Abstract
Background
Neoadjuvant chemoimmunotherapy treatment (NCIT) has achieved great success for non-small cell lung cancer (NSCLC); however, the intrinsic mechanism underlying this treatment remains unclear.
Methods
Thirty-two patients with stage IIA–IIIC NSCLC who underwent surgery after NCIT were included in this retrospective study. Multiplex immunofluorescence (mIF) staining and image analysis assays were performed on the samples collected before and after NCIT for each patient. RNA analyses was applied to confirm the mIF results.
Results
Among the enrolled patients, 14 achieved major pathological response or pathological complete response (pCR) and were defined as the ‘response’ group, whereas 18 patients did not respond well to NCIT and were defined as the ‘nonresponse’ group. The results of the mIF assays revealed an overall increase in tumor immune lymphocytes (TILs) after NCIT in the stroma area (p = 0.03) rather than the tumor area (p = 0.86). The percentage of CD8+ T cells and tertiary lymphoid structure counts in both the response and nonresponse groups increased significantly after NCIT compared with before NCIT. CD3+ T cells and FOXP3+ cells decreased significantly in the response group but remained unchanged or increased in the nonresponse group. A comparison of the response and nonresponse groups showed that CD3, FOXP3+ and CD8+/PD-1+ cells before NCIT may serve as predictors of the response to neoadjuvant immunotherapy. The RNA analyses confirmed the mIF results that TILs were elevated after NCIT.
Conclusions
The infiltration of immune cells before NCIT was correlated with pathologic complete response, which enhanced the TILs as a promising predictor for selecting patients who were more likely to benefit from NCIT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Over the past decade, the wide application of immunotherapy has become one of the most important developments in the treatment of non-small cell lung cancer (NSCLC).1,2,3,4 Immune checkpoints such as programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1) play an important role in the activation or inhibition of T cells. Tumor mutational burden (TMB) and microsatellite instability (MSI) have been identified as potential predictive indicators to guide the utilization of anti-PD-L1 therapy.5,6 However, recent studies have suggested that PD-L1 expression levels7 or TMB8 fail as biomarkers to identify patients with NSCLC who would derive clinical benefit from immune checkpoint inhibitors. Neoadjuvant chemoimmunotherapy treatment (NCIT) has become the dominant form of therapeutics in the neoadjuvant setting and extended its application in clinical practice because of the promising results in terms of the rate of clinical response9 when compared with neoadjuvant chemotherapy (NCT)10 or neoadjuvant immunotherapy11 alone. Distinguishing between patients who may benefit from NCIT remains a critical problem in determining appropriate clinical care for these patients.
The specific killing of tumor cells by the immune cells is a complex process that involves neoantigen production, antigen presentation, and T-cell activation. However, existing biomarkers such as TMB and MSI only reflect the production of neoantigens, whereas PD-L1 expression reflects the suppression function of T cells. As mentioned earlier, the immune-mediated killing of tumor cells is a complex process and goes beyond the aspects of the immune microenvironment represented by these biomarkers. The immune microenvironment mainly includes immune-infiltrating cells such as CD8+ cells, CD4+ cells, Treg (FOXP3+) cells, macrophages, and natural killer cells. Tertiary lymphoid structures (TLS) have also been proven to predict the efficacy of immunotherapy in some tumors. A correlation between CD8+ T-cell infiltration and clinical outcomes has been reported in advanced NSCLC.12 Macrophages with M1 polarization are associated with a better prognosis, whereas the presence of M2 macrophages is associated with a poorer efficacy and higher rate of tumor metastasis in lung cancer.13,14,15 However, it is still hard to predict the efficacy of immunotherapy prior to treatment and more robust biomarkers are needed to improve clinical practice. A recent study reported that neoadjuvant chemotherapy increases cytotoxic T cells, tissue-resident memory T cells, and B-cell infiltration in resectable NSCLC.16 Some studies have focused on changes in tumor immune lymphocytes (TILs) before and after neoadjuvant immunotherapy;17 however, the change in TILs over the period of NCIT remains unclear.
Therefore, this study was aimed at exploring the relationship between TILs and the response to NCIT, and assessing the change in TILs before and after NCIT in Chinese patients with NSCLC.
Material and Methods
Patients and Study Design
A retrospective study was conducted at the Shanghai Chest Hospital, Shanghai, China, on patients undergoing management for NSCLC between July 2019 and January 2021. Patients with stage IIA-IIIC NSCLC as determined by the pathologists who received NCIT were included, as staged using the American Joint Committee on Cancer (AJCC) Lung Cancer Staging, 8th edition (2017).18 Data were retrospectively collected from the patients’ electronic medical records. Patients for whom tissue samples for immunofluorescence testing were not available or those with unavailable clinicopathological data were excluded, as were patients harboring driver genes such as EGFR or ALK. Patient management was left to the discretion of the treating physicians.
This study was conducted with the approval of the Ethics Committee of Shanghai Chest Hospital [ID: KS 1971] and written informed consent was obtained from all patients.
Grade of Treatment Response
Histopathological evaluation of the surgical specimens of the primary resected tumors was performed to assess the grade of treatment response by our dedicated team of pathologists. Pathological complete response (pCR) was defined as the lack of any viable tumor cells on review of hematoxylin and eosin slides after complete evaluation of a resected lung cancer specimen, including all sampled regional lymph nodes, while major pathological response (MPR) was defined as the reduction of viable tumor to the amount beneath an established clinically significant cut-off based on prior evidence according to the individual histologic type of lung cancer and a specific therapy. Generally, patients demonstrating 0% viable residual tumor cells are graded as having pCR, while those with < 10% of viable tumor cells are graded as having MPR. In our study, both pCR and MPR patients were classified as responders (‘response’ group), while the remaining patients were classified as nonresponders (‘nonresponse’ group).19
Tumor Microenvironment by Multiplex Immunofluorescence and Analysis
Multiplex immunofluorescence staining was performed using the Akoya OPAL Polaris 7-Color Automation IHC (immunohistochemistry) kit (NEL871001KT). Formalin-fixed paraffin-embedded (FFPE) tissue slides were first deparaffinized in a BOND RX system (Leica Biosystems) and then incubated sequentially with primary antibodies targeting CD163 (Abcam, ab182422, 1:500), CD68 (Abcam, ab213363, 1:1000), PD-1 (CST, D4W2J, 86163S, 1:200), PD-L1 (CST, E1L3N, 13684S, 1:400), CD3 (Dako, A0452), CD4 (Abcam, ab133616, 1:100), CD8 (Abcam, ab178089, 1:100), CD56 (Abcam, ab75813, 1:100), CD20 (Dako, L26, IR604), FOXP3 (Abcam, ab20034, 1:100), pan-CK (Abcam, ab7753, 1:100), or S100 (Abcam, ab52642, 1:200) [Akoya Biosciences]. This was followed by incubation with secondary antibodies and the corresponding reactive Opal fluorophores. Nuclei were stained with DAPI. Tissue slides that were bound with primary and secondary antibodies, but not fluorophores, were included as negative controls to assess AF. Multiplex-stained slides were scanned using the Vectra Polaris Quantitative Pathology Imaging System (Akoya Biosciences) at 20 nm wavelength intervals from 440 to 780 nm with a fixed exposure time and an absolute magnification of ×200. The scans obtained on each slide were then superimposed to obtain a single image. Multilayer images were imported into inForm v.2.4.8 (Akoya Biosciences) for quantitative image analysis. The tumor parenchyma and stroma were differentiated using pan-CK staining. The quantities of various cell populations were expressed as the number of stained cells per square millimeter and as the percentage of positively stained cells among all nucleated cells. When the data were analyzed, only counts of cells that were positive for the markers were assessed. The same evaluation method has been reported in several published articles.20,21,22 Delta infiltration was calculated as the TILs observed in the post-treatment sample minus those observed in the paired pre-treatment specimen.
RNA Sequencing
RNAs were extracted and purified from FFPE tissues using the RNeasy FFPE Kit (73504, Qiagen, Germany), according to the manufacturer’s instructions, and quantified using the Qubit RNA HS Assay Kit (Thermo Fisher Scientific). RNA quality and integrity were characterized using a Bioanalyzer and High Sensitivity RNA ScreenTape. (Agilent Technologies). RNA-seq libraries were prepared using the KAPA RNA HyperPrep Kit with KAPA RiboErase (KAPA Biosystems). Total RNA (200 ng) was fragmented at 94°C for 6 min, followed by first- and second-strand synthesis, A tailing, adapter ligation, and PCR (using 12 cycles). Final libraries were quantified using the KAPA library quantification kit, Qubit fluorometer, and Agilent 4200 Bioanalyze, and were sequenced on an Illumina NovaSeq6000 sequencer using 2×150 bp cycles with down-machine data size ≥20 G. The raw RNA-Seq reads underwent trimming of adapter sequences and filtering of low-quality bases using FASTP (v0.20.1), followed by mapping to human genome reference hg19 with STAR (v2.7.9a). During alignment, STAR was supplied with transcript models (GENCODE v19) from https://data.broadinstitute.org/Trinity/CTAT_RESOURCE_LIB/__genome_libs_StarFv1.3. The quality control metrics were obtained using FastQC (v0.11.9) [https://www.bioinformatics.babraham.ac.uk/projects/fastqc/], and alignment quality metrics of bam files were measured using RSeQC (v4.0.0). RNA abundance was calculated using RSEM (v1.2.28) and the RSEM results were converted using the Bioconductor package tximport (v4.1).
Differentially expressed genes across groups of samples were calculated separately using DESeq2 (v4.1) and were mapped to Gene Ontology (GO) terms, KEGG pathways, and Molecular Signatures Database (MSigDB) gene sets using clusterProfiler (v4.0.5), org.Hs.eg.db (v3.11.4), and msigdbr (v7.4.1).
Calculation of Immune-Related Cell Infiltration
TPMs (transcripts per kilobase per million mapped reads) were used to measure gene expression levels. TPM values were obtained using the RSEM (v1.2.28),23 where the FASTQ files were mapped to a human reference genome (hg19). The TIMER (tumor immune estimation resource) algorithm (http://timer.comp-genomics.org/) was applied to infer the infiltration of various immune cell subtypes by de-convolution of gene expression data.24
Statistical Analysis
Categorical variables were described as frequencies and percentages, and continuous variables were summarized as means and standard deviations or medians and standard deviations. Student’s t-test was applied to compare the number of TILs of samples obtained from the responder or nonresponder groups, and the paired nonparametric Wilcoxon test was used to compare the TILs before and after NCIT. Two-way analysis of variance (ANOVA) was used to show the overall difference in TILs before and after NCIT.
All analyses were performed using R version 4.1.1 (The R Foundation for Statistical Computing, Vienna, Austria). The receiver operating characteristic (ROC) curve was used to evaluate the power of TILs before, after, or delta (the change in TILs after neoadjuvant treatment) in predicting the clinical benefit of NCIT.
Results
Clinicopathological Characteristics Stratified by Treatment Response
A total of 32 patients were included in this study. A majority of patients were male (28, 88%) and had stage IIIA disease (20, 63%). The predominant histopathological subtype was squamous cell carcinoma (24, 75%), and the expression of PD-L1 was 1–50% in 38% (12/32) of patients. Immune checkpoint inhibitors that were utilized included pembrolizumab (n = 13) and nivolumab (n = 19). The treatment regimen of chemotherapy varied in patients. A total of 14 (44%) patients demonstrated treatment response. The actual numbers for each of these response groups are provided in electronic supplementary material (ESM) Table 1, and the clinicopathological details stratified by treatment response status are summarized in Table 1. There was no statistically significant difference between the responders and nonresponders in terms of age, sex, clinical stage, histologic subtype, and PD-L1 expression (all p > 0.05). The toxicity grading of neoadjuvant treatment-related adverse events has been listed in Table 1. The incidence of immune-related adverse events was low, and events were mainly of grade 1 or 2.
The swimmer’s plot shown in ESM Fig. S1 demonstrates the patient-level follow-up after surgery, and progression of disease, identified radiologically, in the patients included in this study. Thirty of the 32 patients were available for follow-up, and all were alive at last follow-up. Three patients in the nonresponse group experienced disease progression and were treated appropriately.
Multiplex Immunofluorescence
To investigate the impact of NCIT on changes in TILs, multiplex immunofluorescence (mIF) was performed on tissue obtained on biopsy before the administration of NCIT as well as the surgical specimens obtained after NCIT. Changes of TILs before and after NCIT in the response and nonresponse groups are summarized in Fig. 1. The percentage of CD8+ T cells and TLS counts in both the response and nonresponse groups increased significantly after NCIT compared with before NCIT. CD3+ T cells and FOXP3+ cells decreased significantly in the response group but remained unchanged or increased in the nonresponse group. Representative mIF images showed significant differences in the overall TILs and TLS before and after NCIT treatment. These investigations demonstrated an overall increase in TILs after NCIT in the stroma area (p = 0.03) rather than the tumor area (p = 0.86) (Fig. 2, ESM Fig. S2a). In the stroma, a significant increase in the FOXP3+ T cells (p < 0.01) and CD8+/PD-1+ T cells (p < 0.05) was observed. The heatmap shown in ESM Fig. S2b summarized these data. Changes in other lymphocytes that were studied are described in ESM Fig. S3 (tumor) and ESM Fig. S4 (stroma). Among them, the percentage of CD3+ T cells (tumor, p = 0.93; stroma, p = 0.083), CD4+ T cells (tumor, p = 0.25; stroma, p = 0.07), CD8+ T cells (tumor, p = 0.00048; stroma, p = 0.00071), CD8+/PD-1+ T cells (tumor, p = 0.07; stroma, p = 0.011), CD68+/PD-L1+ macrophage (tumor, p = 0.066; stroma, p = 0.95) and FOXP3+ T cells (tumor, p = 0.43; stroma, p = 0.006) were significantly elevated (ESM Fig. S2). The changes in other lymphocytes in the tumor (ESM Fig. S3) and stroma (ESM Fig. S4) were not significantly increased.
Tertiary Lymphoid Structures Infiltration
In addition to the type and enumeration of TILs via mIF, complex lymphocyte structures, such as the TLS, were also analyzed in this study. TLS are ectopic cellular aggregates that resemble secondary lymphoid organs and are mainly composed of T cells (CD3+) and follicular B cells (CD20+). Recent studies have reported a prognostic value of TLS and immunotherapeutic responses in solid tumors.25 TLS in the post-treatment samples exhibited a significant increase compared with the pre-treatment results (0.009/mm2 in pre-NCIT samples and 0.34/mm2 in post-NCIT samples; p < 0.001).
Representative mIF images showed TLS was not a predictive factor for clinical benefit in the pre-NCIT specimens (area under the curve [AUC] 0.44). However, the number of TLS had a trend of difference between the response and nonresponse groups in post-NCIT measurement and the change in values observed (delta NCIT, AUC 0.63) across therapy (ESM Fig. S5). Therefore, further investigation on the predictive power of TLS is warranted.
mIF revealed the increase of TILs after NCIT treatment. a The overall TILs taken into account increased statistically significantly after NCIT in the stroma area but not significantly in the tumor area. b Representative of mIF (patient 15, P15) showed the increase of TILs after NCIT. DAPI 40,6-diamidino-2-phenylindole, mIF multiplex immunofluorescence, H&E hematoxylin and eosin, TILs tumor immune lymphocytes, NCIT neoadjuvant chemoimmunotherapy treatment
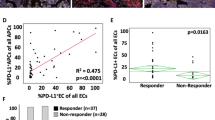
Potential Predictive Role of CD8+/PD-1+ and CD3+ for Patients Undergoing Neoadjuvant Chemoimmunotherapy Treatment (NCIT)
To further explore the difference between the response and nonresponse groups in TILs, we compared certain types of immune cell infiltration between the two groups (Fig. 3). These analyses showed that the CD8+/PD-1+ T cells in the tumor area were significantly different in pre-treatment samples between Response and Non-Response groups (0.05% in response group samples of pre-NCIT, 0.01% in nonresponse group samples of pre-NCIT; p = 0.025). In addition, CD3+ T cell and FOXP3+ cell infiltrations in the tumor area of pre-NCIT samples were more prevalent in the response group (CD3+: 25.5% in the response group vs. 7.2% in the nonresponse group; FOXP3+: 4.6% in the response group vs. 1.7% in the nonresponse group), with statistical significance (p = 0.04 for CD3+T cells; p = 0.045 for FOXP3+ Treg cells) (Fig. 3a, b, c). Compared with the nonresponse group, the percentage of CD68+/CD168+ M2 macrophage infiltration in the stroma decreased more in the response group (p = 0.0019) (Fig. 3d), consistent with a previous study.26
We then conducted an analysis to explore the predictive value of TILs for distinguishing between the response and nonresponse groups. The results showed that CD3+ T cells (AUC 0.72, 95 confidence interval [CI] 0.533–0.9) (Fig. 4a), CD8+/PD-1+ T cells (AUC 0.76, 95 CI 0.586–0.926) (Fig. 4b) and FOXP3+ cells (AUC 0.71, 95 CI 0.527–0.894) (Fig. 4c) had promising sensitivity in predicting the clinical benefit of using NCIT. However, the CD68+/CD163+ M2 macrophages had a weak ability to predict the outcome (AUC 0.58, 95 CI 0.459–0.89), although there was no statistical significance between the response and nonresponse groups (p = 0.153) (Fig. 4d).
RNA Expression
To further validate the changes in TILs between the pre-treatment and post-treatment specimens, we compared the RNA expression profile in these patients. Specimens from six patients, including pre-treatment and post-treatment samples, were evaluated (ESM Fig. S1). We found that CD8+ T cell (p = 0.031), CD4 T-helper (Th) 1 T cell (p = 0.029) and macrophage (p = 0.019) populations were significantly increased in post-treatment samples compared with the pre-treatment samples (Fig. 5a). No significant difference was found in terms of the CD8+ T cells (p = 0.72), CD4 Th1 cells (p = 0.07) and macrophages (p = 0.34) in the pre-NCIT samples (Fig. 5b). CD4 Th1 cells populations in the post-NCIT samples were significantly increased in Response group compared with Non-Response (Fig. 5c). We then explored the potential mechanisms that could drive the benefit of NCIT treatment. The results of the GO enrichment analyses revealed that the differentially expressed genes (DEGs) between samples taken before and after NCIT enrichment were enriched for genes involved in the regulation of chemokine production, humoral immune response, and positive regulation of immune response (Fig. 5d). At the same time, DEGs revealed the enrichment of activation of immune response, the immune response-regulating signaling pathway, and B- or T-cell activation (Fig. 5e).
RNA analyses confirmed the mIF results and revealed the possible mechanism of NCIT. The expression comparison between patients before and after (bottom) NCIT treatment showed a the increase of TILs. The TILs varied in Response and Non-Response groups in both b pre-treatment samples and c post-treatment samples. d GO enrichment; and e the DEGs analysis between samples of before and after NCIT. mIF multiplex immunofluorescence, NCIT neoadjuvant chemoimmunotherapy treatment, TILs tumor immune lymphocytes, Th1 T-helper 1, GO Gene Ontology, DEGs differentially expressed genes
A Representative Case Showed the Increased Infiltration of Tumor Immune Lymphocytes After NCIT
We present a representative case of pCR in NCIT, which was consistent with our results. The radiographic and corresponding mIF images are shown in Fig. 6. The increase in immune cells and TLS (panel 2) between pre-treatment (upper) and post-treatment (bottom) can be appreciated on mIF imaging. The patient was a 62-year-old Asian woman who was diagnosed with a stage IIIB lung adenocarcinoma. She was treated with paclitaxel, carboplatin and sintilimab for two cycles, and demonstrated stable disease on imaging; pCR was observed on histopathologic evaluation. No relapse was observed at the time of last follow-up.
Radiographical and corresponding mIF imaging of one patient (patient 14, P14) before (upper) and after (bottom) NCIT treatment showed the increase of TILs. mIF multiplex immunofluorescence, NCIT neoadjuvant chemoimmunotherapy treatment, TILs tumor immune lymphocytes, DAPI 40,6-diamidino-2-phenylindole
Discussion
The introduction of NCIT in the management of resectable NSCLC has revolutionized the care of these patients. The CheckMate-816 study reported that nivolumab and platinum-doublet chemotherapy in the neoadjuvant setting exhibited a promising 24% rate of pCR in patients with resectable NSCLC;9 however, this study did not provide an in-depth discussion on the mechanisms that could drive these findings.
It is worth mentioning that NCIT has demonstrated improved outcomes over neoadjuvant NCT alone.10 The comparative real-world research published recently revealed that there was a significant difference in the MPR rate plus pCR rate (14.3% vs. 53.2%; p < 0.001) between the NCT and NCIT groups.10 The 2-year disease-free survival (DFS) rates between the two groups were significantly different (39.5% in the NCT group vs. 67.2% in the NCIT group; p = 0.019). Univariate Cox analysis demonstrated that the type of neoadjuvant therapy (hazard ratio 1.922, 95% CI 1.142–3.233; p = 0.014) was associated with DFS.
This study had several limitations, especially in terms of exploring the molecular mechanisms underlying these differences. Our study may help explain these differences. In our study, CD8+/PD-1, CD4+/FOXP3+ Treg, and PD-L1+/CD68+ cells increased after the administration of NCIT treatment. The increased number of immune cell types may explain the better clinical outcomes of NCIT treatment compared with NCT treatment alone.
There has been great improvement in pCR in chemoimmune therapy versus immunotherapy as a neoadjuvant treatment. In patients receiving atezolizumab and chemotherapy for resectable NSCLC, 57% had an MPR,11 which was the first published trial of immune checkpoint inhibitors combined with chemotherapy applied as neoadjuvant treatment in resectable NSCLC patients. In the NEOSTAR study, which enrolled patients receiving nivolumab-based neoadjuvant therapy followed by surgery, 10% of patients achieved a pCR rate and 24% achieved an MPR rate.17 The response rate to single-agent immunotherapy varied from approximately 12% to 20%;27 however, the underlying mechanism remains unclear. Here, we revealed that TILs, including the traditional positive immune markers CD3+T, CD8+ T, CD8+ T/PD-1+, and CD68+/PD-L1+ cells, as well as the traditional negative immune marker CD4+/FOXP3+ T cells, may be responsible for the clinical benefit derived from the use of NCIT. Two-way ANOVA showed that the overall lymphocyte infiltration increased after treatment. Our results revealed the association of improved survival observed in patients receiving NCIT may be explained by increased TILs.
TLS are lymphoid-like organs usually discovered in solid tumor tissues.28,29 The existence of TLS indicates lymphoid neogenesis in solid tumors upon chronic inflammatory exposure mediated by chemokines and cytokines,30,31 which is regarded as an indicator of improved clinical benefit.25,32,33,34 The presence of TLS is thought to be associated with chronic rejection through local enrichment of B cells, which can mediate the killing of tumor cells via the production of antibodies.35,36 Elevated TLS infiltration after NCIT indicates a good clinical outcome, which is consistent with the high rates of MPR in neoadjuvant chemoimmunotherapy clinical trials. Moreover, in our study, the increase in TIL infiltration after treatment was higher in Response group than in Non-Response group, although the difference was not statistically significant (p = 0.06) [ESM Fig. S5], which may be due to the limited sample size. At the same time, the presence of TLS in pre-NCIT samples was rare, which may be associated with the pathology of selected patients, among whom 80% had the squamous cell carcinoma histological subtype.
We performed a comprehensive analysis to explore the role of TILs in predicting clinical outcomes of NCIT. We proposed that the infiltration of lymphocytes in pre-treatment samples could predict a clinical benefit of using this therapy. Our results supported this hypothesis revealed that CD3+ T cells, CD8+/PD-1+ T cells, and FOXP3+ cells had improved ability to predict response to NCIT treatment. Clinical outcomes may be evaluated before NCIT treatment using IHC technology, which includes a precision approach to the management of these patients.
This retrospective study has several limitations. First, the neoadjuvant chemotherapy regimen differed among the patients enrolled in this study. Second, we were limited by the availability of tumor TILs in surgical samples because of the cleavage of tumor cells after neoadjuvant treatment, especially in the response group. Only three patients in the response group underwent TIL evaluation after treatment, which restricted the comparison between the two groups. Third, the sample size restricted the reliability of the conclusions. Further prospective research with a larger sample size is needed to obtain more solid conclusions.
Conclusions
Our research revealed, for the first time, that TILs increased after neoadjuvant treatment using NCIT, which is potentially related to improved clinical outcomes over neoadjuvant immunotherapy or neoadjuvant chemotherapy alone. Assessment of TILs prior to administration of therapy may have a promising role in predicting the clinical benefits of NCIT and can be used as a tool to identify appropriate therapy for patients with NSCLC.
Data Availability
Sequence Archive for National Genomics Data Center (BioProject ID: PRJCA013526, https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA013526; OMIX ID: OMIX002464, https://ngdc.cncb.ac.cn/omix/release/OMIX002464). The data generated and analyzed during this study are described in the ESM (Tables S1 and S2).
References
Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Kim S, Buecher B, Andre T, et al. Atezolizumab plus modified docetaxel-cisplatin-5-fluorouracil (mDCF) regimen versus mDCF in patients with metastatic or unresectable locally advanced recurrent anal squamous cell carcinoma: a randomized, non-comparative phase II SCARCE GERCOR trial. BMC Cancer. 2020;20(1):352. https://doi.org/10.1186/s12885-020-06841-1.
Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5(5):696–702. https://doi.org/10.1001/jamaoncol.2018.7098.
Wang Z, Zhao X, Gao C, et al. Plasma-based microsatellite instability detection strategy to guide immune checkpoint blockade treatment. J Immunother Cancer. 2020;8(2):e001297. https://doi.org/10.1136/jitc-2020-001297.
Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. https://doi.org/10.1056/NEJMoa1613493.
Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–8. https://doi.org/10.1038/s41591-018-0134-3.
Forde PM, Spicer J, Lu S, et al. Abstract CT003: nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CHECKMATE 816 trial. Cancer Res. 2021;81:C003.
Liu Z, Gao Z, Zhang M, et al. Real-world effectiveness and prognostic factors analysis of stages I–III non-small cell lung cancer following neoadjuvant chemo-immunotherapy or neoadjuvant chemotherapy. Ann Thorac Cardiovasc Surg. 2022;28(2):111–20. https://doi.org/10.5761/atcs.oa.21-00143.
Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–95. https://doi.org/10.1016/S1470-2045(20)30140-6.
Fumet JD, Richard C, Ledys F, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119(8):950–60. https://doi.org/10.1038/s41416-018-0220-9.
Kim SR, Chun SH, Kim JR, et al. The implications of clinical risk factors, CAR index, and compositional changes of immune cells on hyperprogressive disease in non-small cell lung cancer patients receiving immunotherapy. BMC Cancer. 2021;21(1):19. https://doi.org/10.1186/s12885-020-07727-y.
Hwang I, Kim JW, Ylaya K, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18(1):443. https://doi.org/10.1186/s12967-020-02618-z.
Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26(4):970–7. https://doi.org/10.1158/1078-0432.CCR-19-1040.
Gaudreau PO, Negrao MV, Mitchell KG, et al. Neoadjuvant chemotherapy increases cytotoxic T cell, tissue resident memory T cell, and B cell infiltration in resectable NSCLC. J Thorac Oncol. 2021;16(1):127–39. https://doi.org/10.1016/j.jtho.2020.09.027.
Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27(3):504–14. https://doi.org/10.1038/s41591-020-01224-2.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009.
Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–40. https://doi.org/10.1016/j.jtho.2020.01.005.
Dai Y, Zhao L, Hua D, et al. Tumor immune microenvironment in endometrial cancer of different molecular subtypes: evidence from a retrospective observational study. Front Immunol. 2022;13:1035616. https://doi.org/10.3389/fimmu.2022.1035616.
Ju WT, Xia RH, Zhu DW, et al. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat Commun. 2022;13(1):5378. https://doi.org/10.1038/s41467-022-33080-8.
Zhang W, Gong C, Peng X, et al. Serum concentration of CD137 and tumor infiltration by M1 macrophages predict the response to Sintilimab plus bevacizumab biosimilar in advanced hepatocellular carcinoma patients. Clin Cancer Res. 2022;28(16):3499–508. https://doi.org/10.1158/1078-0432.CCR-21-3972.
Yu J, Yan J, Guo Q, et al. Genetic aberrations in the CDK4 pathway are associated with innate resistance to PD-1 blockade in Chinese patients with non-cutaneous melanoma. Clin Cancer Res. 2019;25(21):6511–23. https://doi.org/10.1158/1078-0432.ccr-19-0475.
Koeppen H, Yu W, Zha J, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib+/−onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res. 2014;20(17):4488–98. https://doi.org/10.1158/1078-0432.CCR-13-1836.
Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–55. https://doi.org/10.1038/s41586-019-1922-8.
Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp Ther Med. 2019;18(6):4490–8. https://doi.org/10.3892/etm.2019.8068.
Qin H, Wang F, Liu H, et al. New advances in immunotherapy for non-small cell lung cancer. Am J Transl Res. 2018;10(8):2234–45.
Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. https://doi.org/10.1016/j.it.2012.04.006.
Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–62. https://doi.org/10.1038/nri3700.
Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–25. https://doi.org/10.1038/s41568-019-0144-6.
Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35(11):571–80. https://doi.org/10.1016/j.it.2014.09.006.
Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–5. https://doi.org/10.1038/s41586-019-1914-8.
Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–60. https://doi.org/10.1038/s41586-019-1906-8.
Vanhersecke L, Brunet M, Guegan JP, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802. https://doi.org/10.1038/s43018-021-00232-6.
Thaunat O, Field AC, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102(41):14723–8. https://doi.org/10.1073/pnas.0507223102.
Thaunat O, Patey N, Caligiuri G, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185(1):717–28. https://doi.org/10.4049/jimmunol.0903589.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (82072564, 82272679), Project of Shanghai Natural Science Foundation (20ZR1452000), Program of Shanghai Academic Research Leader (22XD142280), Shanghai Municipal Health Commission (2022XD029), Shanghai Youth Talent Support Program, Shanghai Talent Development Fund (2019073), Shanghai Chest Hospital Project of Collaborative Innovative Grant (YJT20190209), The Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212302), Lian Yun Gang Shi Hui Lan Public Foundation (HL-HS2020-65), Guangdong Association of Clinical Trials (GACT)/Chinese Thoracic Oncology Group (CTONG) and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (2017B030314120), National Multi-disciplinary Treatment Project for Major Diseases (2020NMDTP), and Shanghai Chest Hospital Project of Talent Support (2018YNZYJ02)
Author information
Authors and Affiliations
Contributions
TC and ZC contributed equally to this work. YS, JH, SS, SL and ZL participated in the design and performance of this study. FW, XZ and DZ participated in the analysis and interpretation of the data. YC, HC and MH performed the statistical analysis. The manuscript was drafted by TC, FW and DZ, and was reviewed by all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Disclosures
Fengcai Wen, Xiaochen Zhao, Ding Zhang, Mengli Huang, Yanan Chen, and Hao Chen were employed by 3D Medicines-Shanghai Inc. Tianxiang Chen, Zhengqi Cao, Yingjia Sun, Jia Huang, Shengping Shen, Yueping Jin, Long Jiang, Shun Lu, and Ziming Li declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, T., Cao, Z., Sun, Y. et al. Neoadjuvant Chemoimmunotherapy Increases Tumor Immune Lymphocytes Infiltration in Resectable Non-small Cell Lung Cancer. Ann Surg Oncol 30, 7549–7560 (2023). https://doi.org/10.1245/s10434-023-14123-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14123-w