Abstract
Background
Heterogenous nomenclature describing appendiceal neoplasms has added to uncertainty around their appropriate treatment. Although a recent consensus has established the term low-grade appendiceal neoplasm (LAMN), we hypothesize that significant variation remains in the treatment of LAMNs.
Methods
We retrospectively reviewed our prospectively maintained appendiceal registry, identifying patients with LAMNs from 2009 to 2019. We assessed variability in treatment, including whether patients underwent colectomy, spread of disease at presentation, and long-term outcomes.
Results
Of 136 patients with LAMNs, 88 (35%) presented with localized disease and 48 (35%) with disseminated peritoneal disease. Median follow-up was 2.9 years (IQR 1.9–4.4), and 120 (88%) patients underwent pre-referral surgery. Among 26 pre-referral colectomy patients, 23 (88%) were performed for perceived oncologic need/nodal evaluation; no nodal metastases were identified. In patients with resected LAMNs without radiographic evidence of disseminated disease, 41 (47%) underwent second look diagnostic laparoscopy (DL) to evaluate for occult metastases. No peritoneal metastases were identified. Patients with disseminated disease were treated with cytoreductive surgery/heated intraperitoneal chemotherapy (CRS/HIPEC). For patients undergoing CRS/HIPEC, 5-year recurrence-free survival was 94% (95% CI 81–98%). For patients with localized disease, 5-year RFS was 98% (95% CI 85–99%).
Conclusions
Significant variation exists in treatment patterns for LAMNs, particularly prior to referral to a high-volume center. Patients frequently underwent colectomy without apparent oncologic benefit. In the current era of high-quality cross sectional imaging, routine use of DL has low yield and is not recommended. Recurrence in this population is rare, and low-intensity surveillance can be offered. Overall prognosis is excellent, even with peritoneal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent decades, through targeted study and increased recognition of rare diseases, our understanding of the biology of appendiceal neoplasms has evolved significantly. With this evolution, however, has come changes in nomenclature and subsequent confusion in the proper treatment for these rare tumors. In 2016, the International Peritoneal Surface Oncology Group released a consensus report on appendiceal neoplasms and pseudomyxoma peritonei.1 This report standardized the term low-grade appendiceal mucinous neoplasm (LAMN) to describe architecturally low-grade tumors either confined to the appendix or exhibiting the clinical constellation of pseudomyxoma peritonei. While these tumors typically exhibit an indolent course, they have the potential to progress to disseminated intraperitoneal disease. For those with localized disease, these malignancies can be treated with either an appendectomy or right hemicolectomy, while those with disseminated disease are treated with cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC).2,3
Following the standardization of the nomenclature for these rare neoplasms, societal guidelines from the American Society of Colon and Rectal Surgeons have defined optimal and evidence-based care of patients with LAMN.4 To summarize these recommendations, patients who can undergo an appendectomy with negative margins do not require further resection (strong recommendation, based on moderate-quality evidence, 1B). Moreover, there is no clinical utility to lymphadenectomy associated with ileocectomy or right hemicolectomy in these patients given the low rate of reported lymph node metastases. The degree of adoption of this practice to the wider surgical oncology and general surgery community was recently described through a survey noting up to a 20% rate of pre-referral right hemicolectomy.5 These findings, however, have not been validated beyond a survey setting.
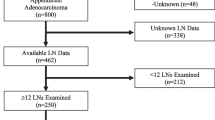
Despite standardization in nomenclature and recent societal guidelines, practice patterns in the treatment of LAMNs continue to exhibit significant variability, with surgeons oftentimes performing more extensive resections than are indicated. We suspect these variations to be due, in part, to the complex nomenclature and management courses needed to treat appendiceal neoplasms as outlined in Fig. 1. To that end, we sought to review the treatment differences and any difference in outcomes for patients with LAMN treated at our quaternary referral center to better understand broader community practice patterns. Through this we intend to not only quantify variation in care seen in patients referred after initial treatment, but to also characterize any difference in outcomes should they exist. We also describe outcomes following referral including outcomes of interval laparoscopy for these patients to ensure adequate resection after initial resection and confirm lack of interval recurrence.
Patients and Methods
This is a retrospective review of our prospectively collected institutional database of appendiceal malignancies. Our review was approved by the MD Anderson Institutional Review Board. All patients with a primary appendiceal tumor classified after as a LAMN by gastrointestinal pathologist at our institution between 2009 and 2019 were included. Pathology slides were independently reviewed by expert pathologists specializing in peritoneal surface malignancy at MD Anderson Cancer Center (M.T., W.C.F.). Patients were clinically classified as either having localized or disseminated disease. Localized disease was defined as disease confined to within the serosa of the appendix. Disseminated disease was defined as extraserosal extension or presence of mucin or visible disease within the peritoneal cavity. Patients with disseminated disease were offered CRS and HIPEC at our institution with a 90-minute perfusion with mitomycin C. Operative reports for patients who had undergone resection prior to presentation were independently reviewed by two surgeons (M.G.W., C.P.S.) to identify reasoning behind any colon resections performed prior to referral. Patient demographic data and tumor characteristics noted on internal pathology review were all prospectively collected. Follow-up data were collected by research coordinators following established database protocols.
Following definitive resection, patients underwent surveillance with serial radiographic imaging and tumor markers (i.e., CEA, CA 125, CA 19-9) annually for 2–3 years and then biennially thereafter. Patients deemed at high risk for local recurrence after initial resection, as determined by the attending surgeon, underwent interval laparoscopy to evaluate for local recurrence or signs of disseminated disease. Changes in treatment course based on this intervention were noted prospectively within the appendiceal database and described below.
Demographic differences between those undergoing appendectomy or right colectomy were compared using Wilcoxon rank-sum test, t-test, or chi-squared test where appropriate. Long-term outcomes such as overall and recurrence-free survival were analyzed using univariate Cox proportional hazards ratios and the Kaplan–Meier method. For all analyses, p < 0.05 was considered significant. All analyses were performed using Stata 13 (StataCorp LLC, College Station, Texas, USA).
Results
Within the study period, 136 patients with LAMN were evaluated in our surgical oncology practice. Among these, 88 (64.7%) patients had localized disease, while 48 (35.3%) were noted to have disseminated disease prior to referral. The demographics of these patient populations are detailed in Table 1 and Supplementary Fig. 1. Of those patients with localized disease, 78 (89%) underwent surgical resection prior to referral. Prior to referral 52 (59%) underwent an appendectomy alone, while 19 (22%) underwent a partial colectomy. Seven (8%) patients with localized disease underwent multiple procedures, including four (5%) appendectomies with oophorectomy (two unilateral, two bilateral) and three (3%) appendectomies with total abdominal hysterectomy with bilateral oophorectomy (TAHBSO). Upon review of prior medical records, of the 73 (53.6%) patients (21 with disseminated disease and 52 with localized disease) who underwent a prereferral appendectomy, only 2 (2.7%) had a positive microscopic margin, while 16 (21.9%) had gross tumor at the margin. Those with a positive margin did not have tumor identified on final pathology in their specimens. The indication for colectomy was noted for nodal evaluation or other oncologic purpose in 17 (89%) cases of colectomy for localized disease. No lymph node metastases were noted in these specimens of patients with localized or disseminated disease. Extent of abdominal assessment or exploration was inconsistently described on outside operative reports.
Of the 48 patients presenting with disseminated disease, one (2%) patient underwent pre-referral definitive resection and HIPEC. Seven (15%) patients with disseminated disease underwent colectomy prior to referral, while 32 (67%) required a colectomy during their subsequent CRS and HIPEC. Another nine (19%) patients underwent multiple procedures, including an appendectomy and TAHBSO in four (8%) patients, an appendectomy and partial debulking in three (6%) patients, and an isolated bilateral oophorectomy in one (2%) patient. Management details of those patients with disseminated disease are described in Table 2. For the 39 patients with disseminated disease who underwent right hemicolectomy either prior to or following their referral, 28 (72%) were for margin status or disease involvement of the base of the appendix, while 10 (26%) were noted to be performed for nodal evaluation, and no clear reason provided for 1 (2%) patient. Among patients undergoing colectomy during CRS and HIPEC, all were performed for direct disease involvement. A total of 46 (96%) of the patients underwent HIPEC, with the additional 2 patients having disease isolated to their ovary treated with bilateral salpingo-oophorectomy and appendectomy alone. No lymph node metastases were noted in the surgical specimens of patients with disseminated disease. While five patients received some chemotherapy for their disseminated disease, on review it appeared to only be indicated for a single patient who developed a recurrence that on histology revealed moderate-to-poorly differentiated appendiceal adenocarcinoma.
During the study period, patients with localized disease considered by the attending surgeon at our referral center to be high risk for local recurrence after their initial resection were offered diagnostic laparoscopy for further evaluation beyond tumor markers and cross sectional imaging. These evaluations occurred between 6 and 12 weeks following their initial prereferral procedure. Of the 78 patients with previously resected localized disease, 35 (45%) of the patients with localized disease underwent diagnostic laparoscopy between 12 and 24 months from their time of presentation to our surgical oncology offices to evaluate for recurrence. Six of the laparoscopic evaluations demonstrated mucin consistent with disseminated disease. There was, however, a high preoperative suspicion based on the patients’ cross sectional imaging. None of those laparoscopies demonstrated findings that resulted in a change in clinical management and no adverse events were encountered. All patients were able to be managed with curative intent for their disseminated disease.
When considering the long-term outcomes of these standard-of-care patients, 5-year recurrence-free survival for patients with localized disease was 98% with an overall survival of 98%. A single death was secondary to cardiac disease in the localized disease group. In the case of patients with disseminated disease, the 5-year recurrence free survival was 80% with an overall survival of 100%. Comparing the two groups, localized disease was associated with an improved recurrence-free survival [hazard ratio (HR) 0.21 95% confidence interval (CI) 0.04–1.11, p = 0.07, Fig. 2]. Of the five recurrences noted during the study period, four were able to undergo repeat CRS and HIPEC with one completeness of cytoreduction (CC) CC-0, one CC-1, one CC-2, and one CC-3 resection.
Discussion and Conclusions
As demonstrated by this cohort of patients and reflective of previous work, outcomes are excellent for patients with localized appendiceal LAMNs as well as disseminated LAMN when treated with CRS and HIPEC.2,6 Indeed, our current experience mirrors that described by our group and others, namely that LAMNs represent slow-growing tumors with excellent 5-year recurrence-free survival (i.e., > 95%) treated with appendectomy alone in the setting of non-ruptured appendix, negative margin appendectomy, and no evidence of peritoneal disease.2,7 Even patients with disseminated disease treated appropriately (i.e., with CRS and HIPEC) demonstrate very good prognosis with low rate of recurrence.2,7
During this study period, we regularly performed second-look laparoscopy for high-risk patients with localized LAMN. Notably, none of these laparoscopies affected the management of these patients. While not a randomized cohort, these findings have resulted in the abandonment of this as routine practice by our group. Given the rarity of LAMNs, it is unlikely that this question will be able to be adequately studied in a randomized fashion in the future.
The excellent prognosis and low recurrence rates of patients with LAMN who undergo appropriate treatment begs the question of how best to follow these patients. Historically, our group has serially deescalated our postoperative surveillance protocols as we have internally evaluated our data. The present data and those from other groups suggest that many of these patients can likely be safely followed in a non-intensive fashion [e.g., serial radiographic imaging and tumor markers (i.e., CEA, CA 125, CA 19-9) annually for 2–3 years and then biennially thereafter]. Unfortunately, given the rare incidence of recurrence, accurately identifying the point after which recurrence is exceedingly unlikely if it has not already occurred, thus allowing for evidence-based further surveillance deescalation, remains challenging and would be left to the discretion of each surgeon in the context of a discussion with each patient.
This study highlights issues surrounding awareness and adoption of evidence-based guidelines in surgical management of neoplasms. Here, the aforementioned ASCRS guidelines regarding management of patients with LAMN recommend appendectomy alone for patients with LAMN without perforation or peritoneal dissemination of disease who undergo a margin-negative resection.4 Notably, however, pre-referral practice patterns vary considerably. Despite guidelines recommending right hemicolectomy only in the setting of direct involvement, it is notable that the majority of pre-referral colectomies within this cohort were performed for lymph node sampling or some degree of perceived oncologic benefit. It is therefore concerning that a number of these procedures appear to not have been clinically indicated. As demonstrated here and reflected in multiple clinical guidelines (ASCRS and Chicago Consensus), right hemicolectomy offers little oncologic benefit aside from when disease invades onto the cecum. When these lesions are encountered, we therefore advocate for a minimalistic approach to treat a patient’s immediate surgical indication (e.g., appendectomy for perforated appendicitis), thorough recording of the patient’s disease burden, and referral of patients to a high-volume peritoneal center for definitive treatment recommendations. Indeed, previous work in the surgical oncology literature has demonstrated that adjusting clinical practice to conform to evidence-based guidelines avoids overtreatment while also reducing cost of care, thereby decreasing patient risk and financial toxicity without clinical benefit.8 As a result, additional educational efforts are warranted to optimize management of LAMNs across the surgical community.
This work should be viewed in light of the limitations of a retrospective review of a prospectively maintained database. Similarly, significant variation in treatment patterns is reflected within our dataset given our institution’s role as a regional and national cancer referral center. The effects of recent guidelines, such as the ASCRS guidelines, on treatment patterns will not be reflected in this work as most patients treated in this study were treated prior to their release in 2019. In the case of second-look laparoscopies, defined criteria for high-risk patients and the time frame of their laparoscopy were not defined a priori. As such, these procedures were performed at the discretion of the attending surgeon after consultation with the patient. Finally, it should be noted that these findings are reflective of patients with LAMN and should not be applied to other higher-risk appendiceal neoplasms, and that these patients with rare malignancies are best managed at high-volume centers.9
From these data we advocate for continued adherence to guidelines that recommend appendectomy alone for the management of LAMNs and advise against segmental colectomy for the clearance of the associated lymph node basin, as this and other studies have not demonstrated a benefit to clearance of the lymph node basin. Future work will be needed to optimize and centralize the care of patients with these rare tumors. While oftentimes presenting with emergent surgical processes, such as perforated appendicitis, we continue to advocate for minimal surgical intervention to treat the emergent process at hand prior to referral to a high-volume referral center.
References
Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, Gonzalez-Moreno S, Taflampas P, Chapman S, Moran BJ, Peritoneal Surface Oncology Group I. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016;40:14–26.
Fournier K, Rafeeq S, Taggart M, Kanaby P, Ning J, Chen HC, Overman M, Raghav K, Eng C, Mansfield P, Royal R. Low-grade appendiceal mucinous neoplasm of uncertain malignant potential (LAMN-UMP): prognostic factors and implications for treatment and follow-up. Ann Surg Oncol. 2017;24:187–93.
Chicago Consensus Working G. The Chicago consensus on peritoneal surface malignancies: management of appendiceal neoplasms. Ann Surg Oncol. 2020;27:1753–60.
Glasgow SC, Gaertner W, Stewart D, Davids J, Alavi K, Paquette IM, Steele SR, Feingold DL. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62:1425–38.
Istl AC, Gage MM, Esquivel J, Ahuja N, Greer JB, Johnston FM. Management of low-grade appendiceal mucinous neoplasms (LAMN): an international survey of surgeons performing CRS and HIPEC. Ann Surg Oncol. 2021;28:3831–7.
Gupta AR, Brajcich BC, Yang AD, Bentrem DJ, Merkow RP. Necessity of posttreatment surveillance for low-grade appendiceal mucinous neoplasms. J Surg Oncol. 2021;124:1115–20.
Li X, Zhou J, Dong M, Yang L. Management and prognosis of low-grade appendiceal mucinous neoplasms: a clinicopathologic analysis of 50 cases. Eur J Surg Oncol: J Eur Soc Surg Oncol Brit Assoc Surg Oncol. 2018;44:1640–5.
Bhutiani N, Mercer MK, Bachman KC, Heidrich SR, Martin RCG 2nd, Scoggins CR, McMasters KM, Ajkay N. Evaluating the effect of margin consensus guideline publication on operative patterns and financial impact of breast cancer operation. J Am Coll Surg. 2018;227:6–11.
Rajeev R, Klooster B, Turaga KK. Impact of surgical volume of centers on post-operative outcomes from cytoreductive surgery and hyperthermic intra-peritoneal chemoperfusion. J Gastrointest Oncol. 2016;7:122–8.
Acknowledgement
We thank the Bidnick Appendix Cancer Research Fund, the Schroeder Appendix Cancer Research Fund, the Volz Appendix Cancer Research Fund, the David Dwyer Research Fund, and the Sunny and Do Lee Research Fund.
Funding
The authors have no competing interests to report.
Author information
Authors and Affiliations
Contributions
MGW and CPS were responsible for study conception and design; MT, WCF, PM, KFF, and CPS were responsible for data collection; MGW, NB, BAH, PFM, CFF, and CPS were responsible for analysis and interpretation of results; and MGW, NB, and CPS were responsible for draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript
Corresponding author
Ethics declarations
DISCLOSURE
The authors have no competing interests to report
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
White, M.G., Bhutiani, N., Helmink, B.A. et al. Treatment Variation and Long-Term Outcomes of Low-Grade Appendiceal Neoplasms. Ann Surg Oncol 30, 8138–8143 (2023). https://doi.org/10.1245/s10434-023-13501-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13501-8