Abstract
Introduction
Despite lymph node metastases (LNMs) being associated with worse survival, adequate lymph node evaluation (LNE) has not been universally adopted for intrahepatic cholangiocarcinoma (ICC). We sought to evaluate trends in LNE, predictors of LNE and LNM, as well as the role of adequate lymphadenectomy in stratifying patients relative to survival.
Methods
Patients who underwent curative-intent liver resection for ICC (2010–2019) were identified from the National Cancer Database and stratified according to LNE: 0, 1–5 (inadequate lymphadenectomy) and ≥6 (adequate lymphadenectomy). Multivariate logistic regression was utilized to assess predictors of LNE and LNM. Overall survival and receipt of adequate lymphadenectomy were assessed relative to LNM and log-odds of lymph nodes (LODDS).
Results
Among 6507 patients, adequate lymphadenectomy was performed in only 1118 (17.2%) patients, although compliance with adequate lymphadenectomy increased over time (2010–2012: 14.2% vs. 2016–2019: 18.9%; p < 0.001). After controlling for relevant factors, region (reference: Northeast; Midwest: odds ratio [OR] 1.90, 95% confidence interval [CI] 1.48–2.44; South: OR 1.64, 95% CI 1.28–2.10; West: OR 1.83, 95% CI 1.37–2.44) and preoperative nodal status (reference: cN0; cNx: OR 2.18, 95% CI 1.68–2.95; cN1: OR 3.88, 95% CI 3.02–4.98) strongly predicted adequate lymphadenectomy. Furthermore, adequate lymphadenectomy resulted in higher odds of detecting ≥1 LNMs (OR 2.63, 95% CI 2.25–3.08), regardless of preoperative nodal status. Adequate lymphadenectomy demonstrated an improved ability to stratify patients relative to 5-year survival based on LNM (N0: 51.3% vs. N1: 30.6% vs. N2: 13.7%; p < 0.001) and LODDS (LODDS1: 50.7% vs. LODDS2: 27.4% vs. LODDS3: 15.7%; p < 0.001).
Conclusions
Compliance with adequate lymphadenectomy at the time of surgery for ICC remains suboptimal with marked regional variations. Adequate lymphadenectomy was associated with higher odds of detecting LNM and improved survival stratification relative to both LNM and LODDS. Greater emphasis on nodal evaluation is required to ensure optimal management of ICC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignancy of the liver that accounts for 10–15% of all primary liver cancers.1 Although ICC has traditionally predominated in Eastern countries, the incidence of ICC has doubled to 2.1/100,000 person-years in Western countries over the last decade.1,2,3,4 Despite advances in multimodal therapy, surgical resection remains the only potentially curative therapeutic strategy.4 However, long-term prognosis remains dismal, in part due to the high rates of locally advanced disease at diagnosis and recurrence after liver resection, resulting in relatively poor 5-year overall survival (OS) of 25–30%.5
Regional lymphadenectomy is a widely adopted surgical practice to stage patients accurately and remove occult nodal metastases across a range of gastrointestinal cancers.6,7,8,9,10,11 While still debated, the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual recommends routine lymph node evaluation (LNE) of at least six lymph nodes (adequate lymphadenectomy) as part of the surgical management of ICC.12 Lymphadenectomy in the setting of ICC has not been universally adopted, despite lymph node metastases (LNMs) being a significant predictor of worse long-term prognosis.12,13 The debate around the adoption of lymphadenectomy for ICC is due in part to the relatively limited evidence on whether LNE confers a survival benefit. While some studies have demonstrated improved survival associated with adequate lymphadenectomy for ICC,14,15 other data have failed to note a survival benefit and have argued that routine LNE is unnecessary at the time of resection.16
To date, trends in the utilization of lymphadenectomy across a large, nationally representative patient sample have not been well-defined. Furthermore, factors associated with lymphadenectomy, as well as ‘adequate’ lymphadenectomy (i.e., ≥6 LNEs), have not been previously examined. As such, the objective of the current study was to define trends and patterns of LNE for ICC using a large, national database. In particular, we sought to characterize the demographic, facility, and clinicopathologic factors associated with ICC LNE and LNM, as well as examine the impact of adequate lymphadenectomy in stratifying patients with ICC relative to long-term prognosis.
Methods
Data Source and Patient Selection
Patients who underwent curative-intent liver resection for ICC between 2010 and 2019 were retrospectively identified from the National Cancer Database (NCDB). The NCDB, formed in 1989, is a cancer database derived from the hospital registry data of more than 1500 Commission on Cancer (CoC)-accredited hospitals. NCDB data represent >70% of all newly diagnosed cancer cases and >40 million historical records in the US. The NCDB Participant User File was utilized to identify patients with ICC using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) primary site and histology codes. Specifically, patients with ICC were identified using the primary site code for liver (22.0) and the histology code for cholangiocarcinoma (8160), along with the primary site code for intrahepatic bile duct (22.1) and histology codes for malignant neoplasm (8000), malignant tumor cells (8001), carcinoma (8010), undifferentiated carcinoma (8020), adenocarcinoma (8140), and cholangiocarcinoma (8160). Furthermore, patients with the site-specific procedure codes 20–60, 65, and 66 were included in the analytical cohort. Patients who underwent palliative surgery, presented with metastatic disease, had macroscopic residual disease at the surgical resection margin (R2 margins), underwent liver resection prior to 2010, or had missing data on LNE were excluded. The cohort was restricted to the post-2010 period as multiple variables of interest were modified in the NCDB database in 2010. The Institutional Review Board of Ohio State University approved this study.
Study Variables, Definitions and Outcomes
Data were collected on patient demographics, including age, sex, race (i.e., White, minority), insurance status (i.e., uninsured, private insurance, governmental insurance, unknown), median income (i.e., < $50,354, ≥$50,354), rurality (i.e., metropolitan, urban, rural), and educational attainment (i.e. no high school diploma: < 10.9%, ≥10.9%). Facility characteristics included facility type (i.e., community cancer program [CCP], comprehensive community cancer program [CCCP], academic/research program, integrated network cancer program [INCP]), facility location (i.e., Northeast, Midwest, South, West) and great circle distance from facility, in miles. The annual surgical hospital case volume was defined as the number of surgical procedures for ICC that each facility performed during the study period divided by the number of years the facility reported at least one case in the database. Hospitals in the top 10th percentile for annual surgical hospital case volume were then designated as high-volume hospitals. Furthermore, data on operative and clinicopathologic variables included year of diagnosis, Charlson–Deyo Score (CDCC; i.e., 0, 1, ≥2), time from diagnosis to definitive surgery in days, receipt of neoadjuvant chemotherapy, histological grade (i.e., well/moderately differentiated, poorly/undifferentiated), resection margin status (i.e., microscopically negative margins [R0], microscopically positive margins [R1]), tumor size in centimeter (cm), lymphovascular invasion, and preoperative lymph node status (i.e., clinically node-negative [cN0], clinically node-positive [cN1] and clinically suspicious nodes [cNx]). Short-term outcomes such as length of stay (LOS) in days, 30-day unplanned readmission, and 90-day mortality were also assessed.
The primary variables of interest were LNE, as well as LNM confirmed on histopathology. LNE was categorized as 0, 1–5 (inadequate lymphadenectomy) and ≥6 (adequate lymphadenectomy), while LNM was classified as N0 (0 LNMs), N1 (1–2 LNMs) and N2 (≥3 LNMs), in line with the recently proposed classification system for LNM in ICC.15 Log-odds of lymph nodes (LODDS) were also calculated using the previously validated formula: logarithm (number of positive lymph nodes + 0.5/number of negative lymph nodes + 0.5),17,18 and categorized into LODDS1 (< 25th percentile), LODDS2 (≥25th percentile to < 75th percentile) and LODDS3 (≥75th percentile).
Statistical Analysis
Continuous data were presented as median (interquartile range [IQR]), while categorical variables were reported as frequencies (%). Patients were stratified according to LNE: 0 LNEs, 1–5 LNEs (inadequate lymphadenectomy) and ≥6 LNEs (adequate lymphadenectomy). Patient and operative characteristics were compared among these groups using the Chi-square test for categorical variables and the Kruskal–Wallis H test for comparisons between continuous variables. The Cochrane–Armitage test was utilized to quantify trends. Multivariate logistic regression was used to identify factors associated with adequate lymphadenectomy (≥6 LNEs), as well as the detection of LNM (≥1 LNM). Odds ratios (OR) with 95% confidence intervals (CIs) were estimated. Only variables significant on univariate logistic regression were entered into the multivariate model. Missing values were treated using multiple imputation with chained equations (MICE). Specifically, variables with ≥10% and < 50% missing values were treated with MICE, while variables with >50% missing values were discarded from subsequent models, in line with recommendations specific to the NCDB.19 The Kaplan–Meier method was utilized to assess OS; OS relative to LNM and LODDS was assessed using the log-rank test. The level of significance was set at α = 0.05 for all statistical analyses. All statistical analyses were performed with STATA, v17 (StataCorp LLC, College Station, TX, USA).
RESULTS
Among 6507 patients who underwent curative-intent resection for ICC, an adequate lymphadenectomy was not performed (i.e., < 6 LNEs) in the majority of patients (n = 5389, 82.8%). Specifically, 2637 (40.5%) patients did not undergo any LNE, 2752 (42.3%) underwent an inadequate lymphadenectomy, while an adequate lymphadenectomy was performed in only 1118 (17.2%) patients. Overall, median age was 66.0 years (IQR 58.0–72.0) and 3010 (46.3%) patients were male. Patients who underwent an adequate lymphadenectomy were younger (0 LNEs: 66.0 years [IQR 59.0–73.0] vs. 1–5 LNEs: 65.5 years [IQR 57.0–72.0] vs. ≥6 LNEs: 65.0 years [IQR 56.0–71.0]; p < 0.001) and less likely to belong to a minority race/ethnicity (0 LNEs: n = 449 [17.0%] vs. 1–5 LNEs: n = 418 [15.2%] vs. ≥6 LNEs: n = 154 [13.8%]; p = 0.028). Furthermore, patients who had an adequate lymphadenectomy were also more likely to be privately insured (0 LNEs: n = 902 [34.2%] vs. 1–5 LNEs: n = 1090 [39.6%] vs. ≥6 LNEs: n = 491 [43.9%]; p < 0.001), reside in an urban area (0 LNEs: n = 286 [11.4%] vs. 1–5 LNEs: n = 324 [12.4%] vs. ≥6 LNEs: n = 158 [15.2%]; p = 0.013) and in areas with high educational attainment (0 LNEs: n = 1248 [55.0%] vs. 1–5 LNEs: n = 1379 [57.9%] vs. ≥6 LNEs: n = 577 [59.6%]; p = 0.029). Of note, patients had to travel a greater distance to obtain an adequate lymphadenectomy (0 LNEs: 15.9 miles [IQR 6.7–42.2] vs. 1–5 LNEs: 19.7 miles [IQR 8.0–53.8] vs. ≥6 LNEs: 20.4 miles [IQR 8.3–67.0]), and were more likely to be treated at a high-volume facility (0 LNEs: n = 562 [23.1%] vs. 1–5 LNEs: n = 703 [27.8%] vs. ≥6 LNEs: n = 322 [31.6%]) [both p < 0001].
Notably, patients who underwent an adequate lymphadenectomy had the shortest time from diagnosis to surgery (0 LNEs: 43.0 days [IQR 20.0–75.0] vs. 1–5 LNEs: 39.0 days [IQR 18.0–75.0] vs. ≥6 LNEs: 35.0 days [IQR 14.0–64.0]) and smallest median tumor size (0 LNEs: 5.0 cm [IQR 3.4–7.5] vs. 1–5 LNEs: 5.5 cm [IQR 3.5–8.3] vs. ≥6 LNEs: 4.7 cm [IQR 2.7–7.5]), yet were more likely to present with lymphovascular invasion (0 LNEs: n = 685 [31.9%] vs. 1–5 LNEs: n = 944 [40.1%] vs. ≥6 LNEs: n = 421 [43.5%]) [all p < 0.001]. Not surprisingly, patients with clinically suspicious or ‘positive’ nodal disease were the most likely to undergo an adequate lymphadenectomy (cN0: n = 554 [13.3%] vs. cNx: n = 112 [25.3%] vs. cN1: 182 [36.1%]; p < 0.001). Of note, adequate lymphadenectomy resulted in the more frequent detection of 1–2 LNMs (N1 disease; 1–5 LNEs: n = 623 [22.7%] vs. ≥6 LNEs: n = 279 [25.0%]) and ≥3 LNMs (N2 disease; 1–5 LNEs: n = 94 [3.4%] vs. ≥6 LNEs: n = 242 [21.6%]) [p < 0.001]. However, patients who had an adequate lymphadenectomy experienced a longer median LOS (0 LNEs: 5.0 days [IQR 5.0–8.0] vs. 1–5 LNEs: 6.0 days [IQR 5.0–9.0] vs. ≥6 LNEs: 7.0 days [IQR 5.0–11.0]; p < 0.001) (Table 1).
Trends in Lymphadenectomy
The majority of operations were performed between 2016 and 2019 (2010–2012: n = 1362 [20.9%] vs. 2013–2015: n = 1860 [28.6%] vs. 2016–2019: n = 3285 [50.5%]), and the overall utilization of lymphadenectomy (≥1 LNE) increased incrementally over the study period (2010–2012: n = 756 [55.5%] vs. 2013–2015: n = 1088 [58.5%] vs. 2016–2019: n = 2026 [61.7%]) [both p < 0.001] (Fig. 1a). Specifically, patients were more likely to undergo adequate lymphadenectomy over time (Cochrane–Armitage test of trend, p < 0.001; 2010–2012: n = 194 [14.2%] vs. 2013–2015: n = 303 [16.3%] vs. 2016–2019: n = 621 [18.9%]; p < 0.001) (Fig. 1b). Furthermore, marked regional variations in adequate lymphadenectomy were noted. Specifically, patients treated in facilities located in the Northeast were the least likely to undergo an adequate lymphadenectomy (Northeast: n = 187 [12.4%] vs. Midwest: n = 324 [19.9%] vs. South: n = 393 [17.3%] vs. West: n = 173 [18.7%]), while being the most likely to not undergo any nodal evaluation (Northeast: n = 689 [45.7%] vs. Midwest: n = 635 [38.9%] vs. South: n = 912 [40.2%] vs. West: n = 350 [37.9%]) [p < 0.001]. Of note, the Northeast continued to lag behind other regions in achieving adequate lymphadenectomy throughout the study periods examined (Fig. 2). Notably, even after adjusting for other factors, marked regional variations persisted as patients in the Midwest (OR 1.90, 95% CI 1.48–2.44), South (OR 1.64, 95% CI 1.28–2.10), and West (OR 1.83, 95% CI 1.37–2.44) were more likely to be offered an adequate lymphadenectomy compared with individuals in the Northeast.
Various other demographic, facility, and clinicopathologic characteristics were associated with receipt of adequate lymphadenectomy. For example, patients who underwent surgery in 2016–2019 (reference: 2010–2013; OR 1.45, 95% CI 1.23–1.70), individuals living in urban areas (reference: metropolitan; OR 1.36, 95% CI 1.06–1.74), patients who underwent surgery at high-volume hospitals (reference: low-volume hospitals; OR 1.67, 95% CI 1.25–2.22), and individuals with preoperative cN1 and cNx nodal status (reference: cN0; cNx: OR 2.18, 95% CI 1.62–2.95; cN1: OR 3.88, 95% CI 3.02–4.98) and lymphovascular invasion (reference: no lymphovascular invasion; OR 1.27, 95% CI 1.07–1.52) were more likely to receive an adequate lymphadenectomy. Interestingly, patients with a high comorbidity burden (CDCC ≥2) [reference: CDCC 0; CDCC 1: OR 0.81, 95% CI 0.66–0.98; CDCC ≥2: OR 0.71, 95% CI 0.54–0.92] and large tumor size (>5.0 cm) [reference: ≤5.0 cm; >5.0 cm: OR 0.74, 95% CI 0.63–0.87) were less likely to have an adequate lymphadenectomy (Table 2).
Predictors of Lymph Node Metastases
Patient demographic and facility characteristics did not impact the odds of detecting LNM. Lymphovascular invasion (reference: no lymphovascular invasion; OR 3.27, 95% CI 2.80–3.83), R1 resection margins (reference: R0 margins; R1 margins: OR 1.80, 95% CI 1.53–2.13), receipt of neoadjuvant chemotherapy (reference: no neoadjuvant chemotherapy; OR 1.29, 95% CI 1.05–1.58) and high comorbidity burden (reference CDCC 0; CDCC ≥2: OR 0.73, 95% CI 0.56–0.95) were associated with the detection of at least one LNM. After controlling for these factors, patients who underwent an adequate lymphadenectomy still had 163% higher odds of detecting ≥1 LNM compared with those who received an inadequate lymphadenectomy (OR 2.63, 95% CI 2.25–3.08) (Table 3). Interestingly, this association persisted regardless of preoperative lymph node status; specifically, patients with an adequate lymphadenectomy had higher odds of detection of ≥1 LNM in both cN0/cNx (OR 2.18, 95% CI 1.75–2.70) and cN1 (OR 2.31, 95% CI 1.30–4.09) [electronic supplementary Table 1].
Impact of Adequate Lymphadenectomy
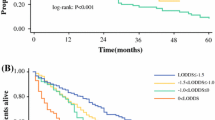
The median OS of the cohort was 44.3 months (95% CI 41.9–46.2), while 5-year OS was 40.5% (95% CI 39.0–42.0%). Adequate lymphadenectomy of ≥6 LNEs, compared with inadequate lymphadenectomy, resulted in improved prognostic stratification among patients with N0 (0 LNMs), N1 (1–2 LNMs) and N2 (≥3 LNMs) disease. Specifically, among patients who underwent inadequate lymphadenectomy, although N1 disease resulted in markedly worse 5-year OS compared with N0 disease (15.6% vs. 44.9%; p < 0.001), no survival differences were noted among patients with N1 and N2 disease (15.6% vs. 21.2%; p = 0.06), respectively. Conversely, patients who underwent adequate lymphadenectomy demonstrated markedly better prognostic stratification among patients with both N0 and N1 disease (51.3% vs. 30.6%; p < 0.001), as well as N1 and N2 disease (30.6% vs. 13.7%; p < 0.001). The improved ability to stratify survival provided by adequate lymphadenectomy was further confirmed using the LODDS classification system. In particular, there was no difference in 5-year OS among patients with LODDS1 and LODDS2 (44.6% vs. 44.2%; p = 0.86) nodal disease among individuals who underwent an inadequate lymphadenectomy, although patients with LODDS2 disease did demonstrate an improved 5-year OS versus LODDS3 in this subset of patients (44.2% vs. 17.0%, p < 0.001). In contrast, among patients who had an adequate lymphadenectomy, there were 5-year OS differences among individuals with both LODDS1 and LODDS2 nodal disease (50.7% vs. 27.4%; p < 0.001), as well as LODDS2 and LODDS3 nodal disease (27.4% vs. 15.7%; p = 0.001) (Fig. 3).
DISCUSSION
The significance of nodal metastases in cancer has evolved over the past century, transitioning from the ‘Halstedian’ concept of nodal metastases representing stage migration, to the Fisher–Cady model of nodal metastases representing poor underlying tumor biology.6 In modern surgical oncology, LNE is an important cornerstone in the accurate staging and prognostic stratification of patients with various gastrointestinal and hepatobiliary cancers, such as colorectal cancer, pancreatic cancer, gastric cancer, fibrolamellar hepatocellular carcinoma (HCC) and gallbladder carcinoma.6,7,8,9,10,11 However, the role of LNE in hepatobiliary tumors remains heterogenous; while not performed for primary HCC or metastatic liver tumors, LNE is recommended for fibrolamellar hepatocellular cancer, perihilar cholangiocarcinoma, and gallbladder cancer.10,20,21 However, the performance of routine LNE and extent of LNE for patients with ICC has remained a subject of debate, despite LNM being a strong adverse prognostic factor.13 ICC was included in the same staging paradigm as HCC until the 7th edition of the AJCC staging manual, while it was not until the 8th edition of the AJCC staging manual that routine LNE of ≥6 lymph nodes was explicitly recommended.12,22 However, compliance with this recommendation has generally been poor.23 The current study was important because it defined the predictors, patterns and impact of LNE across the US using a large, nationally representative cohort. Notably, although adequate lymphadenectomy (≥6 LNEs) was more frequently adopted across the US over time, marked regional variations continued to exist, with the Northeast lagging behind all other regions. Perhaps not surprisingly, suspicious preoperative lymph node status was the strongest predictor of adequate lymphadenectomy at the time of surgery (reference: cN0; cNx: OR 2.18, 95% CI 1.62–2.95; cN1: OR 3.88, 95% CI 3.02–4.98). Furthermore, the performance of an adequate lymphadenectomy resulted in markedly higher odds to detect ≥1 LNM, with this trend persisting regardless of preoperative lymph node status. Of note, adequate lymphadenectomy resulted in improved prognostic stratification based on both LNM and LODDS criteria.
Traditionally, LNE for ICC was largely at the discretion of the treating surgeon, based on clinical judgment and preoperative perception of nodal disease risk. With the current recommendations of the 8th edition AJCC staging manual, there are now standardized recommendations to incorporate routine lymphadenectomy into the surgical management of patients with ICC.12 In particular, adequate lymphadenectomy, defined as the assessment of ≥6 lymph nodes, is now recommended in all patients with ICC.12 Despite these guidelines, the adoption of routine lymphadenectomy of ≥6 lymph nodes for ICC has been slower than anticipated.23,24 The current study expands on previous reports by utilizing a large, nationally representative cohort of patients that included time periods following the publication of the 8th edition AJCC recommendations. Of note, adequate lymphadenectomy, while slightly increasing over time, remained very low across the US (2010–2012: n = 194 [14.2%] vs. 2013–2015: n = 303 [16.3%] vs. 2016–2019: n = 621 [18.9%]; p < 0.001). In fact, despite the increasing trend, adequate lymphadenectomy was still only performed for one in five patients. This was despite the relatively steep rise in incidence of ICC across the study period, with 3285 cases included in the cohort between 2016 and 2019, compared with 1362 cases between 2010 and 2012. The rise in incidence may in part be due to the way time periods were categorized in the current study, with a slightly longer duration of the final time period. However, this trend is consistent with the globally increasing incidence of ICC.1,2,3,4 The underlying cause of this increasing incidence is likely multifactorial, ranging from improved diagnostic techniques to changes in ICD codes over time to increased risk factors for ICC (i.e., obesity, diabetes, etc.).2 Of note, there were marked regional variations in the performance of lymphadenectomy throughout the time periods examined; patients in the Northeast were the least likely to undergo adequate lymphadenectomy across all time periods. In fact, after adjusting for demographic, facility, and clinicopathologic characteristics, patients in the Midwest, South, and West were 90%, 64%, and 83% more likely to undergo adequate lymphadenectomy compared with patients in the Northeast, respectively. Of note, a previous Surveillance, Epidemiology, and End Results (SEER)-based study had similarly reported low utilization of adequate lymphadenectomy in the Northeast versus the Midwest and West.23 Moreover, patients treated at high-volume hospitals were 67% more likely to receive an adequate lymphadenectomy, reaffirming the widely documented relationship of high hospital volume with improved quality of care.25 In aggregate, these data highlight the marked geographic and hospital variation in the adoption of the AJCC recommendations.
Notably, perception of preoperative lymph node “positivity” was the factor most associated with adequate lymphadenectomy at the time of surgery. These results are intuitive as most surgeons would be strongly inclined to perform LNE in patients with a high preoperative suspicion of LNM.2 To this point, in the present study, compared with cN0, patients with cN1 and cNx had 288% and 118% higher odds of receiving an adequate lymphadenectomy, respectively. Moreover, patients who underwent an adequate lymphadenectomy had 163% higher odds of at least one LNM compared with individuals who had an inadequate lymphadenectomy. Notably, adequate lymphadenectomy aided in the detection of pathological LNM in patients with both cN0/cNx and cN1 nodal status. These findings highlight that even patients who did not appear to have suspicious nodal disease on preoperative imaging benefited from adequate lymphadenectomy. While preoperative factors have been utilized in several predictive models of pathological LNM, the overall predictive performance of these models has generally been poor.26,27,28,29,30 As such, given the inability to predict which patient may or may not have LNM in the preoperative setting, even patients with clinically node-negative/low-suspicion nodal disease on preoperative imaging likely benefit from lymphadenectomy, in part due to a greater likelihood of accurate staging.31,32,33
The primary benefits of adequate lymphadenectomy include accurate staging and prognostic stratification post-resection, as well as possible clearance of occult metastases from the locoregional nodal basin.34,35 The extent of nodal evaluation and varied nodal classification systems for ICC remain controversial. Of note, the pathological LNM classification system for ICC differs from other biliary tract cancers in that it only differentiates between LNM (N1) and non-LNM (N0) disease. In contrast, LNM for gallbladder carcinoma, perihilar cholangiocarcinoma, and distal cholangiocarcinoma are classified as N0 (0 LNMs), N1 (1–3 LNMs) and N2 (≥4 LNMs).12 Recently, a new nodal staging system for ICC was proposed that further subdivided LNM into N1 (1–2 LNMs) and N2 (≥3 LNMs) disease.15 In addition, rather than traditional staging systems that stratify prognosis based on the number of LNMs, a growing body of literature has suggested alternative lymph node staging systems such as the LODDS classification system.17,18,36 The benefit of LODDS over LNM is that it also takes into account LNE, resulting in improved prognostic discrimination.36 In the current study, adequate lymphadenectomy was associated with markedly better stratification relative to survival using both the LNM and LODDS systems. As such, these results highlight the benefit of adequate lymphadenectomy in accurately stratifying patient prognosis.
The results of the current study should be interpreted in light of several limitations. As the study utilized retrospective data from an administrative database, it was prone to residual selection bias and coding errors. Furthermore, the NCDB does not provide information on recurrent disease; as such, the impact of adequate lymphadenectomy on overall risk, timing, and patterns of recurrence could not be assessed. Moreover, as the NCDB does not report data on perioperative complications, an analysis on the impact of adequate lymphadenectomy on short-term outcomes could not be conducted. As has been previously documented in the NCDB,19,37 the presence of missing data may pose analytic challenges; specifically, although CA19-9 is a key variable in models related to ICC, it was excluded from the analyses due to a high proportion of missing values (>50%).19 However, the impact of the missing data effect was mitigated through the use of MICE procedures. Only data up until 2019 could be accessed in the present study. Future studies with a longer study duration may be beneficial to evaluate the long-term adherence to guidelines recommending LNE.
CONCLUSION
Although increasingly adopted across the US, performance of lymphadenectomy that includes ≥6 LNs remains extremely low with marked regional variations. Adequate lymphadenectomy was however associated with markedly higher odds of detecting LNM, regardless of preoperative nodal status. Furthermore, compared with inadequate lymphadenectomy, adequate lymphadenectomy resulted in better prognostic discrimination relative to survival using both the LNM and LODDS classification systems. Surgeons should strive to incorporate routine lymphadenectomy of >6 lymph nodes in the surgical management of patients with ICC. Not only would the use of adequate lymphadenectomy for ICC increase compliance with AJCC guidelines, staging of the nodal basin would result in better prognostic stratification of patients, which may inform adjuvant treatment choices and eligibility for clinical trials.
References
Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208(1):134–47. https://doi.org/10.1016/j.jamcollsurg.2008.09.007.
Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–89. https://doi.org/10.1016/j.jhep.2014.01.021.
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. https://doi.org/10.1097/SLA.0b013e318176c4d3.
Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The clinical management of cholangiocarcinoma in the United States and Europe: A comprehensive and evidence-based comparison of guidelines. Ann Surg Oncol. 2021;28(5):2660–74. https://doi.org/10.1245/s10434-021-09671-y.
Bagante F, Spolverato G, Weiss M, et al. Defining long-term survivors following resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2017;21(11):1888–97. https://doi.org/10.1007/s11605-017-3550-7.
Rosen ST (series editor). Cancer Treatment and Research. Volume 168. Springer Nature
Han J, Noh KT, Min BS. Lymphadenectomy in colorectal cancer: Therapeutic role and how many nodes are needed for appropriate staging? Curr Colorectal Cancer Rep. 2017;13(1):45–53. https://doi.org/10.1007/s11888-017-0349-6.
Erdem S, Bolli M, Müller SA, von Flüe M, White R, Worni M. Role of lymphadenectomy in resectable pancreatic cancer. Langenbeck’s Arch Surg. 2020;405(7):889–902. https://doi.org/10.1007/s00423-020-01980-2.
Degiuli M, De Manzoni G, Di Leo A, et al. Gastric cancer: current status of lymph node dissection. World J Gastroenterol. 2016;22(10):2875–93. https://doi.org/10.3748/wjg.v22.i10.2875.
Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215(6):820–30. https://doi.org/10.1016/j.jamcollsurg.2012.08.001.
Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79(5):892–9. https://doi.org/10.1002/(SICI)1097-0142(19970301)79:5%3c892::AID-CNCR4%3e3.0.CO;2-E.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. https://doi.org/10.3322/caac.21388.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. https://doi.org/10.1053/j.gastro.2013.10.013.
De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–5. https://doi.org/10.1200/JCO.2011.35.6519.
Zhang X-F, Xue F, Dong D-H, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann Surg. 2021;274(6):e1187–95. https://doi.org/10.1097/SLA.0000000000003788.
Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;145(4):411–6. https://doi.org/10.1016/j.surg.2008.11.010.
Li R, Lu Z, Sun Z, et al. A nomogram based on the log odds of positive lymph nodes predicts the prognosis of patients with distal cholangiocarcinoma after surgery. Front Surg. 2021;8:757552. https://doi.org/10.3389/fsurg.2021.757552.
Sun Z, Xu Y, Li DM, et al. Log odds of positive lymph nodes: A novel prognostic indicator superior to the number-based and the ratio-based n category for gastric cancer patients with R0 resection. Cancer. 2010;116(11):2571–80. https://doi.org/10.1002/cncr.24989.
Merkow RP, Rademaker AW, Bilimoria KY. Practical guide to surgical data sets: National Cancer Database (NCDB). JAMA Surg. 2018;153(9):850–1. https://doi.org/10.1001/jamasurg.2018.0492.
Amini N, Spolverato G, Kim Y, et al. Lymph node status after resection for gallbladder adenocarcinoma: prognostic implications of different nodal staging/scoring systems. J Surg Oncol. 2015;111(3):299–305. https://doi.org/10.1002/jso.23813.
Bagante F, Tran T, Spolverato G, et al. Perihilar cholangiocarcinoma: number of nodes examined and optimal lymph node prognostic scheme. J Am Coll Surg. 2016;222(5):750–9. https://doi.org/10.1016/j.jamcollsurg.2016.02.012.
Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. https://doi.org/10.1245/s10434-010-0985-4.
Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for intrahepatic cholangiocarcinoma: has nodal evaluation been increasingly adopted by surgeons over time? A national database analysis. J Gastrointest Surg. 2018;22(4):668–75. https://doi.org/10.1007/s11605-017-3652-2.
Zhang XF, Chakedis J, Bagante F, et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg. 2018;105(7):857–66. https://doi.org/10.1002/bjs.10827.
Munir MM, Alaimo L, Moazzam Z, et al. Textbook oncologic outcomes and regionalization among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2023;127(1):81–9. https://doi.org/10.1002/jso.27102.
Navarro JG, Lee JH, Kang I, Rho SY, Choi GH, Han DH. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: Do all require lymph node dissection? Int Hepato-Pancreato-Biliary Assoc. 2020;22(10):1411–9. https://doi.org/10.1016/j.hpb.2020.01.009.
Ji G, Zhu F, Zhang Y, et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol. 2019;29(7):3725–35.
Meng Z-W, Lin X-Q, Zhu J-H, Han S-H, Chen Y-L. A nomogram to predict lymph node metastasis before resection in intrahepatic cholangiocarcinoma. J Surg Res. 2018;226:56–63. https://doi.org/10.1016/j.jss.2018.01.024.
Zhou Y, Zhou G, Gao X, Xu C, Wang X, Xu P. Apparent diffusion coefficient value of mass—forming intrahepatic cholangiocarcinoma: a potential imaging biomarker for prediction of lymph node metastasis. Abdom Radiol. 2020;45(10):3109–18. https://doi.org/10.1007/s00261-020-02458-x.
Yoh T, Hatano E, Seo S, et al. Preoperative criterion identifying a low-risk group for lymph node metastasis in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2018;25(6):299–307. https://doi.org/10.1002/jhbp.552.
Yoh T, Cauchy F, Le Roy B, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: a multicenter study. Surg (United States). 2019;166(6):975–82. https://doi.org/10.1016/j.surg.2019.06.025.
Ke Q, Wang L, Lin Z, et al. Prognostic value of lymph node dissection for intrahepatic cholangiocarcinoma patients with clinically negative lymph node metastasis: a multi-center study from China. Front Oncol. 2021;11:585808. https://doi.org/10.3389/fonc.2021.585808.
Yang F, Wu C, Bo Z, Xu J, Yi B, Li J. The clinical value of regional lymphadenectomy for intrahepatic cholangiocarcinoma. Asian J Surg. 2022;45(1):376–80. https://doi.org/10.1016/j.asjsur.2021.06.031.
Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the national cancer database. Int Hepato-Pancreato-Biliary Assoc. 2016;18(1):79–87. https://doi.org/10.1016/j.hpb.2015.07.006.
Bagante F, Spolverato G, Weiss M, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new eighth edition AJCC staging system. J Gastrointest Surg. 2018;22(1):52–9. https://doi.org/10.1007/s11605-017-3426-x.
Chen X, Rong D, Zhang L, et al. Evaluation of nodal status in intrahepatic cholangiocarcinoma: a population-based study. Ann Transl Med. 2021;9(17):1359. https://doi.org/10.21037/atm-21-2785.
Yang DX, Khera R, Miccio JA, et al. Prevalence of missing data in the national cancer database and association with overall survival. JAMA Netw Open. 2021;4(3):211793. https://doi.org/10.1001/jamanetworkopen.2021.1793.
Funding
No sources of funding were used to assist in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
DISCLOSURE
Zorays Moazzam, Laura Alaimo, Yutaka Endo, Henrique A. Lima, and Timothy M. Pawlik have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moazzam, Z., Alaimo, L., Endo, Y. et al. Predictors, Patterns, and Impact of Adequate Lymphadenectomy in Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 30, 1966–1977 (2023). https://doi.org/10.1245/s10434-022-13044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-13044-4