs/MAs that described an explicit and reproducible methodology including literature search and eligibility, study selection and extraction, quality appraisal, and quantitative or qualitative synthesis). The review included only primary studies with cohort, cross-sectional, and case-control design and secondary analysis of randomized controlled trials.
The review excluded (1) articles reporting studies of patients with recurrent breast cancer, metastatic disease, primary lymphedema, or lymphedema secondary to other diseases; (2) studies that recruited participants with acute lymphedema occurring within 3 months after breast cancer diagnosis or surgery, latent or subclinical lymphedema with an RAVI lower than 3%, or breast or trunk lymphedema; and (3) publications without full-text, conference abstracts, or protocols. No language restrictions were applied. Articles in other languages were translated by google translator for assessment and extraction.
Data Extraction
Two authors (A.S., L.Z.) independently extracted data using a predesigned data extraction form. The following data were extracted: first author, year of publication, country, participants’ characteristics, total number of participants, number of lymphedema cases, search strategy (sources searched, range of years, number of studies included), types of studies included, quality appraisal (instruments and results), outcomes of significance, and results/findings. For meta-analyses, effect sizes (random-effect size and/or fixed-effect size, odds ratio [OR], risk ratio [RR], hazard ratio [HR] for binary measures or standardized mean difference [SDM] for continuous measures, with 95% confidence interval [CI]), value of I2, significance levels, publication bias, and small-study effects also were extracted.
If multiple meta-analyses investigated the same risk factor, we usually chose the most recently published meta-analysis with the largest number of original studies.18 For studies without quantitative synthesis, we documented a summary statement detailing the authors’ primary findings and the rationale for not attempting a quantitative synthesis. All eligible meta-analyses used summary-level data from published literature. Due to the large number of primary studies included, we did not extract the data from the original studies as planned. Any discrepancies were solved through discussion or consultation with a third author.
Methodologic- and Evidence-Quality Assessments
Quality assessment was performed by two authors independently (A.S., J.B.). Any disagreement was resolved through discussion or by consulting a third author to reach a consensus.
Methodologic-Quality Assessment
The Assessing the Methodological Quality of Systematic Reviews-2 (AMSTAR-2) guidelines and checklist21 were used to assess the methodologic quality of SRs/MAs. In AMSTAR-2 (www.amstar.ca), 16 items assess study eligibility criteria, identification and selection of studies, data collection methods, study appraisal methods and findings, and synthesis methods. Each item can be rated as “yes,” “no,” or “partially yes.” Items 2, 4, 7, 9, 11, 13, and 15 are considered to be critical items. Overall confidence can be rated as 1 (high quality: no or only one non-critical weakness), 2 (moderate quality: more than one non-critical weakness, but no critical item weakness), 3 (low quality: one critical item weakness, with or without a non-critical item weakness, and 4 (critically low quality: more than one critical item weakness, with or without a non-critical item weakness).
Risk-of-Bias Assessment
We assessed the risk of bias with the Risk of Bias in Systematic Reviews (ROBIS) tool,22 which consists of three phases: (1) relevance assessment (optional), (2) identification of concerns with the review process, and (3) judgment on the risk of bias. Phase 2 covers four domains: study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. Phase 3 determines the overall risk of bias in the interpretation of review findings while taking into account the limitations identified in phase 2. Signaling questions are included to help judge concerns with the review process, which should be answered as “yes,” “probably yes,” “probably no,” “no,” or “no information. The overall risk of bias is judged as “low,” “high,” or “unclear.”
Evidence-Quality Assessment
We also assessed the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.23 In the GRADE system, the level of evidence is divided into four categories: high, moderate, low, and very low. The quality of evidence is primarily determined by the study design, with observational studies initially assigned a low level of certainty. The certainty of evidence is rated as low when there are no reasons to downgrade, and very low if there is at least one reason to downgrade the certainty of evidence. When there are some reasons to upgrade the certainty of evidence (e.g., strong association), with no other reasons to downgrade, the results of observational studies could be upgraded to the level of ‘moderate.’
Overlap Assessment
The degree of overlap between the included SRs/MAs was assessed by creating citation matrices and calculating the “Corrected Covered Area” (CCA)24 using the following formula:
where n refers to all the original studies included, r denotes all the original studies included after deduplication, and c is the number of studies included in the umbrella review. The overlap can be classified into four levels based on the results of CCA as follows: slight overlap (0–5), moderate overlap (6–10), high overlap (11–15), and very high overlap (> 15). The overlap was reported and recognized as a limitation if necessary.
Data Analysis
We extracted the effect size and a 95% CI for each risk factor from the included SRs/MAs. For instances in which both a random-effects model and a fixed-effects model were applied to analyze the same risk factor, we predominantly extracted the former as the final outcome. The measures of heterogeneity and publication bias in relevant meta-analyses were obtained by extracting the I2 value of the Egger’s test and the P value of the Begg’s test. If these data were absent from the meta-analyses, the I2 statistic was computed to evaluate heterogeneity, and the Egger’s test was performed to assess the publication bias, provided that detailed primary data were available.
Significant heterogeneity was defined as I2 greater than 50%, whereas statistically significant publication bias was indicated by a P value lower than 0.1 for Egger’s or Begg’s test. We assessed whether there was evidence for small-study effects. When the effect size of the largest study was more conservative than the summary effect size of the random-effects meta-analysis and the P value of Egger’s test was less than 0.1, this possibly indicated the presence of small-study effects, in which smaller studies tended to yield significantly larger estimates of effect size than larger studies.25
Results
Study Selection Results
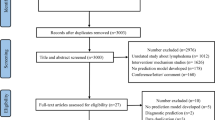
The literature search identified 401 records. One record was obtained by tracking reference lists of the included studies. Before the screening, 175 duplicated records were removed automatically by Endnote and manually by hand. Then, after screening 226 records, the study excluded 167 records. Of the remaining 59 articles, 51 were retrieved for full-text reading. Finally, 14 publications10,11,12,14,26,27,28,29,30,31,32,33,35 were included in the umbrella review (see Supplementary File 3 for studies excluded with reasons). The search results and the selection process are detailed in Fig 1.
Characteristics of the Included Studies
Of the included articles, 4 were SRs without quantitative synthesis,10,14,27,32 and 10 were SRs with meta-analyses.11,12,26,28,29,30,31,33,35 Five SRs/MAs were performed by authors from China, two by authors from America, two by authors from Australia, and the others by authors from Brazil, England, Netherlands, Greece, and Burundi (Tables 1, 2). Of the included SRs/MAs, 79% (11/14) were published in the last 5 years, with the earliest one published in 2013. The number of original studies included in the SRs/MAs ranged from 6 to 72, with 6 to 57 of these original studies related to risk factors for BCRL. The total number of participants recruited ranged from 1379 to 28,615.
Only two SRs/MAs were registered. Five SRs/MAs were reported following PRISMA, with two of them additionally adhering to MOOSE (Checklist for Meta-Analyses of Observational Studies). A total of 283 primary studies were included. After deduplication, 176 primary studies were retained. According to the formula of CCA = (283 – 176)/(176 *14 – 176) = 0.047, the primary studies included were slightly overlapped, which was not likely to have an impact on the conclusion. The citation overlap matrix is shown in Supplementary File STable 3.
Risk Factors for Breast Cancer-Related Lymphedema
The included SRs/Mas reported 39 risk factors. These risk factors could be categorized according to the Health Ecological Model as follows:36 (1) innate personal trait-related factors (n = 29): gene variations, age, race, BMI, presence of comorbidities, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), history of limb damage, tumor stage, lymph node status, pathologic T classification, higher nodal ratio, treatment on the dominant side, axillary lymph node dissection (ALND) (vs. sentinel lymph node biopsy [SLNB]), level of ALND, type of breast surgery (mastectomy vs. lumpectomy), number of lymph nodes (LNs) dissected, number of metastatic LNs, chemotherapy, radiotherapy, axillary radiotherapy, breast reconstruction, tissue expander/implant reconstruction (vs. autologous reconstruction), endocrine therapy, postoperative infection, subcutaneous effusion, presence of at least mild upper-body symptoms, post-radiotherapy moist desquamation; (2) behavioral lifestyle-related factors (n = 4): smoking, non-participation in regular physical activity, non-engagement in preventive self-care activities, blood pressure readings taken on the treated side; (3) interpersonal network-related factors (n = 2): marital status, children in care age 14 years or younger.
(4) Socioeconomic status-related factors (n = 4): education, income, employment status, occupation requiring a high level of hand use.
(5) Macro-environment-related factors (n = 1): no pretreatment education of BCRL received.
Without any additional quantitative synthesis, the four SRs reported evidence supporting associations between BCRL and 23 gene variations (including HGF, VEGF-C, MET, KDR, FLT4, NRP2, GJC2, GJA4, IL1A, IL4, IL6, IL10, IL13, NFKB2, FOXC2, RORC, LCP2, KCNA1, KCNJ3, KCNJ6, KCNK3, SYK, VCAM1), age, BMI, type of breast cancer surgery, and the like based on descriptive synthesis. Kapellas et al.10 updated the results of Visser et al.32 by including two new studies and adding five genes (GJA4, KCNA1, KCNJ3, KCNJ6, KCNK3). Guliyeva et al.14 performed a systematic review including seven studies to evaluate the relationship between age and the development of BCRL. All the authors except Disipio et al.27 declared that quantitative synthesis was not feasible due to significant heterogeneity among methods, study design, and outcome reporting, as well as other differences.
Among the 10 MAs, four articles focused on single risk factors, with two articles on BMI (Manirakiza et al.29, Wu et al.33), one article on breast reconstruction surgery (Siotos et al.30), and the remaining article on radiotherapy (Kanda et al.28). As shown in Table 3, 30 unique meta-analyses on certain risk factors were provided. The median number of included studies was eight (range 2–33). Of the meta-analyses, 20 were performed with the random-effects model and 10 with the fixed-effects model. Half of these meta-analyes showed significant heterogeneity, with an I2 greater than 50%. The 25 meta-analyses with publication bias evaluation (one with a funnel plot and the others with an Egger’s test) had significant publication bias in associations between the level of ALND and postoperation infection and BCRL, with an Egger’s P value lower than 0.1. Small study effects also were detected in these two meta-analyses.
Innate Personal Trait-Related Risk Factors
The majority of the MAs (87%) studied risk factors of innate personal traits, with none relevant to the macro-environments domain and 26 identified as focusing on 22 risk factors for BCRL. Higher BMI, hypertension, advanced tumor stage (stage ≥ II vs. stages 0 and I), advanced pathologic T classification, ALND, expanded level of ALND, more LNs dissected (> 15 vs. ≤ 15), more positive LNs, presence of postoperative complications, postoperative infection, subcutaneous effusion, and reception of chemotherapy and radiotherapy were demonstrated to be risk factors for BCRL. Patients undergoing ALND experienced a 13.7% increase in BCRL incidence compared with those undergoing SLNB (n = 19; pooled SMD, 0.137 [95% CI 0.105–0.168], I2 = 97.40%).26 Breast reconstruction was found to be a protective factor for the occurrence of BCRL (n = 16; pooled OR, 0.66 [95% CI 0.55–0.79], I2 = 23%).30 The associations between age (≥ 60 vs. < 60 years), race (African American vs. Caucasian), COPD, diabetes, type of breast surgery (mastectomy vs. lumpectomy), type of breast reconstruction (tissue expander/implant reconstruction vs. autologous reconstruction), side of treatment (dominant side vs. non-dominant side), and endocrine therapy were not statistically significant.
Behavioral Lifestyle-Related Risk Factors
For behavioral lifestyle-related factors, only one meta-analysis on smoking was included.11 However, pooled analysis showed that smoking was not a risk factor (n = 4; pooled OR, 1.04 [95% CI 0.83–1.30], I2 = 0%). Other potential risk factors such as regular physical activity, preventive self-care activities, and blood pressure readings taken on the treated side were mentioned only in qualitative description without meta-analyses.
Interpersonal Network-Related Risk Factors
Two MAs on marital status (married vs. unmarried) were reported among the included systematic reviews (Zhu et al.,11 Chen et al.34), and both showed insignificant pooled odds ratios. We retained the meta-analysis with more primary studies on marital status (n = 8; pooled OR, 0.88 [95% CI 0.77–1.01], I2 = 43%) from the article of Chen et al.34 Disipio et al.27 reviewed children 14 years of age or younger in care as a possible risk factor with evidence only from a prospective cohort study (OR 0.2).
Socioeconomic Status-Related Risk Factors
Four possible socioeconomic status-related risk factors requiring a high level of hand use (education, income, employment status, and occupation) were identified by the included systematic reviews. Education (high school or above vs. below: n = 6; pooled OR, 1.00; 95% CI 0.71–1.41)\34 and employment status (employed vs. unemployed: n = 3; pooled OR, 1.37; 95% CI 0.86–2.20)11 were supported by meta-analyses. However, the pooled effect sizes of both factors were not statistically significant.
Macro-environments-Related Risk Factors
No meta-analysis was identified for this domain of risk factors. No pretreatment education on BCRL was mentioned as a possible risk factor by one included systematic review (Disipio et al.27), and we classified this factor as a macro-environments-related factor because it reflected the health care quality patients received during breast cancer treatment.
Methodologic Quality, Risk of Bias, and Evidence Quality
With the AMSTAR2, the methodologic quality of seven SRs/MAs was evaluated as low, whereas the remaining seven SRs/MAs were evaluated as critically low. To be specific, not all the SRs/MAs reported on the sources of funding for the included studies. In addition, items on prior established protocols (n = 12), data extraction in duplicate (n = 6), justification of study design (n = 6), meta-analysis assessing the impact of risk of bias (n = 6), interpretation/discussion of results including the risk of bias of studies (n = 5), and investigation of publication bias in the meta-analysis (n = 5) generally were not met, which resulted in overall low methodologic quality (Table 4).
Table 5 and Fig. 2 show the results of the risk-of-bias assessment using ROBIS. The risk of bias was high in 12 of the SRs/MAs. Only one meta-analysis was judged as having a low risk of bias, and the risk of bias in one meta-analysis was unclear. Domain 2 (Identification and Selection of Studies) showed the highest risk of bias, with 10 SRs/MAs classified as a high bias risk. Seven SRs/MAs were at a high bias risk on Domain 4 (Synthesis and Findings). Six SRs/MAs were at a high bias risk on Domain 1 (Study Eligibility Criteria), and three SRS/MAs were evaluated as having a high risk of bias on Domain 3 (Data Collection and Study Appraisal).
This umbrella review identified 30 unique risk factors with meta-analyses. The GRADE assessment of evidence quality identified 22 risk factors as having low-quality evidence and 8 risk factors as having moderate-quality evidence, which were upgraded due to strong associations (OR > 2; Table 3). Additionally, high heterogeneity (15 meta-analyses with I2 > 50%) and the small number of included studies (16 meta-analyses with fewer than 10 studies) also decreased the overall evidence quality.
Discussion
To our knowledge, the current umbrella review is the first effort to comprehensively review the risk factors for BCRL, assess the robustness of associations, and grade the available evidence accordingly. From 14 included SRs/MAs, 39 risk factors for BCRL and 30 associations with meta-analyses were identified. The findings show a statistically significant association of 14 factors with the occurrence of BCRL including BMI, hypertension, tumor stage, pathologic T classification, ALND, level of ALND, number of LNs dissected, number of positive LNs, postoperative complications, postoperative infection, subcutaneous effusion, chemotherapy, radiotherapy, and breast reconstruction.
We classified the identified risk factors for BCRL into five domains based on the Health Ecological Model put forward by Bronfenbrenner.36 The Health Ecological Model emphasizes that the health status and outcome of individuals or populations are the result of multiple and multi-level factors, including innate personal traits, behavioral lifestyle, interpersonal networks, socioeconomic status, and macro-environments.37 However, the majority of the identified factors were related to innate personal traits, with few other domains of influencing factors. This highlights that despite extensive research on the risk factors for BCRL, a lack of attention still is given to behavioral, interpersonal, and socio-environmental-related factors, which are modifiable and valuable for lymphedema prevention. Considering that the development of BCRL is a lifelong risk for breast cancer patients, further original research is necessary to explore the potential impact of these factors on the occurrence and development of BCRL.
Body mass index has always been a highly scrutinized risk factor for BCRL. Two included MAs focused exclusively on BMI.29,33 Seven meta-analyses on the association between BMI and BCRL were identified from seven included publications, with consistent findings. According to our results, a higher BMI is a significant risk factor for BCRL, with the magnitude of risk increasing across higher categories of BMI (< 25, 25–30, ≥ 30 kg/m2).29
The mechanisms underlying the association between higher BMI and lymphedema development remain unclear, but some hypotheses suggest that lipid accumulation may impede lymphatic fluid transport due to chronic inflammation.29 Body weight management is highly beneficial for the prognosis of postoperative breast cancer patients, not only in terms of preventing lymphedema but also in terms of promoting overall health.38 Health care providers should offer guidance and support to help breast cancer patients develop a personalized weight management plan (e.g., dietary control) and exercise guidance.
Controversy exists among multiple studies and SRs/MAs regarding whether age is a contributing factor for BCRL.14 This umbrella review confirmed that older age does not increase the risk of BCRL. However, a systematic review of Guliyeva et al.14 noted that age was possibly associated with the severity of BCRL. This highlights the importance of targeting elderly breast cancer patients as a key population for lymphedema prevention.
Breast cancer patients with hypertension were found to have a 4.76-fold risk of BCRL versus those without hypertension.35 But this association has been supported only by studies of the Chinese breast cancer population. Further research is required to verify this association among other populations.
Cancer- and treatment-related factors dominate the innate personal trait-related factors for BCRL. The association between tumor stage and the risk of BCRL has been supported by many previous studies.27 It could be explained that breast cancer patients with more advanced tumor stages usually undergo more extensive surgery, which would cause more damage to the lymphatic system.31 Similarly, we found that advanced pathologic T classification also increased BCRL risk. Once again, reception of ALND (vs. SLNB or non-ALND), expanded level of ALND, more LNs dissected, more positive LNs, reception of chemotherapy, radiotherapy, and postoperative complications (infection, subcutaneous effusion), which were commonly recognized, have proved to be risk factors for the BCRL.
Moreover, we found that breast reconstruction surgery protected breast cancer patients from BCRL risk. Siotos et al.30 performed a meta-analysis especially on the association between breast reconstruction surgery and the risk for the development of BCRL and showed that breast reconstruction was associated with lower rates of lymphedema than mastectomy or breast-conserving surgery. Identifying the aforementioned risk factors can serve as a reference that health care providers and breast cancer patients can use in making reasonable treatment decisions.
Genetic variations leading to lymphedema were traditionally classified as primary lymphedema, whereas secondary lymphedema often occurs after trauma or cancer treatment, particularly after surgery and/or radiation therapy to the axilla in breast cancer patients.10 Two included SRs examined the genetic predisposition to BCRL. They showed that 23 genes (including HGF, VEGF-C, and the like), mainly related to lymph-angiogenesis and angiogenesis, have genetic variations in patients with BCRL.10,32 A significant overlap was found between these genetic variations and those mutated in primary lymphedema.
These findings highlight the importance of genetic susceptibility in the development of BCRL, altering the traditional perception of its iatrogenic etiology. In this era of precision medicine, taking the genetic perspective into account when the risk of BCRL is assessed provides a novel approach for the precise prediction and management of BCRL. Additional well-designed research is needed given the low level of evidence and the considerable heterogeneity of available evidence.
Recent research has indicated that pathophysiologic factors, such as VEGF-C, MCP-1, and CD4+ cells, may contribute to the development of secondary lymphedema.9 However, none of the included SRs/MAs addressed pathophysiologic factors due to limited primary studies, which also hints the direction for future research.
High-quality SRs/MAs are essential to support health care decision-making. We assessed the methodologic quality of the included SRs/MAs using both AMSTAR2 and ROBIS, which were basically similar, but with some differences.39 However, the overall quality was low with both AMSTAR2 and ROBIS, indicating that the review may have had significant flaws and thus may not be entirely reliable. In the AMSTAR2 assessment, none of the included SRs/MAs scored items regarding sources of funding reports, raising the possibility of potential conflicts of interest with commercial entities.21
Adherence to well-developed protocols reduces the risk of bias in a review, but the protocols of the included SRs/MAs were seldom registered or reported.21 The common reasons for risk of bias based on ROBIS included failure to search unpublished literature, no additional methods to identify relevant records, no bias control in data extraction, and the like.22
It is worth mentioning that the included publications were poorly reported, with only one third following the reporting checklist of PRISMA or MOOSE. We believe this could partially explain the low quality of the SRs/MAs because lack of clarity on methodologic details also lowers the quality. Notably, both AMSTAR2 and ROBIS primarily evaluate the process of conducting SRs/MAs rather than the quality of the included primary studies.
In addition to the methodologic quality assessment, we used GRADE to assess evidence quality of the meta-analyses on each association.23 Given that the SRs of risk factors included only observational studies, the evidence was considered to be low by default. Additionally, the evidence quality of the included meta-analyses was not upgraded by considerations of dose-response relationships, controlling for confounding factors, and strong effect sizes. In summary, future research should focus on adhering strictly to methodologic guidance and reporting checklists to provide high-quality evidence.
This study used an umbrella review to systematically identify potential risk factors for BCRL that can inform the inclusion of variables in BCRL risk-prediction models, thereby enhancing the prediction performance of such models. Additionally, the results of our study can assist physicians and patients in gaining a better understanding of an individual breast cancer patient’s risk of experiencing BCRL, which can facilitate informed treatment decisions and promote patients’ lymphedema self-management adherence.
Furthermore, identification of high-risk populations for BCRL enables the development and implementation of prospective surveillance programs and precise prevention strategies, thus improving the efficiency of BCRL prevention and management. By clarifying currently available risk factors in SRs/MAs and assessing the quality of existing evidence, this umbrella review may contribute to a more thorough understanding of the associations between potential risk factors (from pathophysiologic factors to lifestyle-related behavior factors) and the development of BCRL. Meanwhile, we also enhance the needs and provide directions for future research in genetic predisposition, pathophysiologic factors, and behavioral-, interpersonal-, and environmental-related factors for BCRL.
Study Limitations
Several limitations of this review need to be declared. First, although CCA was calculated to estimate the degree of overlap, its impact cannot be removed, which would have made the results biased by inflating the associations. Second, we considered only evidence synthesized in SRs/MAs, which may have excluded relevant primary studies. Third, we did not extract the data from original studies included in the SRs/MAs, which led to stratification of evidence not being performed as planned. Finally, based on the available SRs/MAs, we failed to synthesize evidence on pathophysiologic factors for the development of BCRL. Future efforts should be made to study possible pathophysiologic factors or the development of BCRL by primary research or systematic reviews if possible.
Conclusions
In summary, this umbrella review identified 39 potential factors for BCRL within five domains of the Health Ecological Model based on 14 SRs/MAs. The risk factors for BCRL were higher BMI, hypertension, advanced tumor stage, higher pathologic T classification, ALND, higher level of ALND, more LNs dissected, more positive LNs, postoperative complications, postoperative infection, subcutaneous effusion, chemotherapy, and radiotherapy. Breast reconstruction was a protective factor. Our findings contribute to a better understanding of the association between potential risk factors and BCRL and can provide valuable information to both health care providers and breast cancer patients regarding BCRL risk prediction, precise prevention, and management. However, considering the low quality of the SRs/MAs, significant risk of bias, and low level of evidence for most associations, we recommend more well-conducted cohort studies and robust meta-analyses. Furthermore, future research should explore other potential unproven risk factors (genetic variations, pathophysiologic factors, and behavioral-, interpersonal-, and environmental-related factors) with rigorous studies.
References
Shen A, Lu Q, Fu X, et al. Risk factors of unilateral breast cancer-related lymphedema: an updated systematic review and meta-analysis of 84 cohort studies. Support Care Cancer. 2022;31:18.
Pappalardo M, Starnoni M, Franceschini G, Baccarani A, De Santis G. Breast cancer-related lymphedema: recent updates on diagnosis, severity, and available treatments. J Pers Med. 2021;11:402.
McLaughlin SA, Brunelle CL, Taghian A. Breast cancer-related lymphedema: risk factors, screening, management, and the impact of locoregional treatment. J Clin Oncol. 2020;38:2341–50.
Rockson SG. Lymphedema after breast cancer treatment. N Engl J Med. 2019;380:694.
Tandra P, Kallam A, Krishnamurthy J. Identification and management of lymphedema in patients with breast cancer. J Oncol Pract. 2019;15:255–62.
Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg. 2018;7:379–403.
Offord DR, Kraemer HC. Risk factors and prevention. Evid Based Ment Health. 2000;3:70–1.
He L, Qu H, Wu Q, Song Y. Lymphedema in survivors of breast cancer. Oncol Lett. 2020;19:2085–96.
Brown S, Dayan JH, Kataru RP, Mehrara BJ. The vicious circle of stasis, inflammation, and fibrosis in lymphedema. Plast Reconstr Surg. 2023;151:330e-e341.
Kapellas N, Demiri E, Lampropoulos A, Dionyssiou D. Genetic predisposition in cancer-related lymphedema: a systematic review. Lymph Res Biol. 2022;20:478–87.
Zhu YQ, Xie YH, Liu FH, et al. Systemic analysis on risk factors for breast cancer-related lymphedema. APJCP Asian Pac J Cancer Prev. 2014;15:6535–41.
Lin Y, Xu Y, Wang C, et al. Loco-regional therapy and the risk of breast cancer-related lymphedema: a systematic review and meta-analysis. Breast Cancer. 2021;28:1261–72.
Guliyeva G, Huayllani MT, Boczar D, et al. Age as a risk factor for breast cancer-related lymphedema: a systematic review. J Cancer Surviv. 2021;17(1):246.
Guliyeva G, Huayllani MT, Boczar D, Avila FR, Forte AJ. Correlation of older age with severity of lymphedema in breast cancer survivors: a systematic review. Breast Dis. 2021;40:191–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Aromataris E, Munn Z. JBI Manual for Evidence Synthesis. JBI; 2020.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Ed. Chichester, UK: John Wiley & Sons; 2019.
Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13:132–40.
Shen A, Lu Q, Zhang L, et al. Risk factors of breast cancer-related lymphoedema: protocol of an umbrella review. BMJ Open. 2023;13:e070907.
Morgan RL, Whaley P, Thayer KA, Schunemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121(Pt 1):1027–31.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Whiting P, Savovic J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34.
Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6.
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368–75.
Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–73.
Che Bakri NA, Kwasnicki RM, Khan N, et al. Impact of axillary lymph node dissection and sentinel lymph node biopsy on upper limb morbidity in breast cancer patients: a systematic review and meta-analysis. Ann Surg. 2022;277:572.
Disipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:1077–4114 ((print)(6): 500–515).
Kanda MH, da Costa Vieira RA, Lima JPSN, Paiva CE, Cunha de Araujo RL. Late locoregional complications associated with adjuvant radiotherapy in the treatment of breast cancer: systematic review and meta-analysis. J Surg Oncol. 2020;121:766–76.
Manirakiza A, Irakoze L, Shui L, Manirakiza S, Ngendahayo L. Lymphoedema after breast cancer treatment is associated with higher body mass index: a systematic review and meta-analysis. East Afr Health Res J. 2019;3:178–92.
Siotos C, Sebai ME, Wan EL, et al. Breast reconstruction and risk of arm lymphedema development: a meta-analysis. J Plast Reconstr Aesth Surg. 2018;71:807–18.
Torgbenu E, Luckett T, Buhagiar MA, Chang S, Phillips JL. Prevalence and incidence of cancer-related lymphedema in low and middle-income countries: a systematic review and meta-analysis. BMC Cancer. 2020;20:1–20.
Visser J, van Geel M, Cornelissen AJM, van der Hulst RRWJ, Qiu SS. Breast cancer-related lymphedema and genetic predisposition: a systematic review of the literature. Lymph Res Biol. 2019;17:288–93.
Wu R, Huang X, Dong X, Zhang H, Zhuang L. Obese patients have higher risk of breast cancer-related lymphedema than overweight patients after breast cancer: a meta-analysis. Ann Translat Med. 2019;7:172.
Chen C, Gu W, Chen Y, et al. Risk factors for postoperative breast cancer-related lymphedema: a meta-analysis. Chin Evid Based Nurs. 2021;7:866–74.
Zhang H, Liu R, Zhu L, et al. Risk factors for breast cancer-related lymphedema in Chinese women: a meta-analysis. Chin Gen Pract. 2021;24:3349–58, 3376.
Bronfenbrenner U. Toward an experimental ecology of human development. Am Psychol. 1997;32:513.
Kennedy W, Fruin R, Lue A, Logan SW. Using ecological models of health behavior to promote health care access and physical activity engagement for persons with disabilities. J Patient Exp. 2021;8:23743735211034030.
Schmitz KH, Troxel AB, Dean LT, et al. Effect of home-based exercise and weight loss programs on breast cancer-related lymphedema outcomes among overweight breast cancer survivors: the WISER survivor randomized clinical trial. JAMA Oncol. 2019;5:1605–13.
Perry R, Whitmarsh A, Leach V, Davies P. A comparison of two assessment tools used in overviews of systematic reviews: ROBIS versus AMSTAR-2. Syst Rev. 2021;10:273.
Acknowledgments
This umbrella review was supported by the National Natural Science Foundation of China (72174011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, A., Qiang, W., Zhang, L. et al. Risk Factors for Breast Cancer-Related Lymphedema: An Umbrella Review. Ann Surg Oncol 31, 284–302 (2024). https://doi.org/10.1245/s10434-023-14277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14277-7