Abstract
Background
D-dimer (DDI) and albumin are prognostic markers for numerous cancers; however, the predictive value of the preoperative DDI-to-albumin ratio (DAR) on the survival and recurrence patterns of gastric cancer (GC) remains unclear.
Objective
The aim of this study was to explore the prognostic value of the DAR in GC.
Methods
Our study included 1766 patients with GC, divided into training and testing cohorts at a ratio of 7:3. Patients were classified into either a high-DAR group (> 0.0145) or low-DAR group (≤ 0.0145) according to the cut-off value of receiver operating characteristic (ROC) curve analysis. The relationship between the DAR and recurrence pattern was analyzed in stage II/III patients.
Results
Eight preoperative hematological factors were included and 17 composite inflammatory markers were constructed. ROC and random forest analyses indicated that among 17 markers, DAR was the best predictor for overall survival (OS) in GC (p < 0.01). High DAR was significantly associated with poor OS (hazard ratio [HR] 1.89, p < 0.001) and recurrence-free survival (RFS; HR 1.85, p < 0.001). Subgroup analysis showed no differences in OS and RFS between the high- and low-DAR groups in stage I or pT1/2 or pN0/1 patients; however, in stage II/III or pT3/4 or pN2/3 patients, the high-DAR group had shorter OS and RFS rates than the low-DAR group (p < 0.001). Similar results were found in the testing cohort. According to the multivariate analysis based on the training cohort, five indices, including DAR, cT stage, cN stage, age and body mass index (BMI), were incorporated to establish a nomogram model to predict the long-term prognosis of GC. The model showed comparable forecast performance in predicting OS (C-index: 0.773 vs. 0.786) and RFS (C-index: 0.788 vs. 0.795) compared with pTNM. Recurrence pattern analysis in stage II/III patients showed that the high-DAR group had a higher incidence of peritoneal implantation and early recurrence (ER) than the low-DAR group, and the post-recurrence survival in the high-DAR group was significantly shorter than that in the low-DAR group (p = 0.016).
Conclusion
The preoperative DAR is a new biomarker for the long-term survival prediction of GC. In advanced GC, a preoperative DAR > 0.0145 aids the timely detection of ER and peritoneal recurrence after surgery, thus guiding individual follow-up strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer (GC) is the fifth most frequently diagnosed cancer and the fourth leading cause of cancer deaths worldwide.1 Systemic inflammation and malnutrition are common in cancer patients. Many studies have shown that C-reactive protein (CRP), fibrinogen (FIB), D-dimer (DDI), neutrophils, platelets, and lymphocytes are associated with the prognosis of GC.2,3,4 Serum pre-albumin, albumin, and other nutritional markers have been widely used to predict the prognosis of cancer.5,6,11 In addition, hypercoagulability is often associated with cancer and is thought to be involved in tumor angiogenesis, growth, and proliferation.7,8,9 Therefore, serum DDI, as a factor characterizing fibrinolysis and coagulation cascade activation, has been widely studied in many tumors such as breast cancer and colorectal cancer, and has been used as a marker to predict tumor load and poor prognosis.10,11
In recent years, an increasing number of studies have attempted to predict the prognosis of tumors by using complex systemic inflammatory markers, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammatory index (SII), platelet-to-albumin ratio (PAR), neutrophil-to-albumin ratio (NAR), FIB-to-albumin ratio (FAR), derived NLR (dNLR), and CRP-to-albumin ratio (CAR), which have been demonstrated to be associated with prognosis in a variety of tumors.3,12,13,14 However, it remains unclear whether any of these are good predictive markers of prognosis in GC.
In the present study, we included eight preoperative hematological indicators (DDI, FIB, platelets, white blood cells, neutrophils, albumin, hemoglobin, and lymphocytes), which were related to the systemic inflammatory response and nutritional and blood hypercoagulability status of GC patients. Receiver operating characteristic (ROC) curve and random forest analyses were conducted to compare the predictive efficacy on overall survival (OS) among 17 composite immune inflammatory markers, which were generated by these eight indicators. The newly discovered DDI-to-albumin ratio (DAR) attracted our attention due to its potential predictive value on long-term survival in GC, and its value in predicting recurrence patterns was further explored.
Methods
Patients and Study Design
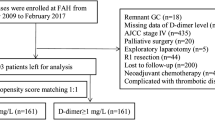
A retrospective analysis was performed on all patients who underwent radical gastrectomy at Fujian Medical University Union Hospital (FMUH) from January 2013 to December 2017. The inclusion criteria were gastric adenocarcinoma was histologically confirmed, no distant metastasis, D2 or modified D2 lymph node dissection was performed, and postoperative pathology confirmed R0 resection, while the exclusion criteria were preoperative neoadjuvant chemotherapy, non-radical (R1/2) resection, presence of other malignant tumors (even in situ cancers), preoperative thrombotic disease and/or long-term anticoagulant therapy, autoimmune diseases and recent corticosteroid therapy, a pathological T stage of T4b, and combined multiple organ resection or incomplete case records. All surgical procedures, including the scope of lymph node dissection, were performed according to the Japanese Gastric Cancer Association guidelines, while staging was performed according to the corresponding 8th edition of the American Joint Committee on Cancer (AJCC) staging manual.15 In this study, patients with stage II/III disease were treated according to the East Asia standard (D2 gastrectomy followed by postoperative adjuvant chemotherapy),16,17,18 even though perioperative chemotherapy is the preferred treatment option for these patients in Western countries.19 Adjuvant postoperative chemotherapy based on 5-fluorouracil (mostly oxaliplatin with either Xeloda or S-1) was recommended for all patients with stage II or higher advanced GC.20 The present study was approved by the Ethics Committee of FMUH.
Blood samples were collected and assayed (including DDI, FIB, platelets, white blood cells, neutrophils, albumin, hemoglobin, and lymphocytes) within 7 days before surgery. The eight hematological indicators were divided into five upregulated indicators (neutrophils, platelets, white blood cells, FIB, and DDI) and three downregulated indicators (albumin, hemoglobin, and lymphocytes) according to their characteristics in GC development. The 17 composite immune inflammatory markers were obtained by the ratio of each upregulated indicator to each downregulated indicator. Detailed information is presented in Fig. 1a.
Definition
A total of 1766 patients were included in this study. We divided patients into either a training cohort (1236 patients) or a testing cohort (530 patients) at a ratio of 7:3 using the random numbering method attached to the R package.21 ROC curves with Youden’s index correction were generated to estimate the optimal threshold for DAR as a risk factor for OS based on the training cohort. The cut-off value was also applied in the testing cohort. Patients were divided into either a high-DAR group (DAR > 0.0145) or a low-DAR group (≤ 0.0145) according to the cut-off value from the ROC analysis.
OS was measured from the date on which the patient underwent surgery until the date of death from any cause (i.e., deaths not related to cancer were not censored) or the last known follow-up for patients who were still alive. Disease recurrence was defined strictly as evidence of any suspicious lesion found within the body on cross-sectional imaging that suggested a recurrence of disease, with or without tissue-biopsy pathologic confirmation. Recurrence-free survival (RFS) was defined as the period from the date of surgery to the date of recurrence or last follow-up without recurrence. For RFS, patients who died without known tumor recurrence were censored at the last documented evaluation. Recurrences were categorized by the site involved, i.e. locoregional, peritoneal, distant, or multiple, as previously described. 22 The presence of recurrent disease at two or more sites was defined as multiple. Multiple recurrences at the same site were not categorized as ‘multiple’ sites of recurrence. 23 Early recurrence (ER) was defined as recurrence occurring within 12 months, 5 and post-recurrence survival was defined as the period from the date of recurrence to the date of death or final follow-up. For patients with recurrence, there were 13 cases without exact sites or sites of recurrence. We only received information on whether the patients had recurrence at other hospitals; therefore, they were excluded from the analysis of recurrence patterns.
Follow-Up
A postoperative follow-up assessment was performed every 3 months for 2 years and then every 6 months during years 2–5. Most routine follow-up appointments included a physical examination, laboratory testing (including cancer antigen [CA] 19-9, CA72-4, and carcinoembryonic antigen [CEA] level measurements), chest radiography, and abdominopelvic ultrasonography or computed tomography, in addition to an annual endoscopic examination. The final follow-up evaluation was conducted in March 2021 and the median follow-up time was 45.9 months (range 2.1–101.6 months).
Construction of the Nomogram
A Cox proportional hazards regression model was used to identify the independent prognostic factors associated with OS and RFS in the training cohort. Variables with a p value < 0.05 in the univariate analysis were subsequently included in a multivariate analysis. Based on the results of the multivariable analysis, nomograms were formulated by R for predicting 3-year OS and RFS.
Validation and Calibration of the Nomogram
The nomogram was subjected to 1000 bootstrap resamples for internal validation of the primary training cohort. The model performance for predicting outcome was evaluated by calculating the concordance index (C-index) and area under the curve (AUC). Calibration of the nomogram, which measures the discrepancy between the actual and predicted survival of patients, was assessed using a calibration plot by plotting the predicted probability of the nomogram; bootstrapping was used for bias correction.23
Statistical Analysis
Continuous variables were reported as the mean ± standard deviation (SD) or median (interquartile range). Categorical and continuous variables were compared using the Chi-square test or Fisher’s exact test and a t test, respectively. To determine the optimal cut-off threshold of the inflammatory markers for OS, ROC curves with Youden’s index correction were established. To calculate the standard error of the AUC and the difference between two AUC values, ROC curve analysis was performed. Survival analysis was performed using the Kaplan–Meier and log-rank tests. Statistical analyses were performed using SPSS v.25.0 for Windows (IBM Corporation, Armonk, NY, USA) and R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria). P values < 0.05 were considered statistically significant.
Results
Patient Characteristics
A total of 1766 patients with GC were divided into either training or testing cohorts at a ratio of 7:3. The clinicopathological data of the two cohorts were equally comparable (Table 1). Overall, 1236 patients were included in the training cohort, with an average age of 61.6 ± 0.3 years, while the testing cohort consisted of 530 patients with an average age of 61.7 ± 0.5 years. The median follow-up time of all patients was 44.1 months (training cohort 45.5 months; testing cohort 47.2 months).
Screening of Composite Immune Inflammatory Markers
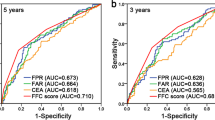
As shown in Fig. 1a, the ratio of each upregulated indicator to each downregulated indicator was calculated to construct 17 composite inflammatory markers. Pearson correlation tests showed that the DAR was not significantly correlated with other indicators (electronic supplementary Fig. S1). ROC analysis based on the training cohort showed that all 17 composite markers could predict the OS of GC. Although the AUC for the DDI-to-hemoglobin ratio (DHR: 0.680) and DDI-to-lymphocyte ratio (DLR: 0.675) was approximately close to the DAR, the DAR was more accurate, with an AUC of 0.683 than the other 16 combinations, including those of classical markers reported in the literature (Fig. 1b and electronic supplementary Fig. S2). Furthermore, the random forest model analysis indicated that the DAR was the most important marker in predicting the OS of GC compared with other markers (electronic supplementary Fig. S3). We therefore focused on the DAR to evaluate its prognostic value for patients with GC.
Association between the Preoperative D-Dimer-to-Albumin Ratio (DAR) and Clinicopathological Characteristics
Based on the training cohort, the optimal cut-off value of the DAR was determined to be 0.0145 according to ROC analysis. Accordingly, 845 (47.8%) patients (581 in the training cohort and 264 in the testing cohort) were classified into the high-DAR group, and 921 (52.2%) patients (655 in the training cohort, 266 in the testing cohort) were classified into the low-DAR group. A scatter plot showed that the preoperative DAR value increased with the progression of pathological stages (electronic supplementary Fig. S4), and the proportion of high-DAR patients also increased with the progression of T, N, and pTNM stages (electronic supplementary Fig. S5). Further analysis of the correlation between the DAR and clinicopathological factors indicated that the high-DAR group showed more progressive pathological stages and larger tumor diameters than the low-DAR group in both the training and testing cohorts (p < 0.05) (electronic supplementary Table S1).
Association between the Preoperative DAR and Survival
The median survival time of the entire group was 35.0 months. Survival analysis in both the training and testing cohorts confirmed a significantly poorer OS and RFS in the high-DAR group than in the low-DAR group (OS: 63.8 vs. 44.2%; RFS: 62.4 vs. 46.6%; p < 0.001) (electronic supplementary Fig. S6). Stratified analysis by overall stage, T stage, and N stage (Fig. 2a–f) revealed no differences in OS and RFS between the low- and high-DAR groups in stage I, pT1-2, or pN0-1 patients. However, in stage II/III, pT3/4, or pN2/3 patients, OS and RFS in the high-DAR group were significantly poorer than those in the low-DAR group (p < 0.05). Although there was significant overlap by definition between stage II/III and pT3-4 or pN2-3 patients, except those with pT2N1 stage, no similar trend in prognosis was found in pT2N1 patients. Accordingly, we compared the prognostic difference between the high- and low-DAR groups in the pT2N1 patients, which revealed that DAR did not have a similar trend in prognosis in this pT2N1 population (electronic supplementary Fig. S7). Multivariate Cox analysis (electronic supplementary Tables S2 and S3) further revealed that the preoperative DAR was an independent risk factor not only for OS (training cohort: HR 1.47, 95% CI 1.20–1.80, p < 0.001; testing cohort: HR 1.50, 95% CI 1.09–2.07, p = 0.014) but also for RFS (training cohort: HR 1.43; 95% CI 1.17–1.75, p < 0.001; testing cohort: HR 1.59, 95% CI 1.14–2.22, p = 0.006).
KAPLAN–Meier curves of a, c, e OS and b, d, f RFS according to DAR and a, b pTNM, c, d pT, and e, f pN stage among GC patient subgroups in the training (left) and testing (right) cohorts. P values were calculated using a two-sided log-rank test. DAR D-dimer to albumin ratio, GC gastric cancer, OS overall survival, RFS recurrence-free survival
According to the Cox multivariate analysis, OS was independently predicted by the preoperative DAR, age, body mass index (BMI), and cT and cN stage. Therefore, using the training cohort, a nomogram was constructed to predict OS and RFS. The calibration curves for the nomogram showed good agreement between the estimations and clinical outcomes. The C-index value of the model indicated that the nomogram had comparable forecast performance in predicting OS (0.773 vs. 0.786) and RFS (0.788 vs. 0.795) compared with pTNM. Further ROC analysis showed that the nomogram model had a relatively good predictive performance for OS and RFS, with AUC values of 0.810 and 0.837, respectively. Similar results were found in the testing cohort (Fig. 3 and electronic supplementary Fig. S8).
a Nomogram based on the training cohort, for predicting 1-, 3-, and 5-year OS after radical gastrectomy for gastric cancer. Nomogram properties. b, c Calibration and d, e ROC curves of the nomogram for the b, d training and c, e testing cohorts. OS overall survival, ROC receiver operating characteristic, DAR D-dimer to albumin ratio, BMI body mass index, KM Kaplan–Meier, AUC area under the curve
Association between the Preoperative DAR and Recurrence Pattern in Stage II/III Patients
To explore whether the DAR could affect the recurrence pattern of GC, we analyzed the relationship between the preoperative DAR value and recurrence pattern in stage II/III patients. In the present study, 601 (46.9%) of the 1282 patients had recurrence after gastrectomy, and 588 patients had detailed recurrence information. Electronic supplementary Fig. S9 shows the distribution of the first recurrence sites after gastrectomy, including 124 (21.0%) with locoregional recurrence, 108 (18.3%) with peritoneal recurrence, 244 (41.5%) with distant metastasis, and 112 (19.1%) with multiple recurrences. There were no differences in locoregional recurrence (27.7 vs. 35.2%, p = 0.095), distant metastasis (57.6 vs. 59.1%, p = 0.726), or multiple recurrence (18.1 vs. 19.9%, p = 0.095) between the high- and low-DAR groups (p = 0.591). However, the incidence of peritoneal recurrence was significantly higher in the high-DAR group than in the low-DAR group (35.7 vs. 23.9%, p = 0.002) (Fig. 4). In addition, patients in the high-DAR group had significantly worse post-recurrence survival than those in the low-DAR group (p = 0.01) (electronic supplementary Fig. S10).
ER was defined as recurrence occurring within 12 months, as previously described. Compared with patients in the low-DAR group, patients in the high-DAR group exhibited a higher risk of ER (p = 0.005) (electronic supplementary Fig. S11). Further multivariate analyses (Table 2) revealed that DAR (HR1.378, 95% CI 1.04–1.83; p = 0.026), lymphovascular invasion, pT stage, pN stage, and more than three cycles of adjuvant chemotherapy were independent predictive factors for ER.
Discussion
Although the incidence of GC has decreased globally in recent years, it is still the fourth leading cause of cancer deaths in both sexes worldwide.1 Studies have shown that preoperative hematological immune inflammatory indicators are closely related to tumor prognosis;24,25 however, it is still not clear which marker is the best predictor of prognosis in GC. The present study developed a novel composite inflammatory marker based on plasma DDI and albumin, and assessed its prognostic value in GC. Our data first demonstrated that the preoperative DAR is significantly associated with long-term survival and could act as an independent predictive marker for ER and peritoneal recurrence in advanced GC.
Hypercoagulability is common in malignant tumors. Previous studies have shown that changes in the activation state of the coagulation/procoagulant system are related to tumor angiogenesis, invasion, and metastasis.26 The DDI is the final degradation product of fibrin and represents coagulation activation and hypercoagulation states. It has been confirmed that DDI is associated with cellular signaling and can promote cell growth and angiogenesis.27 Researchers believe that DDI may reflect the presence of micrometastases or circulating tumor cells, which may be responsible for tumor recurrence.28,29 Di Nisio et al.30 reported that DDI could also induce the spread of tumors by stimulating tumor cells to adhere to endothelial cells. Previous studies have reported that DDI levels are elevated in the plasma of various solid tumor patients, including breast, lung, melanoma, colorectal, esophageal, and GC patients.10,11,31,32 In most tumors, elevated DDI levels indicate a more advanced stage and an increased risk of death. Blackwell and colleagues first discovered that plasma DDI levels are markers of lymphovascular invasion, clinical stage, and lymph node involvement in operable breast cancer.10 In patients with colorectal cancer, DDI levels have been shown to correlate with the depth of tumor invasion at the time of surgical excision.33,34 We also found that preoperative DDI elevation was associated with a more advanced pathological stage (p < 0.05). These results suggested that DDI level may be correlated with tumor burden.
In addition to hypercoagulability, malnutrition is also a common complication in malignant tumors. Albumin has been commonly used to determine the nutritional status of patients, while hypoalbuminemia may lead to the downregulation of immune defense mechanisms and resistance to antitumor therapy.35 In addition, serum albumin is influenced by the systemic inflammatory response.36 Thus, preoperative serum albumin levels, as a marker of inflammatory and nutritional status, are also widely used in predicting the prognosis in many malignant tumors. Previous studies have shown that hypoalbuminemia is associated with poor survival in numerous solid cancers, including gastrointestinal, lung, and breast cancers.7,25,37 However, instead of applying albumin alone, combining albumin with other immune inflammatory indicators has been more widely used to predict cancer prognosis in previous studies. For example, Morhij et al.38 combined albumin with CRP to construct a Glasgow prognostic score (GPS), which predicts the prognosis of non-small cell lung cancer (NSCLC). Lin et al.14 found that the fibrin-to-albumin ratio (FAR) has good predictive value for the prognosis of GC.
In the present study, we combined DDI and albumin to develop DAR, which simultaneously represents hypercoagulability and malnutrition in GC. An elevated preoperative DAR may be indicative of an increase in systemic inflammatory response or hypercoagulable state, a decrease in nutritional states due to tumor consumption, or both. Although the AUC of DAR was < 0.7, it was still superior to many indicators reported in the literature. Similar to other inflammatory markers reported in previous studies,39,40,41 DAR represents a surrogate of tumor burden and may be a dynamic variable such that it can change over time with treatment. Thus, we believe that the DAR is more accurate in predicting the long-term prognosis of GC. Recently, a study by Zhang et al.42 revealed that the albumin-to-DDI ratio may be a useful marker for prognosis and chemotherapy efficacy in advanced GC patients with neoadjuvant chemotherapy. Our data showed that the correlation coefficient of DAR and tumor size was 0.85, suggesting that there was strong multicollinearity between DAR and tumor size. However, we included tumor size and DAR in the Cox proportional hazards regression model using the stepwise forward logistic regression method, which resulted in high DAR being an independent risk factor for OS and RFS in GC, especially for those with advanced stage disease. Furthermore, in the high-DAR population, we found that the OS and RFS of the chemotherapy group was significantly better than that of the non-chemotherapy group in stage III patients (p < 0.05), while no significant difference was observed in stage I/II patients (electronic supplementary Figs. S12 and S13). This result indicated that adjuvant chemotherapy may overcome the adverse prognosis seen in high-DAR patients with stage III disease, but not in those with stage I/II disease. Our results suggested that DAR was a cost effective and accessible indicator for prognosis and chemotherapy benefit prediction, and should also be routinely assessed after surgery or during the follow-up period.
The process of metastasis and tumor recurrence requires several steps to occur within a favorable host environment. Malignant cells must first leave the primary tumor, migrate into the lymphovascular and circulatory systems or plant onto other organs, and establish a new blood supply at the metastatic site to survive.11,43 Elevated preoperative DDI levels are related to the depth of tumor invasion and lymphatic vascular invasion.10,44 Furthermore, severe malnutrition leads to unstable DNA replication during cell growth, which may affect tumor recurrence and metastasis.45 Thus, we hypothesized that the preoperative DAR may influence the recurrence patterns of GC. For the first time, we explored the relationship between the preoperative DAR and recurrence patterns in patients with stage II/III GC. Our data showed that patients in the high-DAR group were more likely to undergo peritoneal implantation after surgery because they generally had a more aggressive depth of tumor invasion, which may increase the risk of peritoneal implantation after surgery. In addition, we found that a high DAR was an independent risk factor for ER in patients with stage II/III disease. These findings suggest more aggressive adjuvant therapy and closer follow-up for advanced GC patients with elevated preoperative DAR levels.
Our study had some limitations. First, this was a single-center, retrospective study. Although we divided patients into training and testing cohorts for internal validation, further validation by external data is needed. Second, studies exploring the mechanism of the association between the DAR and recurrence patterns should be further explored. Third, patients with neoadjuvant chemotherapy were excluded since the number of patients was quite low in our center between 2013 and 2017, and the effect of adjuvant chemotherapy on recurrence patterns and post-recurrence survival was not investigated in the present study. Fourth, we failed to include CRP, pre-albumin, and interleukin-6, which are critical inflammatory indicators in the previous literature, and we failed to explore the relationship between the prognosis and dynamic changes of these dynamic variables over treatment period in this retrospective study due to the lack of data. Therefore, future studies should consider incorporating additional blood examination markers to select more reliable indicators.
Conclusion
Our findings demonstrated that the preoperative DAR, based on plasma DDI and albumin, is a promising predictive biomarker for long-term prognosis in GC patients. We also concluded that the preoperative DAR is associated with peritoneal recurrence and may serve as an independent predictive marker for ER in advanced GC. It is rational to suggest that GC patients with increased pretreatment DAR levels may require timely adjuvant therapy and closer surveillance, especially those with advanced disease. Therefore, the results of the present study should be further verified by external data, especially data from Western countries.
References
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012. https://doi.org/10.3390/ijms21114012.
Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–64. https://doi.org/10.3324/haematol.2011.054718.
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. https://doi.org/10.1186/s12916-020-01817-1.
Saito R, Shoda K, Maruyama S, et al. Platelets enhance malignant behaviours of gastric cancer cells via direct contacts. Br J Cancer. 2021;124(3):570–3. https://doi.org/10.1038/s41416-020-01134-7.
Lu J, Xu BB, Zheng ZF, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer. 2019;22(3):536–45. https://doi.org/10.1007/s10120-018-0892-0.
Pang H, Zhang W, Liang X, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: a two-center cohort study. Adv Ther. 2021;38(9):4917–34. https://doi.org/10.1007/s12325-021-01870-z.
Lin Y, Liu Z, Qiu Y, et al. Clinical signifificance of plasma D-dimer and fifibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(10):1494–503. https://doi.org/10.1016/j.ejso.2018.07.052.
Kawai K, Watanabe T. Colorectal cancer and hypercoagulability. Surg Today. 2014;44(5):797–803. https://doi.org/10.1007/s00595-013-0606-5.
Hu C, Chen R, Chen W, et al. Thrombocytosis is a significant indictor of hypercoagulability, prognosis and recurrence in gastric cancer. Exp Ther Med. 2014;8(1):125–32. https://doi.org/10.3892/etm.2014.1699.
Blackwell K, Haroon Z, Broadwater G, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18(3):600–8. https://doi.org/10.1200/JCO.2000.18.3.600.
Blackwell K, Hurwitz H, Liebérman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101(1):77–82. https://doi.org/10.1002/cncr.20336.
Xu BB, Lu J, Zheng ZF, et al. The predictive value of the preoperative C-reactive protein-albumin ratio for early recurrence and chemotherapy benefit in patients with gastric cancer after radical gastrectomy: using randomized phase III trial data. Gastric Cancer. 2019;22(5):1016–28. https://doi.org/10.1007/s10120-019-00936-w.
Lin GS, Huang XY, Lu J, et al. A good preoperative immune prognostic index is predictive of better long-term outcomes after laparoscopic gastrectomy compared with open gastrectomy for stage II gastric cancer in elderly patients. Surg Endosc. 2022;36(3):1814–26. https://doi.org/10.1007/s00464-021-08461-7.
Lin GT, Ma YB, Chen QY, et al. Fibrinogen-albumin ratio as a new promising preoperative biochemical marker for predicting oncological outcomes in gastric cancer: a multi-institutional study. Ann Surg Oncol. 2021;28(12):7063–73. https://doi.org/10.1245/s10434-021-10027-9.
Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th edn. New York: Springer; 2016.
Iwasaki Y, Terashima M, Mizusawa J, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24(2):492–502. https://doi.org/10.1007/s10120-020-01136-7.
Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350–7. https://doi.org/10.1200/JCO.2015.63.7215.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21. https://doi.org/10.1016/S0140-6736(11)61873-4.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. https://doi.org/10.1056/NEJMoa055531.
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93. https://doi.org/10.1200/JCO.2011.36.5908.
Zheng H, Zhao Y, He Q, et al. Multi-institutional development and validation of a nomogram to predict recurrence after curative resection of gastric neuroendocrine/mixed adenoneuroendocrine carcinoma. Gastric Cancer. 2021;24(2):503–14.
Jiao X, Wang Y, Wang F, Wang X. Recurrence pattern and its predictors for advanced gastric cancer after total gastrectomy. Medicine. 2020;99(51):e23795. https://doi.org/10.1097/MD.0000000000023795.
Lu J, Xu BB, Zheng CH, et al. Development and external validation of a nomogram to predict recurrence-free survival after R0 resection for stage II/III gastric cancer: an international multicenter study. Front Oncol. 2020;10:574611. https://doi.org/10.3389/fonc.2020.574611.
Piazuelo MB, Riechelmann RP, Wilson KT, Algood HMS. Resolution of gastric cancer-promoting inflammation: a novel strategy for anti-cancer therapy. Curr Top Microbiol Immunol. 2019;421:319–59. https://doi.org/10.1007/978-3-030-15138-6_13.
Kanda M, Tanaka C, Kobayashi D, et al. Proposal of the coagulation score as a predictor for short-term and long-term outcomes of patients with resectable gastric cancer. Ann Surg Oncol. 2017;24(2):502–9. https://doi.org/10.1245/s10434-016-5544-1.
He SS, Wang Y, Yang L, et al. Plasma fibrinogen correlates with metastasis and is associated with prognosis in human nasopharyngeal carcinoma. J Cancer. 2017;8(3):403–9. https://doi.org/10.7150/jca.17028.
Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(10):1494–503. https://doi.org/10.1016/j.ejso.2018.07.052.
Batschauer APB, Figueiredo CP, Bueno EC, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21(6):1267–72. https://doi.org/10.1093/annonc/mdp474.
Dai H, Zhou H, Sun Y, et al. D-dimer as a potential clinical marker for predicting metastasis and progression in cancer. Biomed Rep. 2018;9(5):453–7. https://doi.org/10.3892/br.2018.1151.
Di Nisio M, Klerk CP, Meijers JC, Büller HR. The prognostic value of the D-dimer test in cancer patients treated with and without low-molecular-weight heparin. J Thromb Haemost. 2005;3(7):1531–3. https://doi.org/10.1111/j.1538-7836.2005.01413.x.
Liu DQ, Li FF, Jia WH. Cumulative scores based on plasma D-dimer and serum albumin levels predict survival in esophageal squamous cell carcinoma patients treated with transthoracic esophagectomy. Chin J Cancer. 2016;35:11. https://doi.org/10.1186/s40880-015-0062-2.
Desch A, Gebhardt C, Utikal J, Schneider SW. D-dimers in malignant melanoma: association with prognosis and dynamic variation in disease progress. Int J Cancer. 2017;140(4):914–21. https://doi.org/10.1002/ijc.30498.
Edwards CM, Warren J, Armstrong L, Donnelly PK. D-dimer: a useful marker of disease stage in surgery for colorectal cancer. Br J Surg. 1993;80(11):1404–5. https://doi.org/10.1002/bjs.1800801116.
Kilic L, Yildiz I, Sen FK, et al. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark. 2015;15(4):405–11. https://doi.org/10.3233/CBM-150477.
Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. 2021;22(9):4496. https://doi.org/10.3390/ijms22094496.
Barreiro E. Models of disuse muscle atrophy: therapeutic implications in critically ill patients. Ann Transl Med. 2018;6(2):29. https://doi.org/10.21037/atm.2017.12.12.
Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. 2017;36(5):1333–8. https://doi.org/10.1016/j.clnu.2016.08.023.
Morhij R, Mahendra A, Jane M, McMillan DC. The modified Glasgow prognostic score in patients undergoing surgery for bone and soft tissue sarcoma. J Plast Reconstr Aesthet Surg. 2017;70(5):618–24. https://doi.org/10.1016/j.bjps.2017.01.016.
Lin JX, Wang ZK, Huang YQ, et al. Dynamic changes in pre- and postoperative levels of inflammatory markers and their effects on the prognosis of patients with gastric cancer. J Gastrointest Surg. 2021;25(2):387–96. https://doi.org/10.1007/s11605-020-04523-8.
Wang Q, Tan X, Deng G, Fu S, Li J, Li Z. Dynamic changes in the systemic immune-inflammation index predict the prognosis of EGFR-mutant lung adenocarcinoma patients receiving brain metastasis radiotherapy. BMC Pulm Med. 2022;22(1):75. https://doi.org/10.1186/s12890-022-01866-7.
Zhou ZQ, Pang S, Yu XC, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci. 2018;38(5):798–808. https://doi.org/10.1007/s11596-018-1946-6.
Zhang L, Wang Z, Xiao J, Zhang Z, Li H, Li F, Zhang L, Wang Y. Prognostic value of albumin to D-dimer ratio in advanced gastric cancer. J Oncol. 2021;2021:9973743. https://doi.org/10.1155/2021/9973743.
Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. https://doi.org/10.1186/s12943-018-0858-1.
Oya M, Akiyama Y, Yanagida T, Akao S, Ishikawa H. Plasma D-dimer level in patients with colorectal cancer: its role as a tumor marker. Surg Today. 1998;28(4):373–8. https://doi.org/10.1007/s005950050144.
Lu Z, Fang Y, Liu C, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39(7):748–56. https://doi.org/10.1200/JCO.20.01254.
Acknowledgements
The authors are grateful to the Chinese Gastric Cancer Association, the Chinese Society of Laparo-Endoscopic Surgery, and the Chinese Society of Gastrointestinal Surgery for their scientific support.
Author information
Authors and Affiliations
Contributions
Guosheng Lin and Jun Lu contributed to the conception and design of the study. Dong Wu, Bin-Bin Xu, Zhen Xue, Jia Lin, Lili Shen, and Hualong Zheng contributed to the follow-up and acquisition, analysis and interpretation of data. Guosheng Lin and Jun Lu participated in drafting the article. Ping Li, Jian-Wei Xie and Qi-Yue Chen participated in revising the article critically for important intellectual content. Chang-Ming Huang and Chao-Hui Zheng provided final approval of the version to be published.
Corresponding author
Ethics declarations
Disclosure
Guo-Sheng Lin, Jun Lu, Jia Lin, Hua-Long Zheng, Bin-Bin Xu, Zhen Xue, Dong Wu, Lili Shen, Chao-Hui Zheng, Ping-Li, Jian-Wei Xie, Qi-Yue Chen, and Chang-Ming Huang have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, GS., Lu, J., Lin, J. et al. Value of the Preoperative D-Dimer to Albumin Ratio for Survival and Recurrence Patterns in Gastric Cancer. Ann Surg Oncol 30, 1132–1144 (2023). https://doi.org/10.1245/s10434-022-12625-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12625-7