Abstract
Background/Purpose
Malignant small bowel obstruction (mSBO) is a common consequence of advanced malignancies. Surgical consultation is common, however data on the outcomes following an operation are lacking. We investigated a specific operative approach—intestinal bypass—to determine the outcomes associated with this intervention.
Methods
Patients with a preoperative diagnosis of mSBO who underwent intestinal bypass between 2015 and 2021 were included. Isolated colonic obstruction was excluded as was gastric outlet obstruction. Perioperative and postoperative outcomes were measured, including complications, overall survival, return to oral intake, and return to intended oncologic therapy. Patients were additionally grouped as to whether the operation was performed as elective or as inpatient.
Results
Overall, 55 patients were identified, with a mean age of 61.2 ± 14 years. The most common primary malignancy was colorectal cancer (65.5%) and 80% of patients had a preoperative diagnosis of metastatic disease. Small bowel to colon was the most common bypass procedure (51%). Severe complications occurred in 25.5% of patients with three in-hospital mortalities (5.5%). Survival rates at 30, 90, and 180 days were 91%, 80%, and 62%, respectively. The majority of patients were discharged to home (85.5%) and were tolerating an oral diet (74.6%). Twenty-seven patients (49.1%) returned to some form of oncologic treatment.
Conclusions
Patients with mSBO face a potentially terminal condition. In this study, approximately 75% of patients who underwent intestinal bypass were able to regain the ability to eat, and 49% returned to oncologic therapy. Although retrospective, these data suggest the approach is efficacious for palliation of this difficult sequela of advanced cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Malignant small bowel obstruction (mSBO) represents advanced progression of intra-abdominal malignancy. The incidence varies based on primary tumor site, however approximately 10–30% of patients with gastrointestinal (GI) malignancies will develop an mSBO.1,2,3 Surgical consultation for obstruction is common, representing up to 15% of hospitalizations in some studies.4,5,6 Despite this high incidence, data on optimal management and outcomes remain limited.4,6,7,8,9 The combination of inadequate data with a terminal cancer diagnosis creates a difficult decision-making process and the definition of success can be nebulous.5,10
Traditional quantitative outcome measures are clearly important but do not capture the nuanced goals of an invasive palliative procedure. For example, a quantitative measure such as mortality does not clarify if the disease process or surgical complications were the driver of mortality. Furthermore, these metrics do not capture the broader goals of palliative care, such as relief of nausea, vomiting, and the ability to eat, which can improve quality of life even in the absence of prolonged survival.5,11,12 Additionally, time spent at home may be valued more than absolute time alive.13,14,15 A patient-centered approach to the goals of care is critical in the determination of ‘success’ in each situation.10,16
Treatment options for patients with mSBO include medical management, treatments through interventional radiology or endoscopy, and operative approaches.17,18,19 For patients with small bowel obstruction, venting gastrostomy tubes may provide symptomatic relief but usually do not allow for sufficient caloric intake.19 Surgical procedures aim to address symptoms and potentially allow for the opportunity to resume oncologic therapy; however, mortality rates are reported to be as high as 30%, morbidity is significant, and recurrence of obstruction is common.1,20,21 For patients deemed operative candidates, options may include resection with primary anastomosis, proximal diversion, or intestinal bypass.
Surgical bypass has the theoretical advantage of less manipulation of diseased bowel and avoids complications related to resection of an advanced tumor; however, there are limited data on this approach in comparison with other operative options. In most retrospective mSBO studies, <10% of patients undergoing surgical intervention are defined as receiving an intestinal bypass.22 Other studies have been underpowered, with fewer than 35 patients included in the bypass cohorts.22,23,24,25,26 As bypass is a common approach for the treatment of mSBO at our institution, we determined further study was needed to address this gap in knowledge regarding the effectiveness of intestinal bypass for mSBO. Thus, the specific aim of this study was to assess outcomes associated with small bowel bypass in a large cohort of mSBO patients. To overcome the issue of measuring ‘success’ in this cohort of palliative patients, this study incorporated traditional quantitative measures (e.g., postoperative morbidity, readmission, and survival) as well as less commonly reported qualitative measures (e.g., diet at 30 days, return to oncologic therapy).
Methods
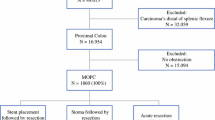
This was a retrospective study of patients taken to the operating room for the management of presumed mSBO between 2015 and 2021 at a National Cancer Institute (NCI)-designated comprehensive cancer center. The study was approved by Institutional Review Board review. A Health Insurance Portability and Accountability Act (HIPAA) wavier of informed consent was approved due to the retrospective nature of the study. Operative records from GI surgical oncologists were reviewed for any procedure involving ‘bypass’ or ‘entero-enterostomy’. In this retrospective study, there was no predefined algorithm for determination of whom to offer an operation. In general, patients were considered if they demonstrated good performance status and had imaging demonstrating a relatively focal point of obstruction. Patients with multiple sites of peritoneal disease were not automatically excluded; however, evidence of multiple levels of obstruction (i.e. multifocal) is generally considered a contraindication to operation. Other factors such as duration of prior chemotherapy and the availability of additional treatment options are considered individually. Patients were included if they demonstrated signs and symptoms of obstruction and an intestinal bypass (defined as entero-enterostomy or entero-colostomy) was performed. If patients underwent multiple intestinal bypasses (i.e. small bowel to small bowel, and small bowel to colon), they were included in the study, as were patients who underwent intestinal bypasses performed in combination with a stoma or gastrostomy tube. Patients with clinical or operative findings of gastric outlet obstruction or an isolated large bowel obstruction were excluded, as were patients in whom the treatment was primary resection and anastomosis. Cases that were aborted without a bypass being performed were not captured in this cohort.
Patients were additionally divided into two subpopulations: elective and non-elective. The elective group was defined as patients with a planned admission for surgical intervention due to chronic obstructive symptoms and with malignant findings concerning for impending obstruction, whereas the non-elective group was defined as patients with an unplanned admission to the hospital for obstructive symptoms, including hospital transfers, who underwent surgical intervention. Cases were then reviewed for inclusion in the cohort; 55 patients who meet the inclusion criteria of operative intervention for mSBO with a small bowel bypass were identified.
Data collected included patient characteristics, preoperative clinical characteristics, perioperative data, including complications, and survival outcomes. Cancer diagnoses and stages are reported as defined on admission, regardless of intraoperative findings. Preoperative variables included whether a patient was undergoing active treatment including chemotherapy, immunotherapy, and/or radiation. Preoperative variables also included admission characteristics, laboratory values, preoperative imaging, preoperative interventions, and surgical consult timing if the procedure was non-elective. Perioperative data included the procedure performed, remaining small bowel length if recorded, intraoperative transfusions, and reason for obstruction, including malignant, adhesions, or other if not defined in the operative report. Return to diet at discharge and 30 days was categorized into PO (per os) only, PO with supplementation by tube feeds or parenteral nutrition, or NPO (nil per os). Postoperative variables included complications as categorized by the Clavien–Dindo (CD) classification, palliative care consults, unplanned readmissions, urgent care visits, discharge location, hospital length of stay (LOS), and 30- and 90-day mortality.27 Hospital LOS was defined as total time admitted, whereas surgical LOS was defined as time from operation to discharge. Percentage of hospital days was defined as total hospital days divided by survival, where hospital days were defined as surgical LOS with the addition of any inpatient readmission days up until 1 year. For those patients with survival past 1 year, survival was defined as 365 days.
Statistical Analysis
All continuous variables were assessed for normality. Demographic and clinical variables for elective and non-elective patients were evaluated using Chi-square tests for categorical variables and two-sided t-tests or the Wilcoxon rank-sum test for continuous variables, as appropriate. Univariate analysis using the entire population was performed to determine differences in 30-day diet, 30-day mortality, and 90-day mortality. Kaplan–Meier curves were generated for the elective and non-elective subgroups. All statistical analyses were conducted using STATA version 17.0 (StataCorp LLC, College Station, TX, USA).
Results
The cohort consisted of 55 patients who met the inclusion criteria. Demographic data are presented in Table 1. The majority of patients were Caucasian (76%) and male (56%), and colorectal cancer was the most common primary cancer, representing approximately two-thirds of the cohort. Most patients had private insurance (55%) or were government funded (29%), with 16% of patients defined as ‘charity’. Two-thirds of cases were non-elective (65%). Eighty percent of patients had a known stage IV cancer diagnosis upon presentation, with the remaining 20% having a preadmission diagnosis of stage II or III cancer. Of those 11 patients with stage II or III disease, 6 were upstaged to stage IV at the time of surgery. Sixty-four percent of all patients presented with an albumin level <3.5 g/dL, with a significant difference in those patients undergoing non-elective operations (16% elective vs. 89% non-elective; p < 0.001).
As outlined in Table 2, the most common surgical bypass was small bowel to colon. Twenty-five percent of patients had a gastrostomy tube placed at the time of the procedure, with one patient in the non-elective group having a pre-existing gastrostomy tube (G-tube). While a larger proportion of non-elective patients received a G-tube, this did not reach statistical significance (33 vs. 10.5%; p = 0.07). Small bowel length after bypass was reported in 30 patients, and the average length was 208 cm of bowel proximal to the bypass.
The overall complication rate was 76.4% (Table 2), with severe complications (CD grade 3 or higher) occurring in 25.5% of patients. Most patients (60%) received total parenteral nutrition (TPN) during the postoperative period, although the usage was different depending on whether the procedure was elective or not (26.3% elective vs. 77.8% non-elective; p < 0.001). The total LOS was significantly higher for the non-elective cohort (9.1 days elective vs. 18.7 non-elective; p < 0.001); however, surgical LOS was not statistically different between the elective and non-elective cohorts (9 vs. 11.8 days; p = 0.14).
Seventy-five percent of patients were tolerating a PO diet at the time of discharge (Table 3); three patients were discharged with TPN. At 30 days, 68% of patients were tolerating PO, with 26% of patients supplementing their nutrition with either TPN or tube feeds. On univariate analysis, no patient characteristics or preoperative factors were found to be significantly different between those tolerating a diet at 30 days or not. In this cohort of patients with predominantly stage IV disease, 20% of patients had a palliative care consult documented during inpatient admission. Approximately half of patients (49.1%) were able to resume some form of additional therapy, including chemotherapy or immunotherapy.
Median OS was 199 days (6.6 months), and the 30- and 90-day mortality rates were 9.1% and 20%, respectively (Table 3). Sixty-two percent of patients were alive at 6 months, and there were three in-hospital (CD grade 5) mortalities (5.5%). Readmission rates were 26% and 38.6% of patients alive at 30 and 90 days, respectively. Additionally, 23 patients (46%) were seen in urgent care at 30 days, either at our own institution or at another as identified by records review. Figure 1a shows survival between elective and non-elective cases was not different. For patients who underwent surgery and then were able to start some additional therapy, median OS was 357 days, compared with 123 days for surgery alone (p < 0.036).
We assessed time spent in hospital for the year following surgery, including inpatient stay and readmission days (Fig. 2). Excluding in-hospital deaths, patients spent an average of 11% of their remaining time alive in the hospital. For those patients alive at 1 year, that number was 3.5%, compared with 17% in patients who died within 1 year of surgery (p = 0.001). There was no significant difference between elective and non-elective cases.
The average percentage of time each cohort of patients spent either in the hospital or at home during the year after surgery. The mean was 11% for the overall population. For those patients who died within 1 year, the mean time in the hospital was 17.14% ± 17.25 compared with 3.47% ± 1.83 for those alive at 1 year (p < 0.001).
Discussion
The management of patients with malignant bowel obstruction is challenging with limited evidence to help guide decisions. As survival following the development of a malignant obstruction is poor, interventions are primarily aimed at management of symptoms, specifically the inability to tolerate enteral nutrition. Thus, understanding the trade-off between the extent of interventions and their efficacy in this regard is essential. Our study investigated outcomes associated with a specific surgical management strategy, namely intestinal bypass.
The median OS in this cohort was 6.6 months, with 42% of patients alive at the 1-year mark. The 91% 30-day survival in our study is on the high end of a previously reported range.1,2,3,4 One possible explanation is that our study solely evaluates patients who underwent intestinal bypass, while most other studies regarding malignant bowel obstructions tend to have a small proportion of approximately 10% of patients undergoing bypass. Studies in which intestinal bypass mortality has been specifically assessed report 30-day mortalities as <10%, with median survival of 2.7–6.5 months, consistent with our data.24,25 The morbidity of operative intervention remains considerable—25% experienced a severe complication in this study and 5.5% died postoperatively. Thus, despite being potentially less invasive than a complete exploration and resection of tumor, these data suggest one must still be cautious when considering patients for this approach.
Our study also evaluated additional, alternative measures of quality of life in this cohort. While not prospectively collected, nutritional status has been shown to be a strong predictor of quality of life in cancer patients and the ability to tolerate food may be used as a surrogate quality-of-life metric in the mSBO population.28 In this cohort, 41 (75%) patients were able to tolerate PO at discharge following surgery. This decreased slightly at 30 days, but nearly 70% were still tolerating at least some diet at that time. This is in line with Prost et al. who found 68% of patients were able to tolerate PO; however, only 50% could eat without some digestive intolerance.29 Fewer than 5% of our patient population was entirely NPO at 30 days. As ability to tolerate some type of food is a key goal of many patients, this measure offers a patient-centered approach to defining success.
Many view malignant obstructions as an obstacle to starting or continuing systemic therapy.30 Thus, one potential motivation in this group is the ability to temporize the obstruction to allow for such treatment. An intestinal bypass theoretically allows for resolution of the intestinal failure without the need for chronic TPN. In our patient population, half of the patients were able to proceed with additional therapy after their operation. Helyer et al. reported a series in which 16 of 47 patients operated on for mSBO underwent postoperative chemotherapy (34%); however, that study included patients with bypass, resection and stoma formation,30 and also showed survival benefit to those patients in whom chemotherapy could be restarted. In the clinical context, the ability to resume therapy is often an important consideration. Our data suggest that half of patients will be able to derive a potential survival benefit from surgery (bypass) and restarting treatment.
Another important patient-centered factor to consider is the cost—both financial as well as time-burden of care—to patients and their families. A large contributor to cost as well as quality of life is medical admissions (and readmissions). A study by Englert et al. found hospital admission costs for patients undergoing intestinal bypass to be an average of $51,000.24 Medical management has been found to be associated with shorter lengths of stay of around 8 days, with varying data on readmissions.17,21 Our study found an average of 10.8 days for surgical LOS, with no statistical difference between our elective versus non-elective subpopulations. Additionally, one-quarter of the patients were readmitted within 30 days, with the number reaching one-third by 90 days. Accounting for index surgical LOS and subsequent readmissions, patients who died within 1 year of the operation spent 17% of their remaining life in the hospital, versus 3.5% for those alive at 1 year (p = 0.001). These data emphasize the need for continued refinement of patient selection to offer appropriate palliation without significant loss of time at home.
Support with an integrated palliative care team is also an important consideration in the care of patients with an advanced cancer diagnosis, including those with mSBO; however, only a small proportion (20%) of our patients received a defined palliative care consult during admission. While this number does not capture any palliative support received on an outpatient basis, this team’s guidance should be integral to the patient’s admission and care decisions. This number seems consistent with larger population-based studies, including the study by Lilley et al. that noted fewer than 5% of patients received a palliative care consult.17 Moreover, as 35% of the patients were initially admitted to a medical service, early and frequent multidisciplinary discussion of these patients and their management, including operative and non-operative options, is important.30 In the future, approaching this as a quality improvement initiative is consistent with institutional goals of delivery of goal-concordant care.31
We understand these data utilize a very selected population and should be interpreted in the setting of a ‘successful’ surgical bypass. Importantly, this was a retrospective study limited to a single institution with selection and potential reporting biases. As the selection criteria identified only patients who underwent surgical bypass by our GI oncology team, our demographics are highly skewed towards GI cancers, with fewer cases involving gynecologic cancers. This study was aimed to describe surgical outcomes and thus, as our patients were selected from operating room data, we were unable to include those managed non-operatively. We were also unable to capture patients who were not amenable to bypass after exploration or those who only received a venting gastrostomy tube. Based on the data acquired in this study, we do plan to investigate the global management of mSBO at our institution (including medical, interventional, and surgical approaches). Additionally, we do not have a sense of the extent of peritoneal disease. The Peritoneal Carcinomatosis Index (PCI) could be a metric for this type of assessment but was not recorded systematically. Due to the retrospective nature of the dataset, we do not have reliable data on reobstruction rates. Finally, quality of life was not measured prospectively and surrogate measures such as return to oral intake and the percentage of remaining life spent in hospital were used. Despite the limitations, the data reflect our practice of offering bypass to patients we consider good candidates based on appropriate preoperative risk.
Conclusion
Small bowel bypass in our patient population with mSBO was associated with resolution of symptoms, and the majority of patients were able to tolerate diet following surgery. The OS of approximately 6 months highlights the terminal nature of mSBO and the difficulty in affecting the natural history of peritoneal disease. However, if one considers 62% of patients were alive at 6 months and spent the majority of this time at home, it is reasonable to consider an intestinal bypass as effective palliation. These data support our practice of offering surgical exploration to selected patients with the intent to palliate symptoms and to potentially allow return to intended oncologic therapy. A prospective study to investigate management of mSBO would be ideal, particularly with respect to quality-of-life metrics. Our data showing the benefit of return to chemotherapy after bypass can inform future studies about the importance of this metric. Additionally, these data can inform difficult end-of-life goals of care regarding symptom relief and survival.
References
Banting SP, Waters PS, Peacock O, et al. Management of primary and metastatic malignant small bowel obstruction, operate or palliate. A systematic review. ANZ J Surg. 2021;91(3):282–90.
Franke AJ, Iqbal A, Starr JS, Nair RM, George TJ Jr. Management of malignant bowel obstruction associated with GI cancers. J Oncol Pract. 2017;13(7):426–34.
Song Y, Metzger DA, Bruce AN, et al. Surgical outcomes in patients with malignant small bowel obstruction: a national cohort study. Ann Surg. 2022;275(1):e198–205.
Badgwell BD, Smith K, Liu P, Bruera E, Curley SA, Cormier JN. Indicators of surgery and survival in oncology inpatients requiring surgical evaluation for palliation. Support Care Cancer. 2009;17(6):727–34.
Williams LA, Bruera E, Badgwell B. In search of the optimal outcome measure for patients with advanced cancer and gastrointestinal obstruction: a qualitative research study. Ann Surg Oncol. 2020;27(8):2646–52.
Alese OB, Kim S, Chen Z, Owonikoko TK, El-Rayes BF. Management patterns and predictors of mortality among US patients with cancer hospitalized for malignant bowel obstruction. Cancer. 2015;121(11):1772–8.
Badgwell BD, Contreras C, Askew R, Krouse R, Feig B, Cormier JN. Radiographic and clinical factors associated with improved outcomes in advanced cancer patients with bowel obstruction. J Palliat Med. 2011;14(9):990–6.
Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159–69.
Chicago Consensus Working G. The Chicago consensus on peritoneal surface malignancies: palliative care considerations. Ann Surg Oncol. 2020;27(6):1798-1804.
Miller P, John PR, Ganai S. How should surgical palliative success be defined? AMA J Ethics. 2021;23(10):E778-782.
Lee KC, Sokas CM, Streid J, et al. Quality indicators in surgical palliative care: a systematic review. J Pain Symptom Manage. 2021;62(3):545–58.
Stacey CL, Pai M, Novisky MA, Radwany SM. Revisiting “awareness contexts” in the 21st century hospital: How fragmented and specialized care shape patients’ Awareness of Dying. Soc Sci Med. 2019;220:212–8.
Groff AC, Colla CH, Lee TH. Days spent at home: a patient-centered goal and outcome. New Eng J Med. 2016;375(17):1610–2.
Shen E, Rozema EJ, Haupt EC, et al. Assessing the concurrent validity of days alive and at home metric. J Am Geriatr Soc. 2021. https://doi.org/10.1111/jgs.17506.
Suskind AM, Zhao S, Boscardin WJ, Smith A, Finlayson E. Time spent away from home in the year following high-risk cancer surgery in older adults. J Am Geriatr Soc. 2020;68(3):505–10.
Chesney TR, Haas B, Coburn NG, et al. Patient-centered time-at-home outcomes in older adults after surgical cancer treatment. JAMA Surg. 2020;155(11):e203754.
Lilley EJ, Scott JW, Goldberg JE, et al. Survival, healthcare utilization, and end-of-life care among older adults with malignancy-associated bowel obstruction: comparative study of surgery, venting gastrostomy, or medical management. Ann Surg. 2018;267(4):692–9.
Pujara D, Chiang YJ, Cormier JN, Bruera E, Badgwell B. Selective approach for patients with advanced malignancy and gastrointestinal obstruction. J Am Coll Surg. 2017;225(1):53–9.
Shaw C, Bassett RL, Fox PS, et al. Palliative venting gastrostomy in patients with malignant bowel obstruction and ascites. Ann Surg Oncol. 2013;20(2):497–505.
Francescutti V, Miller A, Satchidanand Y, Alvarez-Perez A, Dunn KB. Management of bowel obstruction in patients with stage IV cancer: predictors of outcome after surgery. Ann Surg Oncol. 2013;20(3):707–14.
Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg. 2014;149(4):383–92.
Wancata LM, Abdelsattar ZM, Suwanabol PA, Campbell DA Jr, Hendren S. Outcomes after surgery for benign and malignant small bowel obstruction. J Gastrointest Surg. 2017;21(2):363–71.
Legendre H, Vanhuyse F, Caroli-Bosc FX, Pector JC. Survival and quality of life after palliative surgery for neoplastic gastrointestinal obstruction. Eur J Surg Oncol. 2001;27(4):364–7.
Englert ZP, White MA, Fitzgerald TL, Vadlamudi A, Zervoudakis G, Zervos EE. Surgical management of malignant bowel obstruction: at what price palliation? Am Surg. 2012;78(6):647–52.
Shariat-Madar B, Jayakrishnan TT, Gamblin TC, Turaga KK. Surgical management of bowel obstruction in patients with peritoneal carcinomatosis. J Surg Oncol. 2014;110(6):666–9.
Ito Y, Fujitani K, Sakamaki K, et al. QOL assessment after palliative surgery for malignant bowel obstruction caused by peritoneal dissemination of gastric cancer: a prospective multicenter observational study. Gastric Cancer. 2021;24(5):1131–9.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Lis CG, Gupta D, Lammersfeld CA, Markman M, Vashi PG. Role of nutritional status in predicting quality of life outcomes in cancer: a systematic review of the epidemiological literature. Nutr J. 2012;11:27.
Prost ADJ, Douard R, Malamut G, Mecheri F, Wind P. Small bowel obstruction in patients with a prior history of cancer: predictive findings of malignant origins. World J Surg. 2014;38(2):363–9.
Helyer LK, Law CH, Butler M, Last LD, Smith AJ, Wright FC. Surgery as a bridge to palliative chemotherapy in patients with malignant bowel obstruction from colorectal cancer. Ann Surg Oncol. 2007;14(4):1264–71.
McNiff KK, Caligiuri MA, Davidson NE, et al. Improving goal concordant care among 10 leading academic U.S. Cancer Hospitals: a collaboration of the alliance of dedicated cancer centers. Oncologist. 2021;26(7):533–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Meagan Read, Benjamin D. Powers, Jose M. Pimiento, Danielle Laskowitz, Erin Mihelic, Iman Imanirad, Sophie Dessureault, Seth Felder, and Sean P. Dineen declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Read, M., Powers, B.D., Pimiento, J.M. et al. Management of Malignant Small Bowel Obstruction: Is Intestinal Bypass Effective Palliation?. Ann Surg Oncol 29, 6980–6987 (2022). https://doi.org/10.1245/s10434-022-12204-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12204-w