Abstract
Background
Primary lung mucinous adenocarcinomas (LMAs) could be subclassified as the pure-solid, part-solid, and pneumonic types according to the findings of high-resolution computed tomography. This study aimed to expound on the clinicopathologic, radiologic, and prognostic characteristics of LMAs based on radiologic classification within a large set of patients.
Methods
From November 2009 to December 2016, this study enrolled 294 resected LMAs, which were divided into the pure-solid (n = 169), part-solid (n = 87), and pneumonic (n = 38) types. The clinicopathologic and radiologic characteristics of the tumors were evaluated, and patient prognosis was determined through follow-up evaluation. Survival outcomes were calculated by Kaplan-Meier curves and compared using log-rank tests. The prognostic impact of clinicopathologic variables, including radiologic presentations, were evaluated by establishing a Cox proportional hazards model.
Results
The LMAs were infrequently associated with lymph node metastasis (5.4 %), lymphatic/vascular invasion (4.4 %), or visceral pleural invasion (5.1 %). During the median 71-month follow-up period, recurrence was observed in 62 patients and death in 44 patients. The patients with pneumonic-type LMAs had a poorer prognosis (5-year recurrence-free survival [RFS], 23.7 %; 5-year overall survival [OS], 44.7 %) than those with the pure-solid type (RFS, 83.2 %; OS, 100 %) or part-solid type (RFS, 93.7 %; OS, 100 %). Besides, lymph node metastasis, emphysema, and clinical T stage were independent predictors of RFS and OS.
Conclusion
Solitary-type LMA patients had excellent prognoses, whereas the survival outcomes for pneumonic-type LMA patients were dismal. Furthermore, pneumonic-type LMA patients were prone to intrapulmonary metastasis by means of aerogenous dissemination rather than distant metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Primary lung mucinous adenocarcinoma (LMA), formerly known as mucinous bronchioloalveolar carcinoma (BAC), is histologically characterized by tumor cells that have a goblet or columnar cell morphology with abundant intra-cytoplasmic mucin.1 In the 2011 classification system for lung adenocarcinomas presented by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS), LMA was classified as a variant of lung adenocarcinoma.2 Lung mucinous adenocarcinoma can be distinguished from non-mucinous adenocarcinoma mainly because of the great differences in clinical, radiologic, pathologic, and genetic characteristics.3,4,5,6,7
Based on high-resolution computed tomography (HRCT) findings, previous studies classified LMA into two types as follows: the solitary type, including pure-solid and part-solid subtypes, in which the shadows represent solitary nodules or masses, and the pneumonic type, in which the shadows represent consolidation with or without air bronchogram, mainly occupying the lung lobe.8,9,10 A previous study reported that LMA had a better prognosis than non-LMA.4 However, several noticeable differences in survival were reported among different radiologic subtypes.8,9,10,11
Although prognosis has been discussed previously, the characteristic HRCT findings of LMA have been poorly elaborated. When pneumonic-type LMA is found in clinical practice, it is difficult to distinguish it from pneumonia.9,12,13
Delay in diagnosis and treatment results in an aggressive progression within a short time. Meanwhile, pneumonic-type LMA has shown a strong predilection for aerogenous dissemination,14 which could lead to high rates of recurrence and intrathoracic metastasis after surgery.6,8,15,16,17 Hence, the prognosis for patients with pneumonic-type LMA is poor, and death is more likely to be secondary to respiratory failure due to tumor airway spread rather than distant tumor metastasis. Therefore, early diagnosis of pneumonic-type LMA is vital for treatment. To date, few studies have concentrated on the radiologic features and factors affecting postoperative recurrence of pneumonic-type LMA.
Comprehensive radiologic studies on LMA have been limited because the histology of LMA is relatively rare compared with other subtypes of lung adenocarcinoma.18,19 In the current retrospective study, we aimed to elucidate the HRCT imaging findings and clinicopathologic characteristics of patients with LMA. Meanwhile, through correlation analysis of clinicopathologic and radiologic characteristics as well as prognosis among the HRCT-based subgroups, we were able to clarify the risk factors related to recurrence-free survival (RFS) and overall survival (OS) and reduce the misdiagnosis rate, which would be highly beneficial, especially for the diagnosis and treatment of pneumonic-type LMA.
Materials and Methods
The current cross-sectional study was approved by the Ethics Committee of Shanghai Pulmonary Hospital. The requirement of informed consent was waived by the Ethics Committee due to the retrospective nature of the study.
Study Population
From November 2009 to December 2016, we retrospectively searched adenocarcinoma and mucous keywords from the pathologic diagnostic system and enrolled 392 patients in our study. The exclusion criteria ruled out mixed mucinous/non-mucinous adenocarcinomas (n = 35), mucinous adenocarcinomas of the gastrointestinal tract metastases to the lung (n = 29), a history of malignancy (n = 2), incomplete clinical and radiologic data (n = 17), and patients lost to follow-up evaluation (n = 15). Finally, 294 patients with pathologically confirmed primary LMA were included in the current retrospective study.
For all the patients, the following clinical features were recorded: age at diagnosis, gender, smoking history, symptoms at diagnosis, and surgical procedures. The clinical T stage of each tumor was determined according to the eighth edition of the tumor-node-metastasis (TNM) classification system for lung cancer.
Preoperative Staging Protocol
Lymph nodes larger than 10 mm in the short axis on the chest computed tomography (CT) scan were clinically defined as metastasis-positive. Mediastinoscopy or positron emission tomography (PET) scan was not routinely performed preoperatively during the period of the study. All the patients except those with early lung cancer underwent a systemic workup that included a cranial contrast-enhanced CT scan or magnetic resonance imaging (MRI), bone scintigraphy, and contrast-enhanced whole-abdominal CT scan or contrast-enhanced MRI of the upper-abdominal. If patients underwent a PET-CT scan of the whole body, bone scintigraphy and enhanced whole-abdominal CT scan often were skipped. All the patients preoperatively underwent an electrocardiogram and a respiratory function test for a cardiopulmonary workup.
Radiologic Evaluation of HRCT
All HRCT scans were reviewed by two chest radiologists (W.L. and J.Y.S., with respectively 10 and 31 years of experience in radiology) who were blinded to the research purpose. The readings were based on the lung window setting (window level, –450 Hounsfield units [HU]; width, 1500 HU) and mediastinal window setting (window level, 40 HU; width, 400 HU).
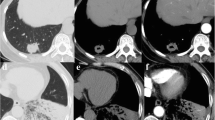
In our study, LMA was classified into three subtypes according to the HRCT findings as follows: pure-solid subtype (representing a pure-solid nodule or mass without a ground-glass opacity [GGO] component), part-solid subtype (representing a solitary nodule with peripheral GGO), and pneumonic subtype (representing mixed GGO and/or consolidation and multi-lobar and bilateral involvement, mimicking pneumonia) (Fig. 1).
a An air-containing part-solid type LMA in the right lower lobe in a 68-year-old man. b A pneumonic-type LMA in the right lower lobe in a 49-year-old woman. c,d An air-containing pure-solid type LMA in the right lower lobe in a 67-year-old man. The tumor maximal diameter in the mediastinal window (2 cm) was smaller than that in the lung window (2.5 cm). The mean computed tomography (CT) attenuation was 15 HU. This also suggested that radiologic pure-solid nodular LMA contains air and a large amount of mucin, with the result that the pathologic invasive size was smaller than the clinical tumor size. Besides, none of the patients showed lymph node metastasis, LVI, or VPI. Gene analysis indicated KRAS mutation in all the patients. LMA, lung mucinous adenocarcinoma; HU, Hounsfield unit; LVI, lymphatic/vascular invasion; VPI, visceral pleural invasion; KRAS, Kirsten rat sarcoma viral oncogene
The following CT parameters were recorded: concomitant interstitial pneumonia, emphysema, tumor distribution and location, CT attenuation of the solid component, and air bronchogram. The solid component or consolidation was defined as an area of increased opacification that completely obscured the underlying vascular markings. We measured the maximum solid component or consolidation diameter for each subtype on the mediastinal window as a clinical T stage. For the pneumonic-type LMA, if consolidation was present, it was evaluated as sub-segmental, multiple segmental single lobar, and multi-lobar consolidations. The presence of accompanying discrete nodules at the peripheral consolidation was assessed, with nodules investigated as GGO, focal consolidation, or both. In addition, we also evaluated the radiologic features of the recurrent lesions and classified them as GGO, focal consolidation, or both.
Surgical Criteria
In our institution, limited surgery was occasionally selected by the attending physicians with consideration of factors such as patient age, lung function, and pre-existing diseases. For pure-solid appearance and pneumonic-type LMA, most of the resections performed were lobectomies. For part-solid nodular LMA, some patients received anatomic segmentectomies or wedge resections, and some patients underwent lobectomies.
In terms of surgical approach, most of the patients (98.3 %, 289/294) underwent video-assisted thoracic surgery (VAST), whereas only a few patients accepted thoracotomy. Meanwhile, a majority (86.7 %, 255/294) of the patients underwent systematic lymph nodal dissections, and the remaining patients (13.3 %, 39/294) accepted lymph node sampling.
Pathologic Evaluation
All tumors were completely sampled and submitted for microscopic evaluation after surgical resection. Because some patients had undergone surgery before 2011, one thoracic pathologist (Mr. Huikang Xie, with 18 years of experience in thoracic pathology) re-evaluated paraffin-embedded sections of the entire tumor (while blinded to the original paraffin-embedded section) and made a final pathologic diagnosis based on the new pathologic criteria of IASLC/ATS/ERS. Meanwhile, pathologic subtypes, pathologic nodal involvement, visceral pleural invasion (VPI), and lymphatic/vascular invasion (LVI) were analyzed.
Follow-up Evaluation
The patients were evaluated at 6-month intervals for the first 2 years, followed by annual check-ups. The last follow-up visit was performed in December 2021. Survival and disease progression were assessed according to the medical records or a telephone interview. The recurrence was diagnosed based on the physical examination and diagnostic imaging findings, and the diagnosis was further confirmed histologically when clinically feasible.
Recurrence-free survival was defined as the time between the date of surgical resection and the date of first lung cancer recurrence (local, regional, distant metastasis) or death (irrespective of the cause). Overall survival was defined as the time between the date of surgery and the date of death from any cause. For the participants who remained alive or/and whose disease had not recurred, RFS and OS were censored on the date of the last visit/contact with disease assessment.
Statistical Analysis
Clinicopathologic characteristics and HRCT findings were summarized as mean ± standard deviation or as frequency (%), as appropriate. Differences between groups were compared using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables and using the chi-square test or Fisher’s exact test for categorical variables.
Kaplan-Meier curves were plotted to show the cumulative probability of experiencing events (RFS and OS) over time, and differences between groups were compared using the log-rank test. The hazard ratios (HRs) with 95 % confidence intervals (95 % CIs) associated with prognosis were estimated using the stepwise Cox proportional hazards model. Schoenfeld's global test was used to examine the proportional-hazards assumption in the Cox proportional hazards model. Because the number of events was available, variables were imported into the multivariable Cox regression model, and those selected were primarily based on univariate relationships with outcomes as well as clinical specialty.
All statistical analyses were performed using R 4.1.2 statistical software (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value lower than 0.05 was considered statistically significant.
Results
Baseline Clinicopathologic Characteristics of LMA Patients
Among the 294 LMA patients (196 women and 98 men) the mean age was 58.8 years (range, 28–83 years). Additionally, in terms of smoking status, 256 non-smokers and 38 smokers were involved. Regarding tumor location and distribution, tumors were located in the lower lobes in 66 % (194/294) of the patients, and the majority (89.5 %, 263/294) of the patients had a strong tendency for a peripheral distribution. Only three patients with pneumonic-type LMA presented with multi-lobar involvement.
In terms of clinical T stage, according to the eighth edition of the TNM classification system for lung cancer, 185 patients had cT1 disease, 47 patients had cT2 disease, 37 patients had cT3 disease, and 25 patients had cT4 disease. The radiologic types of lesions included 87 lesions (29.6 %, 87/294) of the part-solid subtype, 169 lesions (57.5 %, 169/294) of the pure-solid subtype, and 38 lesions (12.9 %, 38/294) of the pneumonic-type LMA. The box-and-whisker plots in Fig. S1 show the distribution of solid component size according to radiologic subtypes. The median, upper-whisker, and lower-whisker solid component size of the pneumonic-type LMA were significantly larger than those of the solitary-type LMA.
According to the IASLC/ATS/ERS criteria, no case of adenocarcinomas in situ (AIS) was diagnosed, and only one case of minimally invasive adenocarcinomas (MIA) was detected. Pathologic lymph node metastasis (5.4 %, 16/294), VPI (5.1 %, 15/294), and LVI (4.4 %,13/294) were less frequent in LMA. Genetic examinations were performed for 125 (42.5 %, 125/294) of the patients, and the most common driver gene was Kirsten rat sarcoma viral oncogene (KRAS) mutation. The clinicopathologic characteristics, symptoms, tumor location, tumor distribution, clinical T stage, surgical procedures, lymph node metastasis, VPI, LVI, and adjuvant therapy showed significant differences in HRCT findings among the three groups (Table 1).
Prognosis
The median follow-up time was 71 months. Recurrence was observed in 62 patients. and death for 44 patients (Table 2). In terms of the recurrence mode, the majority (88.7 %,55/62) were thoracic cases (M1a), and seven cases (11.3 %,7/62) involving recurrence in lymph nodes and distant organs (M1b or M1c) were diagnosed. Most lung cancer-related deaths were observed in the pneumonic-subtype group or pure-solid-subtype group. No recurrence was detected in the part-solid-subtype group. The 5-year RFS and OS rates were 100 % and 100 % in the part-solid subtype group and 83.2 % and 92.3 % in the pure-solid group, respectively, which were significantly higher than those in the pneumonic-subtype group (RFS, 23.7 % [P < 0.0001]; OS, 44.7 % [P < 0.0001]).
Furthermore, we evaluated survival outcomes based on the clinical T stage and found significant differences in the 5-year RFS rates (c-T1 stage [96.8 %], c-T2 stage [80.9 %], c-T3 stage [46.0 %], c-T4 stage [8.0 %]; P < 0.0001) and the 5-year OS rates (c-T1 stage [99.5 %], c-T2 stage [93.6 %], c-T3 stage [67.6 %], c-T4 stage [24.0 %]; P < 0.0001) (Figs. 2 and 3).
The dismal prognosis of pneumonic-type LMA highlighted the importance of early diagnosis and timely treatment to achieve local control. Therefore, the Cox proportional hazards model was used to analyze the risk factors associated with RFS and OS. As a result, the univariable analysis indicated that symptom, radiologic type, distribution, emphysema, CT value, lymph node metastasis, LVI, and clinical T stage were significant prognostic factors for RFS and OS (Table 3). The multivariable logistic regression analysis showed that lymph node metastasis, emphysema, and clinical T stage were dependently significant prognostic factors for RFS and OS (Table 4).
Discussion
The current study concentrated on primary LMA. We investigated the clinicopathologic characteristics and survival outcomes based on the radiologic subtypes in the light of the 2011 classification system for lung adenocarcinomas presented by the IASLC/ATS/ERS. Our study showed that surgical resection of LMA with distinct radiologic and clinicopathologic features was accompanied with promising survival outcomes. The following results could be achieved: (1) different radiologic subtypes of LMA had distinguishable clinicopathologic features and biologic behaviors; (2) LMA showed a low risk of lymph node involvement, and LVI, VPI, and distant metastatic diseases (M1b or M1c) were remarkably less frequent; (3) cases with solitary-type LMA showed an excellent prognosis, whereas survival outcomes were dismal for pneumonic-type LMA patients, who had a higher rate of recurrence and intrathoracic metastasis (M1a); and (4) lymph node metastasis, emphysema, and clinical T stage were independent predictors of RFS and OS.
From the prospective of radiologic findings, resected LMA was classified as pneumonic- and solitary-type LMA. The HRCT findings of pneumonic-type LMA showed alveolar consolidation with pulmonary GGO or focal consolidation. In clinical trials, it is difficult to distinguish pneumonic-type MLA from pneumonia.
In a previous study of solitary pulmonary lesions, air-containing pulmonary lesions were observed more frequently in LMAs than in non-LMAs.20 Moreover, LMAs were reported to be associated with more pulmonary cystic airspace lesions than non-LMAs.3 Miyata et al.9 reported that air-containing pulmonary lesions consisted of air bronchogram and dilated alveolar spaces, which might be generated by bronchial ectasia due to fibrotic collapse and mucin accumulation. It was precisely because of the large amount of mucin accumulation, in which the solid components were found with a relatively low density on HRCT. However, previous studies have demonstrated that the GGO and the solid part on HRCT findings are strongly correlated with the lepidic growth and invasive component on pathology, respectively.2,21 Based on the afore-mentioned results, we could assume that for LMAs with the same solid component diameter in HRCT, the pathologically invasive part may be smaller than that of non-LMAs.
Specifically, LMAs were more likely to be associated with a worse prognosis due to the metastatic potential and consolidation on HRCT.2,22 Due to the low incidence and the limited survival data for LMAs, the results of previous reports had been controversial.22,23 Shim et al.6 reported that LMAs could not be aggressive tumors and showed a tendency for a better RFS. In line with some previous studies, the results of our research demonstrated that the prognosis of resected LMAs was relatively satisfactory because LMAs were not frequently associated with lymph node metastasis, LVI, or VPI. Indeed, several studies reported that the incidence of lymph node metastasis in LMAs was relative low, although most have had small samples.4,24
Meanwhile, in our study, the majority of cases (78.9 %, 232/294) were at a relatively lower clinical T stage, leading to promising treatment outcomes. Although the overall prognosis was excellent, the patients with pneumonic-type LMA had a higher rate of recurrence and a poorer prognosis than those with other types of LMA. The pneumonic-type LMA was more prone to recurrence and death due to a higher rate of aerogenous dissemination than lymph node metastasis, LVI, or VPI. Lung mucinous adenocarcinomas can spread via tumor cells floating in pools of abundant extracellular mucin, replacing air spaces. Microscopically, alveolar lumina are filled with abundant mucin in pneumonic-type LMAs. Tumor cells with mucin spread aerogenously throughout the alveoli. With pneumonic-type LMAs, due to the aggregation of surrounding mucin and different distributions of mucin, tumor cells are more likely to spread through fluid mucin, resulting in tumor diffuse distribution, which could be manifested as multicentric, multilobar, or bilateral lung involvement on radiologic findings.
Because of its relatively low pathologic invasiveness, surgical resection was the rational and preferred treatment for primary LMA, even for pneumonic types with a relatively higher clinical T stage. However, for LMA with high clinical T stage, it still had a strong tendency for recurrence due to aerogenous dissemination rather than hematogenous and lymphoid metastases.
Regarding genetic mutations, LMA was noted to be strongly associated with KRAS mutations and absence of EGFR mutations.25,26,27,28 Meanwhile, LMA had been reported to respond poorly to EGFR-tyrosine kinase inhibitors (EGFR-TKIs) as well as radiation, limiting the treatment options.29 Cha et al.15 concentrated on stage IV LMA patients who received EGFR-TKIs and found that none of these patients achieved partial response (PR) and that the median time to progressive disease (PD) was within 1 month, which could result from the lack of targetable mutations. In addition, the results showed that chemotherapy had no positive effect on OS or RFS for LMA patients.
In our study, few patients with postrecurrence of LMA benefit from EGFR-TKIs, chemotherapy, radiation therapy, or even immunotherapy (Fig. 4). Because no appropriate drug therapy currently exists for these patients, especially for those with pneumonic-type LMA, surgical resection is the rational and preferred treatment for such patients, although LMA has a relatively higher T stage. Moreover, because LMA is frequently accompanied with hematogenous and lymphoid metastases, some scholars have proposed lung transplantation for terminal-stage LMA patients.30 However, pneumonic-type LMA is prone to relapse after surgical resection or single- or double-lung transplantation due to aerogenous dissemination. Therefore, knowing how to predict disease recurrence after lung transplantation on radiologic imaging is crucial.
Serial CT images in pneumonic-type lung mucinous adenocarcinoma. a A 62-year-old woman came to our hospital for a CT examination because of repeated cough and sputum. The CT images showed consolidation with a diameter greater than 7 cm in the right lower lobe. This tumor was classified as clinical T4N0M0 after PET-CT examination. Anti-inflammatory treatment was ineffective. Therefore, surgical resection was performed on 10 October 2015. The lesion was pathologically confirmed as LMA without lymph node metastasis and lymphatic and vascular involvement and carried a KRAS mutation. In addition, this patient was treated with chemotherapy in the first, third, and fourth months after surgery. b In routine reexamination, few new lesions in the bilateral lung were detected. A metastatic nodule was suspected and treated with chemotherapy. c Bilateral nodules were manifested as ground glass opacity or focal consolidation. The nodules were increased and enlarged compared with the previous CT scan. d Disease progression. Lesions in the left upper lobe underwent a biopsy and were confirmed as IMA. The patient received targeted treatment and immunotherapy, respectively. e The recurrent lesions had aggressive progression, and CT images showed multicentric opacities or consolidation as well as multilobar and bilateral lung involvement. Unfortunately, the patient died of respiratory failure in September 2018, and the overall survival (OS) was about 35 months. CT, computed tomography; PET, positron emission tomography; LMA, lung mucinous adenocarcinoma; KRAS, Kirsten rat sarcoma viral oncogene; IMA, invasive mucinous adenocarcinoma
Among various qualitative and quantitative CT findings, emphysema and size of consolidation (clinical T stage) were independent predictors for recurrence. Tumor size is one of the most evident prognostic factors in a clinical T descriptor, which is an independent predictor for survival.31,32 A higher clinical T stage was associated with a higher risk of relapse. In our study, pneumonic-type LMA or pure-solid-subtype LMA was relatively more likely to recur, partly due to the larger size of consolidation. Larger clinical T stage pneumonic-type LMA showed a strong tendency for multicentric, multilobar, and bilateral GGO or focal consolidation lesions after surgical resection, which might reflect aerogenous metastasis. Such patients experience mainly respiratory failure due to postoperative recurrence and bilateral lung involvement through aerogenous dissemination. Therefore, for pneumonic-type LMA patients with a higher clinical T stage, further clinical prospective studies should be conducted to indicate whether surgical resection or the single-lung transplantation is reasonable or whether double-lung transplantation is essential.
The current study had several limitations. First, in this retrospective study, the data were obtained from a single institution. Second, because the incidence of LMA is low in clinical practice, our sample size, especially for pneumonic-type LMA, was not very large. In addition, due to the high cost of the genetic testing and no pathologic assessment of tumor size (pT), and spread through air space (STAS) for some patients, a future integrated, postoperative, pathologic, and genetic analysis of patients’ data is necessary to validate our results.
In conclusion, radiologic classification and differentiation of imaging features of LMA could be advantageous for clinical treatment. Solitary-type LMA showed an excellent prognosis, whereas pneumonic-type LMA was found to be more prone to aerogenous dissemination and recurrence, with intrapulmonary metastasis (M1a) rather than distant metastasis (M1b or M1c). The clinical T factor had a greater effect on postoperative outcomes.
References
Ichinokawa H, Ishii G, Nagai K, et al. Clinicopathological characteristics of primary lung adenocarcinoma predominantly composed of goblet cells in surgically resected cases. Pathol Int. 2011;61:423–9.
Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Lee HY, Lee KS, Han J, et al. Mucinous versus nonmucinous solitary pulmonary nodular bronchioloalveolar carcinoma: CT and FDG PET findings and pathologic comparisons. Lung Cancer. 2009;65:170–5.
Lee HY, Cha MJ, Lee KS, et al. Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J Thorac Oncol. 2016;11:1064–73.
Boland JM, Maleszewski JJ, Wampfler JA, et al. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma: a clinicopathological and molecular genetic study with survival analysis. Hum Pathol. 2018;71:8–19.
Shim HS, Kenudson M, Zheng Z, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2015;10:1156–62.
Geles A, Gruber-Moesenbacher U, Quehenberger F, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis, and survival. Virchows Arch. 2015;467:675–86.
Watanabe H, Saito H, Yokose T, et al. Relation between thin-section computed tomography and clinical findings of mucinous adenocarcinoma. Ann Thorac Surg. 2015;99:975–81.
Miyata N, Endo M, Nakajima T, et al. High-resolution computed tomography findings of early mucinous adenocarcinomas and their pathologic characteristics in 22 surgically resected cases. Eur J Radiol. 2015;84:993–7.
Shimizu K, Okita R, Saisho S, Maeda A, et al. Clinicopathological and immunohistochemical features of lung invasive mucinous adenocarcinoma based on computed tomography findings. Onco Targets Ther. 2017;10:153–63.
Wang T, Yang Y, Liu X, et al. Primary invasive mucinous adenocarcinoma of the lung: prognostic value of CT imaging features combined with clinical factors. Korean J Radiol. 2021;22:652–62.
Aquino SL, Chiles C, Halford P, et al. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: Do CT criteria work? AJR Am J Roentgenol. 1998;171:359–63.
Jung JI, Kim H, Park SH, et al. CT differentiation of pneumonic-type bronchioloalveolar cell carcinoma and infectious pneumonia. Br J Radiol. 2001;74:490–4.
Gaikwad A, Souza CA, Inacio JR, et al. Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. AJR Am J Roentgenol. 2014;203:W570–82.
Cha YJ, Kim HR, Lee HJ, Cho BC, et al. Clinical course of stage IV invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2016;102:82–8.
Gaeta M. Patterns of recurrence of bronchioloalveolar cell carcinoma after surgical resection: a radiological, histological, and immunohistochemical study. Lung Cancer. 2003;42:319–26.
Oka S, Hanagiri T, Uramoto H, et al. Surgical resection for patients with mucinous bronchioloalveolar carcinoma. Asian J Surg. 2010;33:89–93.
Moon SW, Choi SY, Moon MH, et al. Effect of invasive mucinous adenocarcinoma on lung cancer-specific survival after surgical resection: a population-based study. J Thorac Dis. 2018;10:3595–608.
Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6:508–12.
Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–76.
Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol. 2014;9:74–82.
Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64.
Warth A, Muley T, Meister M, et al. The novel histologic international association for the study of lung cancer/American thoracic society/European respiratory society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–46.
Matsui T, Sakakura N, Koyama S, et al. Comparison of surgical outcomes between invasive mucinous and non-mucinous lung adenocarcinoma. Ann Thorac Surg. 2021;112:1118–26.
Ichinokawa H, Ishii G, Nagai K, et al. Distinct clinicopathologic characteristics of lung mucinous adenocarcinoma with KRAS mutation. Hum Pathol. 2013;44:2636–42.
Sakuma Y, Matsukuma S, Yoshihara M, et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–8.
Kadota K, Yeh YC, D’Angelo SP, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol. 2014;38:1118–27.
Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007;9:320–6.
Miller VA, Hirsch FR, Johnson DH, et al. Systemic therapy of advanced bronchioloalveolar cell carcinoma: challenges and opportunities. J Clin Oncol. 2005;23:3288–93.
De Perrot M, Chernenko S, Waddell TK, et al. Role of lung transplantation in the treatment of bronchogenic carcinomas for patients with end-stage pulmonary disease. J Clin Oncol. 2004;22:4351–6.
Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:990–1003.
Zhang J, Gold KA, Lin HY, et al. Relationship between tumor size and survival in non-small cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thorac Oncol. 2015;10:682–90.
Acknowledgment
The authors gratefully acknowledge the help of Mr. Huikang Xie (Department of Pathology, Shanghai Pulmonary Hospital, Tongji University School of Medicine), who kindly evaluated the pathologic retrospective data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Yang, Y., Yang, M. et al. Clinicopathologic Features and Survival Outcomes of Primary Lung Mucinous Adenocarcinoma Based on Different Radiologic Subtypes. Ann Surg Oncol 31, 167–177 (2024). https://doi.org/10.1245/s10434-023-14193-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14193-w