Abstract
Background
The aim of this study was to investigate the prognostic impact of mismatch repair (MMR) status, programmed death-ligand 1 (PD-L1) expression, and Epstein–Barr virus (EBV) status in stage II/III gastric cancer after surgery.
Methods
This study included 679 patients diagnosed with pathological stage II/III gastric cancer who underwent curative gastrectomy followed by adjuvant chemotherapy (AC) or observation between 2007 and 2015. Clinical outcomes were retrospectively reviewed and compared with stratification by AC and other clinicopathological factors.
Results
Patients were divided into AC (n = 484) or surgery alone (SA; n = 195) groups and were further stratified by MMR and EBV status: MMR-deficient (DMMR) and MMR-proficient (PMMR) groups. Comparing the AC-DMMR group versus the AC-PMMR group, 5-year overall survival was 92.0% versus 74.0% (log-rank p < 0.01), and comparing the SA-DMMR group versus the SA-PMMR group, 5-year overall survival was 71.1% versus 73.7% (log-rank p = 0.89). DMMR (hazard ratio 0.25, 95% confidence interval 0.07–0.81) was identified as an independent prognostic factor in the AC group but not in the SA group. In the subgroup analysis, PD-L1-negative patients among the EBV-positive patients or in the DMMR group had a poor prognosis in both the AC and SA groups. The prognosis of the PMMR and EBV-negative patients was similar regardless of PD-L1 expression.
Conclusions
DMMR was associated with a favorable prognosis in stage II/III gastric cancer after surgery and adjuvant therapy. PD-L1 expression may affect the prognosis of DMMR and EBV-positive gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Cancer Genome Atlas project reported that gastric cancer can be divided into four subtypes: Epstein–Barr virus (EBV), microsatellite instability (MSI), genomically stable, and chromosomal instability. Previous studies suggested that high MSI (MSI-H) status is observed in 7–22% of patients with gastric cancer.1,2,3 MSI-H status is caused by mismatch repair deficiency (DMMR), which results from either germline somatic mutations or epimutation in mismatch repair (MMR) genes and is closely associated with tumor mutation burden. Therefore, immune checkpoint inhibitors (ICIs) are considered highly effective in these patients. Furthermore, the therapeutic effect of chemotherapy for patients with MSI-H is reported to be poor in advanced gastric cancers.4,5,6,7 Additionally, adjuvant chemotherapy (AC) is standard treatment for stage II/III gastric cancer in East Asia.8,9,10 In Europe, perioperative chemotherapy is the standard treatment for resectable gastroesophageal cancer.11 Previous reports on colon cancer suggested that fluorouracil-based AC does not improve the survival outcomes of MSI-H patients; therefore, measurement of MSI is recommended for stage II colorectal cancer.12,13,14,15 Similar findings have been reported for AC in MSI-H gastric cancers;2,3 however, information regarding the clinicopathological features of MSI status in patients with stage II/III gastric cancer undergoing S-1-based AC is limited.
Programmed death-ligand 1 (PD-L1) and EBV are predictive biomarkers for ICI efficacy.16,17,18 Antigen escape and overexpression of immune checkpoint proteins observed in gastric cancer are the basis of immunotherapy antibody targeting programmed death-1 (PD-1) and PD-L1.19,20 Moreover, MSI and EBV status were associated with higher PD-L1 expression, and the combination of MSI and PD-L1 was a reported predictive factor for prognosis.21,22 However, the association between PD-L1 expression and prognosis in gastric cancer is still controversial.
In this study, we investigated the impact of MMR status and PD-L1 expression for MMR status or EBV status, on survival outcomes in patients with stage II/III gastric cancer after surgery.
Methods
Patients and Methods
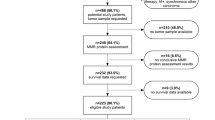
This was a single-institutional, retrospective, case-control study. We retrospectively reviewed the clinical records of 679 patients who underwent gastrectomy with R0 resection for stage II/III gastric cancer between 2007 and 2015, using prospectively collected data from an in-house database at the National Cancer Center Hospital East, Kashiwa, Japan. Patients who received neoadjuvant chemotherapy were excluded. To construct the tissue microarrays (TMAs), two representative tumor cores obtained from the infiltrated area of the tumor were formalin-fixed and embedded in paraffin. Serial sections were cut at 4-μm intervals and used for immunohistochemistry (IHC) and in situ hybridization. All tissue cores were evaluated by a pathologist. Tumor staging was performed in accordance with the Union for International Cancer Control (UICC) 8th TNM classification,23 and the numbering of lymph node stations was performed in accordance with the classification of the Japanese Gastric Cancer Association (3rd English version).24 This study was approved by the Institutional Review Board of the National Cancer Center, Japan (IRB file no. 2017-416; approval date: 2 March 2018).
Immunohistochemistry
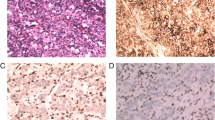
The following primary antibodies were used for IHC: anti-PD-L1 (22C3) rabbit monoclonal antibody (PD-L1 IHC 22C3 pharmDx; Agilent Technologies, Carpinteria, CA, USA), anti-mutLhomolog 1 (MLH1; ES05) monoclonal antibody, anti-mutShomolog 2 (MSH2; FE11) monoclonal antibody, anti-postmeiotic segregation increased 2 (PMS2; EP51) monoclonal antibody, and anti-mutShomolog 6 (MSH6; EP49) monoclonal antibody (Dako, Copenhagen, Denmark) on the Dako autostainer.
Evaluation of Programmed Death-Ligand 1 Expression
Combined positive score (CPS) was determined by the number of PD-L1-positive cells, including tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells. For cases that had two scores that exhibited different PD-L1 expression scores, the highest score was selected.
Evaluation of Mismatch Repair Status
Expression of MLH1, PSM2, MSH2, or MSH6 was determined in the tumor cell nucleus. The absence of expression of any of the following, i.e. MLH1, PSM2, MSH2, or MSH6, was considered DMMR, whereas tumors expressing all markers were considered mismatch repair proficient (PMMR).
Epstein–Barr Virus In Situ Hybridization
EBV-encoded RNA was analyzed using in situ hybridization with an INFORM EBER probe (Ventana, Tucson, AZ, USA). EBER in situ hybridization was performed with an iViewBlue detection kit (Ventana) using the BenchMark ULTRA staining system (Ventana).
Statistical Analysis
All statistical analyses were performed using R version 3.6.1 (www.r-project.org). Fisher’s exact test and the Mann–Whitney U test were used for the statistical analyses. All p values <0.05 were considered statistically significant. Survival curves were constructed using the Kaplan–Meier method, and the log-rank test was used to assess survival differences. Data were censored on 23 September 2021.
Results
Baseline Characteristics and Survival Outcomes of the Patients
First, we divided the patients into either the AC group (n = 484) or the surgery alone (SA) group (n = 195) because S-1-based AC has been the standard treatment for patients with stage II/III gastric cancer in Japan since 2007. Overall, 484 patients (71.2%) received AC, which included S-1 monotherapy (96.5%). The patients’ demographic information is summarized in Table 1. The AC group comprised patients who were eligible for standard treatment, and the SA group comprised patients who were not eligible for standard treatment mainly owing to older age, the presence of pathological stage II cancer, or patients had no wish to receive AC. The SA group had a significantly higher proportion of PD-L1 expression and a lower proportion of DMMR-positive patients than those in the AC group. The ratio of deaths due to other diseases to the total number of deaths was 50.8% (30/59) in the SA group and 7.9% (11/138) in the AC group. Second, the AC and SA groups were further divided into two groups according to MMR status (AC-DMMR group [n = 41] and AC-PMMR group [n = 443], and the SA-DMMR group [n = 30] and SA-PMMR group [n = 165]). The demographic information of the AC group is shown in Table 2. The AC-DMMR group was older and had a significantly higher proportion of PD-L1 expression compared with the AC-PMMR group. Comparing the AC-DMMR group versus the AC-PMMR group, the 5-year overall survival rate was 92.0% versus 74.0% (log-rank p < 0.01), and the relapse-free survival rate was 90.0% versus 62.7% (log-rank p < 0.01) [Fig. 1].
The demographic information of the SA-DMMR (n = 30) and SA-PMMR groups (n = 165) is shown in Table 3. The SA-DMMR group was older and had a significantly higher proportion of PD-L1 expression compared with the SA-PMMR group. Comparing the SA-DMMR group versus the SA-PMMR group, the 5-year overall survival rate was 71.1% versus 73.7% (log-rank p = 0.89), and the relapse-free survival rate was 68.2% versus 67.9% (log-rank p = 0.95) [Fig. 1].
Five-year overall survival (Fig. 2) and 5-year relapse-free survival (electronic supplementary Fig. 1) were stratified for stage II and III cancer in the AC and SA groups. Comparing the AC-DMMR group versus the AC-PMMR group, the 5-year overall survival rate was 100.0% versus 87.8% (log-rank p < 0.01) for stage II, and 83.8% versus 65.8% (log-rank p = 0.08) for stage III. Comparing the SA-DMMR group versus the SA-PMMR group, the 5-year overall survival rate was 84.4% versus 81.0% (log-rank p = 0.72) for stage II, and 41.7% versus 50.1% (log-rank p = 0.41) for stage III. Of the four patients in the stage III AC-DMMR group with recurrence, two patients were pN3 and one patient was pT4b, and of the five patients in the stage III SA-DMMR group with recurrence, two patients were pN3 and two patients were pT4b.
Five-year OS of the AC-DMMR and AC-PMMR groups in a stage II and b stage III gastric cancer, and 5-year OS of the SA-DMMR and SA-PMMR groups in c stage II and d stage III gastric cancer. AC adjuvant chemotherapy, DMMR mismatch repair-deficient, OS overall survival, PMMR mismatch repair-proficient, SA surgery alone
The 5-year overall survival rates based on PD-L1 expression for each MMR status or EBV status are shown in Fig. 3, and the 5-year relapse-free survival rates are shown in electronic supplementary Fig. 2. The patients’ demographic information is summarized in electronic supplementary Tables 1 and 2. In the subgroup analysis, PD-L1-negative patients in the DMMR or EBV-positive groups had a poorer prognosis than that of the PD-L1-positive patients in the same groups. In particular, in the EBV-positive group, the prognosis of PD-L1-negative cases was significantly poorer than that of the PD-L1-positive cases in the AC group (55.6% vs. 92.9%; log-rank p = 0.04) and the SA group (0% vs. 77.8%; log-rank p < 0.01).
Five-year OS of patients based on PD-L1 expression for each MMR status or EBV status in the a, b AC group and c, d SA group. AC adjuvant chemotherapy, DMMR mismatch repair-deficient, EBV Epstein–Barr virus, MMR mismatch repair, OS overall survival, PD-L1 programmed death-ligand 1, PMMR mismatch repair-proficient, SA surgery alone
Multivariate Analysis
The results of the multivariate analysis for overall survival in the AC and SA groups are shown in Table 4. In the AC group, DMMR status (hazard ratio [HR] 0.25, 95% confidence interval [CI] 0.07–0.81) was identified as an independent prognostic factor; however, PD-L1-positive status (CPS ≥1; HR 0.81, 95% CI 0.53–1.23) and EBV-positive status (HR 0.63, 95% CI 0.25–1.58) were not independent prognostic factors. However, in the SA group, DMMR status (HR 1.39, 95% CI 0.61–3.18), PD-L1-positive status (CPS ≥1; HR 0.66, 95% CI 0.36–1.24), and EBV-positive status (HR 2.15, 95% CI 0.68–6.80) were not identified as independent prognostic factors.
Discussion
In this study, we investigated the MMR status, PD-L1 expression, and EBV status of 679 patients in our institution who underwent mainly S-1-based AC as the standard treatment for stage II/III gastric cancer. Most of the patients who did not receive AC were elderly, were diagnosed with stage II gastric cancer, or had no wish to receive AC. Previous reports indicated that fluorouracil-based AC is not effective for MSI-H colon cancer and gastric cancer.12,13,14,15 In colon cancer, a previous report confirmed an overall survival benefit of oxaliplatin as AC for stage III colon cancer, whereas another report showed no improvement in prognosis with oxaliplatin.25,26 In a post hoc analysis of the CLASSIC trial data, AC was not effective for MSI-H stage II/III gastric cancer. Additionally, in an exploratory analysis of the MAGIC trial data, perioperative chemotherapy was associated with a negative prognostic effect in patients with MSI-H-resectable gastroesophageal cancer.2,3 In our study, it was difficult to verify the effect of AC owing to the large selection bias in the AC and SA groups. However, DMMR gastric cancer was associated with a better prognosis than PMMR gastric cancer in patients who received S-1-based AC. This result is consistent with that of a previous report of a subanalysis of the CLASSIC trial data.3
In the AC group, DMMR was identified as a prognostic factor, whereas in the SA group, DMMR was not a prognostic factor in patients with stage II/III gastric cancer. PD-L1 expression and EBV status were not identified as prognostic factors in the AC and SA groups. A systematic review reported that the prognosis of DMMR gastric cancer was better than that of PMMR gastric cancer, with a high proportion of lymph node metastasis in stage III/IV gastric cancer. In contrast, DMMR and PMMR had a similar prognosis in patients with stage II gastric cancer and a low proportion of lymph node metastasis.27 Therefore, in this study, the 5-year overall survival rate was stratified for each stage in the AC and SA groups. In the AC group, the DMMR group had better survival than that in the PMMR group in both stage II and III gastric cancer. The 5-year overall survival of the SA-DMMR and SA-PMMR groups were almost the same in stage II patients. In contrast, although there was no statistical difference, the 5-year overall survival rate of the SA-DMMR group was lower than that in the SA-PMMR group in stage III patients. The reason for this may be, at least in part, that patients in the SA group were older than patients in the AC group because postoperative AC was not performed for elderly patients. Hence, the ratio of deaths due to other diseases to the total number of deaths was higher in the SA group than in the AC group. In the SA group, the rate of death due to other diseases was higher than in the AC group, and age was also considered one of the causes of the poor prognosis in the SA-DMMR group. We also further evaluated recurrent cases in the stage III AC-DMMR group and SA-DMMR group. Most cancers were stage pT4b or patients had multiple lymph node metastases. Even in the DMMR gastric cancer group, stage pT4b and multiple lymph node metastasis were considered risk factors for recurrence.
The association between PD-L1 expression and prognosis in gastric cancer is controversial. It has also been reported that EBV and MSI-H gastric cancers have a higher proportion of immune infiltration and that MSI-H is associated with a favorable prognosis in metastatic gastric cancer patients.28,29 However, in the subgroup analysis in the present study, the prognosis of PD-L1-negative patients in the DMMR-positive or EBV-positive groups was poor in both the AC and SA groups (Fig. 3). Patients with PD-L1-negative status may have a poor prognosis owing to a poor immune response even for DMMR-positive and EBV-positive cases.
There are some limitations to the current study. First, this was a retrospective study performed in a single institution. There was a large selection bias for patients receiving AC, and our results should be validated using larger multicenter datasets. Second, a major limitation of this study is that we investigated only a small portion of the total tumor volume using TMAs. Third, we determined the cut-off value for PD-L1 positivity based on findings in previous reports; however, it was unclear whether this cut-off value could be applied to assess the prognostic importance of PD-L1 expression. Studies using multicenter datasets with larger samples are warranted to reach definitive conclusions.
Conclusions
PD-L1 expression may affect DMMR- and EBV-positive gastric cancer, and further treatment should be considered in addition to S-1-based AC.
Disclosures
Takeshi Kuwata reports grants from Daiichi Sankyo, Roche Diagnostics, and Ono Pharm, and personal fees from Astellas Pharma, AstraZeneca, MSD, Celltrion Healthcare, Ono Pharmaceutical, Bristol-Myers Squibb, and Daiichi Sankyo outside the submitted work. Kohei Shitara reports grants from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, MSD, Amgen, Eisai, and Med Science, and personal fees from Eli Lilly, Bristol-Myers Squibb, Takeda Pharmaceuticals, Pfizer, Ono Pharmaceutical, Novartis, AbbVie, Daiichi Sankyo, Taiho Pharmaceutical, GlaxoSmithKline, Amgen, Boehringer Ingelheim, MSD, Astellas, Guardant Health Japan, and Janssen outside the submitted work. Akihito Kawazoe reports personal fees from Daiichi Sankyo, Ono, Bristol-Myers Squibb, Lilly, Taiho, and Merck Serono Biopharma outside the submitted work. Genichiro Ishii reports grants from Daiichi Sankyo, Ono Pharmaceutical, Noile-Immune Biotech, Takeda Pharmaceutical, Sumitomo Dainippon Pharma, and Nihon Medi-Physics, and personal fees from Takeda Pharmaceutical, Roche Diagnostics, Chugai Pharmaceutical, Novartis, Oncolys Biopharma, Daiichi Sankyo, Eli Lilly, Takeda Pharmaceutical, AstraZeneca, and Riken Genesis outside the submitted work. Eigo Akimoto, Naoya Sakamoto, Atsushi Ochiai, and Takahiro Kinoshita have no disclosures to declare.
References
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3(9):1197–203.
Choi YY, Kim H, Shin SJ, et al. Microsatellite instability and programmed cell death-ligand 1 expression in stage II/III gastric cancer: post hoc analysis of the CLASSIC randomized controlled study. Ann Surg. 2019;270(2):309–16.
Chao J, Fuchs CS, Shitara K, et al. Assessment of Pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7(6):895–902.
Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8(1):49–58.
Kim SY, Choi YY, An JY, et al. The benefit of microsatellite instability is attenuated by chemotherapy in stage II and stage III gastric cancer: results from a large cohort with subgroup analyses. Int J Cancer. 2015;137(4):819–25.
Kubota Y, Kawazoe A, Sasaki A, et al. The impact of molecular subtype on efficacy of chemotherapy and checkpoint inhibition in advanced gastric cancer. Clin Cancer Res. 2020;26(14):3784–90.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2012;379(9813):315–21.
Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Cunningham D, Allum WH, Stenning SP, Thompson JN, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45(10):1890–6.
Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57.
Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant chemotherapy in colon cancer. J Clin Oncol. 2010;28(20):3219–26.
An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131(2):505–11.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Eng J Med. 2015;372(26):2509–20.
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Amatatsu M, Arigami T, Uenosono Y, et al. Programmed death-ligand 1 is a promising blood marker for predicting tumor progression and prognosis in patients with gastric cancer. Cancer Sci. 2018;109(3):814–20.
Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein–Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–15.
Liu X, Choi MG, Kim K, et al. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract. 2020;216(4):152881.
Morihiro T, Kuroda S, Kanaya N, et al. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer. Sci Rep. 2019;9(1):4633.
Brierley JD, Gospodarwivz MK, Wittekind C. TNM classification of malignant tumours. 8th edn. Oxford: Wiley Blackwell; 2017.
Sano T, Kodera Y. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Andre T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–87.
Gevin PG, Colangelo LH, Fumagalli D, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res. 2012;18(23):6531–41.
Choi YY, Bae JM, An JY, et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014;110(2):129–35.
Yang N, Wu Y, Jin M, et al. Microsatellite instability and Epstein-Barr virus combined with PD-L1 could serve as a potential strategy for predicting the prognosis and efficacy of postoperative chemotherapy in gastric cancer. PeerJ. 2021;9:e11481. https://doi.org/10.7717/peerj.11481.
De Rosa S, Sahnane N, Tibiletti MG, et al. EBV+ and MSI gastric cancer harbor high PD-L1/PD-1 expression and high CD8* intratumoral lymphocytes. Cancers. 2018;10(4):102.
Acknowledgment
The authors thank Yuka Nakamura for technical assistance, and also thank Georgia Lenihan-Geels, PhD, and Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Number (C) 21K06939.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akimoto, E., Kuwata, T., Shitara, K. et al. Impact of Programmed Death-Ligand 1 Expression on Mismatch Repair Deficiency and Epstein–Barr Virus Status on Survival Outcomes in Patients with Stage II/III Gastric Cancer After Surgery. Ann Surg Oncol 30, 5227–5236 (2023). https://doi.org/10.1245/s10434-023-13266-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13266-0