Abstract
Background
The use of the robotic approach is increasing for colorectal cancer operations, but the added cost of the platform has the potential to introduce challenges in its dissemination. We hypothesized that adoption of the robot is introducing new disparities in access to minimally invasive surgery (MIS) for colorectal cancer, especially across patient insurance groups.
Methods
This cross-sectional study analyzed surgical cases of stage I–III colorectal cancer from the National Cancer Database (NCDB) between 2010 and 2019. The primary outcome was surgical approach (robotic, laparoscopic, or the composite “MIS”). The predictor was a patient’s primary payor. Potential confounders included sociodemographics, tumor characteristics, and the facility. Hierarchical multivariable models were generated, and sensitivity analyses were performed.
Results
For colorectal cancer operations, the MIS approach increased from 39% in 2010 to 73% in 2019, driven predominantly by an increase in the robotic approach from 2 to 24%. For laparoscopy, the size of the disparity between patients with Private insurance and Medicaid shrank from 11% (2010) to 4% (2019), whereas this disparity increased for the robotic approach from 1% (2010) to 5% (2019). On adjusted analysis, patients with Medicaid (odds ratio [OR] 0.86 [CI 0.79–0.95]) and the Uninsured (OR 0.67 [CI 0.56–0.79]) had lower odds of receiving a robotic operation than those with Private insurance in 2019. This disparity remained consistent across five sensitivity analyses.
Conclusions
As the field of colorectal cancer surgery shifts away from laparoscopy and toward robotics, new inequities across patient insurance are emerging. Proactive efforts are needed to ensure all patients benefit from a minimally invasive approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
General surgery, including colorectal surgery, is the fastest growing segment for the robotic platform.1,2 For surgeons there are clear advantages to the robot, including ergonomics, flexibility, and vision. Randomized trials have demonstrated that robotic surgery is comparable to other approaches for both postoperative and oncologic outcomes.3 It is increasingly evident that robotics is here to stay and being at the forefront of this learning curve is important for up-and-coming surgeons.
However, there are barriers to widespread adoption of the robotic platform. Perhaps the most salient are issues related to cost. The robotic platform is expensive to purchase or lease and has high ongoing instrument and service fees.4 Several studies have suggested the robotic platform is more expensive than laparoscopy or open surgery.5,6 Further, added costs associated with the robot may not be recuperated. Because insurance companies do not pay more for the robotic approach than for the equivalent laparoscopic procedure, any added cost of the robotic approach is borne by the hospital. This creates a financial risk to the institution if they are not adequately compensated by the patient’s insurance.
Similar concerns existed when laparoscopy was first introduced and disseminated, and disparities in access—especially on the basis of a patient’s insurance—were well documented.7,8,9,10 There is some evidence that this disparity is finally closing.11 We hypothesized that dissemination of the robot may be introducing new disparities in access to minimally invasive surgery, especially across patient insurance. We used colorectal cancer as our model disease given its high volume and the rapid adoption of the robotic platform in its treatment. We aimed to understand the association of a patient's insurance on chances of receiving a minimally invasive operation, specifically, receipt of a robotic approach.
Methods
Database and Ethics
This study is presented in accordance with STROBE guidelines (Supplemental Table 1)12 and followed recommendations regarding best practices for database research.13,14,15 The study database was the National Cancer Database (NCDB).15 The NCDB is a joint project of the Commission on Cancer (COC) of the American College of Surgeons (ACS) and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The ACS and the COC have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. This study was approved by the MD Anderson Cancer Center Institutional Review Board and was granted a waiver of informed consent (IRB#2020-0512).
Sample, Inclusion and Exclusion Criteria
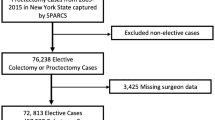
A flow diagram is included in Fig. 1. The starting sample included all cases of colon and rectal cancer currently available in the NCDB from 2004 to 2019. A list of the associated ICD-O-3 primary sites is included in Supplemental Table 2. Cases prior to 2010 did not have information about surgical approach and were excluded. Additional exclusion criteria included: cases that did not receive surgery, cases with a missing or unknown surgical approach, and cases with stage IV disease or missing analytic stage.
Outcome
The primary outcome was surgical approach, classified as open, laparoscopic, or robotic. Cases that were converted (i.e., robotic to open) were classified based on their initial approach, as the focus of this analysis was patient access to a specific surgical approach. The term “minimally invasive surgery” (MIS) refers to a composite outcome including either a laparoscopic or robotic initial approach.
Primary Predictor
The predictor of interest was the patient’s primary payor at time of initial diagnosis and/or treatment, categorized as Private, Medicare, Medicaid, or Uninsured. NCDB also lists Other Government and Unknown categories; these were excluded from most analyses and represented 1.0% (Other) and 1.4% (Unknown) of the total population.
Covariates
Variables that were hypothesized to correlate with both the primary predictor (insurance) and the outcome (receipt of the robotic approach) included: Patient age (continuous, trimmed at 90 years), patient sex, patient race/ethnicity (categorized into Non-Hispanic White/Black/Other and Hispanic), comorbidity profile (measured using Charlson–Deyo comorbidity scores categorized as 0, 1, 2, 3+), tumor location (categorized as colon or rectal; rectosigmoid was included in the latter as both require low anterior resection), cancer stage (three categories: I, II, and III), facility type (COC categories including community cancer program, academic/research program, etc.), and year of analysis. Additional variables used in sensitivity analyses included the patient zip code's median household income based on 2012 American Community Survey data, and two additional facility-level metrics: colorectal robotic volume and proportion of privately insured patients.
Analytic Approach and Statistics
First, we created a description of the entire population including the outcome, primary predictor, and covariates. Second, we analyzed rates of surgical approach over time stratified by insurance (bivariate analysis). Third, we created hierarchical multivariable regression models to assess the association between insurance and receipt of a robotic approach controlling for potential confounders. Multivariable models were run only on 2019 data, given the strong influence of time on rates of robotic surgery. Nonlinear relationships were assessed by including quadratic terms for continuous variables (e.g., age) and assessing significance. Facility was included as a random effect in the multivariable model to adjust for clustering of observations within facilities while controlling for facility-level fixed effects (e.g., facility type). All analyses were performed with STATA v15.1 using two-sided tests and an alpha of 0.05.
Sensitivity Analyses
Several additional analyses were run to assess the robustness of findings. First, we ran a fixed effects model with facility as a dummy variable to reduce bias. Hospitals with no variation (all robot or no robot) were omitted from this analysis. Second, we modified the original model by omitting hospitals with zero robotic operations performed in 2019. Third, we limited analysis to patients with early stage (I or II) cancers that should, in theory, be amenable to an MIS approach. Fourth, we included zip code median income quartile as a covariate. This was not included in the primary analysis because of a moderate (> 10%) amount of missing data. Fifth, and finally, we performed a facility-level analysis looking at the impact of a facility’s robotic volume and insurance distribution on the relationship between insurance and receipt of robotic approach. Robotic tertiles (low volume = 1–5 robotic colorectal operations, mid = 6–15, and high > 15) and private insurance tertiles (low 0–27% privately insured patients, mid 27–38%, and high > 38%) were determined empirically.
Results
Sample
A description of the analytic cohort is included in Table 1. Patients had an average age of 66 years, approximately half (49%) were female, and the majority were Non-Hispanic White (76%). Tumors were predominantly colon (74%) with a near even distribution between stage I, II, and III (32%, 33%, and 35%, respectively). Over the 10-year time period, 59% received an initial MIS approach including 11% robotic and 48% laparoscopic. Medicare was the primary payor for the majority (53%), followed by Private insurers (36%), Medicaid (6%), and Uninsured (3%).
Bivariate Analysis Over Time
The proportion of patients receiving an MIS approach increased across all years of the study from 38.9% in 2010 to 72.7% in 2019. The proportion of patients receiving a laparoscopic approach initially increased from 36.8% in 2010 to a peak of 52.4% in 2016, but has since declined to 48.9% in 2019. The robotic approach increased across all study years from 2.0% in 2010 to 23.9% in 2019.
Bivariate analysis showing rates of MIS approach over time, stratified by insurance status, is shown in Fig. 2. The proportion of patients receiving an MIS approach increased across all insurance types, but there is a persistent disparity over time. For example, the gap in rates of MIS between patients with Private versus Medicaid insurance was approximately the same in 2010 (11.6%, CI 9.6–13.6%) as the gap in 2019 (9.0%, CI 7.6–10.4%). This appears to be driven largely by an increasing disparity in access to the robotic approach, with the gap between Private and Medicaid patients growing from 0.8% (CI 0.1–1.4%) in 2010 to 5.4% (CI 3.9–6.9%) in 2019. Conversely, the gap for laparoscopic surgery has been declining over time, from 10.8% (CI 8.9–12.8%) in 2010 to 3.7% (CI 2.0–5.3%) in 2019.
Multivariable Models and Sensitivity Analyses
The multivariable model including aforementioned patient, tumor, and facility characteristics showed that, compared with those with Private insurance, patients with Medicaid and no insurance had lower odds (0.87 [95% CI 0.78–0.96] and 0.67 [CI 0.57–0.80]) of receiving a robotic approach in 2019 (Table 2). Five sensitivity analyses were performed as described in the Methods. Across all analyses, the direction, magnitude, and significance of the coefficients for patients with Medicaid or those Uninsured were largely the same (Table 3).
”
Facility-level Analysis
As expected, the proportion of patients receiving robotic surgery varied based on the underlying facility-level colorectal robotic volume (Fig. 3), with much higher rates in the highest-volume centers compared with the lowest-volume centers (40.0% vs. 7.7%, p < 0.001). In that context, the largest disparity in robotic surgery across insurance types was seen in the highest-volume centers.
The same relationship held across tertiles of private insurance, with the highest proportion centers performing proportionally more robotic surgery than the lowest (27.0% vs. 19.6%, p < 0.001). However, the size of the disparity between rates of robotic surgery for Private versus Medicaid/Uninsured was roughly the same across the three tertiles.
Discussion
In 2019, nearly three out of four patients with nonmetastatic colorectal cancer now benefit from a minimally invasive operation. However, this study demonstrates a new and enlarging disparity for the robotic approach across patient insurance. As a result, even though disparities in access to laparoscopy across patient insurance have been significantly reduced, the disparity in access to a minimally invasive approach persists and will likely grow if the robotic approach supplants laparoscopy in colorectal surgery.
In general, minimally invasive surgery for colorectal cancer has been shown to improve patient outcomes, including less pain, shorter length of stay, and reduced perioperative mortality, as well as improving chances of receiving adjuvant therapy among higher-stage tumors.16,17,18 Expanding access to these benefits should remain a priority, but the expansion in use of the robot introduces new challenges.
The bivariate analysis performed in this study demonstrates a clear disparity in access to robotic surgery by patient insurance, but perhaps the more relevant inquiry is where the disparity originates. Is the disparity reflective of access to (or choosing to go to) a facility that performs more robotic surgery or, within a given facility, are patients being offered (or choosing to receive) robotic surgery preferentially based on insurance? The present analysis suggests that both are contributory factors. Across all multivariable analyses, the effect size of insurance is less than would have been expected based on the bivariate analysis. This suggests that some of the initial disparity identified is based on complex interactions between a patient's primary insurer, their sociodemographic and comorbid factors, their tumors, and the facilities at which they receive treatment. At the same time, the fact that we consistently found lower odds of receiving a robotic approach among the Uninsured and those with Medicaid across our facility-level analyses and especially the fixed effect model indicate that some of this disparity is occurring within hospitals. Robotic rates can differ for two similar patients at the same facility, with the only observable difference being insurance.
Previous studies have demonstrated the importance of focusing on patient insurance as a gateway to reducing disparities in laparoscopy. A difference-in-difference analysis looking at the effect of Massachusetts healthcare reform found that insurance expansion led to an elimination in racial disparities for access to laparoscopic appendectomy and cholecystectomy.11 The same may hold true for robotic surgery. Alternatively, expanding the Current Procedural Terminology system to include distinct codes for robotic surgery may be a potential policy lever to increasing reimbursement for robotic surgery and improving access. Although not a focus of the present analysis, this study reconfirms racial/ethnic disparities with Non-Hispanic Black individuals having much lower odds of receiving a robotic approach compared to Non-Hispanic White individuals. Insurance may again be a mutable target for helping to reduce racial/ethnic disparities for robotic surgery.
This study has several limitations. First, as an association study, we cannot determine directionality. Patients may be offered a robotic approach at equal rates across insurance types, but patients may elect not to receive the robotic approach for myriad of reasons. Second, NCDB has very little granularity in type of insurance. The analysis is limited to four broad insurance categories, yet there is countless variation within each that may contribute to the disparity identified. Third, while NCDB does capture a large fraction of new cancer diagnoses in the USA, its sampling methodology is prone to bias and therefore should not be considered representative of the entire US population.19 Despite these limitations, the large sample of the study cohort as well as the consistent findings across multiple sensitivity analyses suggest a real disparity for the majority of patients receiving surgery for colorectal cancer in this country.
Conclusions
Insurance appears to be a significant, and growing, barrier to access to a robotic approach for colorectal cancer and is driving a persistent disparity in access to minimally invasive surgery in general. As the field shifts away from laparoscopy and towards robotics, proactive efforts are needed to ensure that all patients benefit from a minimally invasive approach.
References
Childers CP, Maggard-Gibbons M. Trends in the use of robotic-assisted surgery during the COVID 19 pandemic. Br J Surg. 2021;108(10):e330–1.
Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3(1):e1918911.
Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318(16):1569–80.
Childers CP, Maggard-Gibbons M. Estimation of the acquisition and operating costs for robotic surgery. JAMA. 2018;320(8):835–6.
Jeong IG, Khandwala YS, Kim JH, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA. 2017;318(16):1561–8.
Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309(7):689–98.
Barnes WA, Carter-Brooks CM, Wu CZ, Acosta DA, Vargas MV. Racial and ethnic disparities in access to minimally invasive gynecologic surgery for benign pathology. Curr Opin Obstet Gynecol. 2021;33(4):279–87.
Greenstein AJ, Moskowitz A, Gelijns AC, Egorova NN. Payer status and treatment paradigm for acute cholecystitis. Arch Surg. 2012;147(5):453–8.
Kim J, ElRayes W, Wilson F, et al. Disparities in the receipt of robot-assisted radical prostatectomy: between-hospital and within-hospital analysis using 2009–2011 California inpatient data. BMJ Open. 2015;5(4):e007409.
Price JT, Zimmerman LD, Koelper NC, Sammel MD, Lee S, Butts SF. Social determinants of access to minimally invasive hysterectomy: reevaluating the relationship between race and route of hysterectomy for benign disease. Am J Obstet Gynecol. 2017;217(5):572.
Loehrer AP, Song Z, Auchincloss HG, Hutter MM. Massachusetts health care reform and reduced racial disparities in minimally invasive surgery. JAMA Surg. 2013;148(12):1116–22.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Childers CP, Maggard-Gibbons M. Same data, opposite results?: a call to improve surgical database research. JAMA Surg. 2021;156(3):219–20.
Haider AH, Bilimoria KY, Kibbe MR. A checklist to elevate the science of surgical database research. JAMA Surg. 2018;153(6):505–7.
Merkow RP, Rademaker AW, Bilimoria KY. Practical guide to surgical data sets: National Cancer Database (NCDB). JAMA Surg. 2018;153(9):850–1.
Braga M, Vignali A, Gianotti L, et al. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236(6):759-766; discussion 767.
Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477–84.
Zheng Z, Jemal A, Lin CC, Hu CY, Chang GJ. Comparative effectiveness of laparoscopy vs open colectomy among nonmetastatic colon cancer patients: an analysis using the National Cancer Data Base. J Natl Cancer Inst. 2015;107(3).
Palma DA. National Cancer Data Base: an important research tool, but not population-based. J Clin Oncol. 2017;35(5):571.
Author information
Authors and Affiliations
Contributions
Dr. Childers had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Childers, Tran Cao. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Childers. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Childers. Obtained funding: NA. Administrative, technical, or material support: NA. Study Supervision: Tran Cao.
Corresponding author
Ethics declarations
Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Childers, C.P., Uppal, A., Tillman, M. et al. Insurance Disparities in Access to Robotic Surgery for Colorectal Cancer. Ann Surg Oncol 30, 3560–3568 (2023). https://doi.org/10.1245/s10434-023-13354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13354-1