Abstract
Background
Right hemicolectomy (RHC) for nodal staging is recommended for nonmucinous adenocarcinoma of the appendix (NMACA), but it is unclear whether a subgroup of patients at low risk for lymph node (LN) metastasis exists who may be managed with a less extensive resection.
Patients and Methods
Patients with NMACA without distant metastases who underwent margin negative resection via either RHC or appendectomy/partial colectomy (A/PC) were evaluated from the National Cancer Database (2004–2016). Patients at low risk for LN metastasis were identified. Multivariable survival analysis was performed, and 5-year overall survival (OS) was estimated.
Results
Of the 2487 patients included, 652 [26.2%; 95% confidence interval (CI) 24.5–28.0%] had LN metastases. T4 T stage [odds ratio (OR) 4.2, p = 0.032], poorly/undifferentiated histology (OR 2.2, p = 0.004), and lymphovascular invasion (LVI) (OR 4.4, p < 0.001) were associated with LN positivity. One hundred and thirteen patients (4.5%) had tumors at low risk for LN metastasis (T1 T stage, well/moderately differentiated tumors without LVI), and the rate of LN metastasis for this group was 1.8% (95% CI 0.5–6.2%). Conversely, the LN metastasis rate among the 2374 non-low-risk patients was 27.4% (95% CI 25.6–29.2%). Performance of A/PC instead of RHC was associated with a survival disadvantage among all patients (hazards ratio 1.5, p = 0.049), but among the low-risk cohort, 5-year OS did not differ based on resection type (88.3% A/PC versus 92.7% RHC, p = 0.305).
Conclusions
Although relatively uncommon, early, pathologically favorable NMACA is associated with a very low risk of LN metastasis. These select patients may be managed with a less extensive resection without compromising oncologic outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Primary cancer of the appendix is a rare disease entity, not infrequently diagnosed as an incidental finding on abdominal imaging or on final pathology following appendectomy for acute appendicitis or other indications.1,2 Multiple histologic subtypes exist, of which mucinous adenocarcinoma is the most common and accounts for 37% of all cases.1 Colonic type adenocarcinoma, also commonly known as nonmucinous adenocarcinoma of the appendix (NMACA), accounts for 25–27% of cases, and is thought to arise from an adenoma similar to primary colon cancer.3 Due to the rarity of primary appendiceal cancer in general, no dedicated evidence-based management guidelines exist, and thus the National Comprehensive Cancer Network (NCCN) recommendations for management of NMACA largely parallel those for colon cancer.4
Among patients with either mucinous adenocarcinoma of the appendix or NMACA, lymph node (LN) metastases at the time of initial diagnosis have been reported in 20–67% of cases, with a higher likelihood of being present in NMACA.5 Given the frequency of LN positivity, full oncologic resection via right hemicolectomy (RHC) to obtain appropriate LN staging is currently recommended for all patients diagnosed with NMACA.5,6 For early adenocarcinomas with favorable features in other anatomic locations, current guidelines support less extensive resections as a surgical option due to the low associated rate of LN metastases for these tumors.4,7,8,9 However, limited data exist describing the rate of LN metastasis for patients with early NMACA with similar favorable features, and thus whether a less extensive resection may be an appropriate surgical option for these patients is unclear.10
Using the National Cancer Database (NCDB), the current study sought to evaluate the rate of LN metastasis among patients with NMACA, and to identify whether a subgroup of patients exists with early NMACA who may be at low risk of developing LN metastases, and thus might be amenable to a less extensive resection without compromising oncologic outcomes.
Patients and Methods
Data Source and Patient Selection
The NCDB colon participant user file was used for this study. The NCDB is a joint project of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons, composed of cases from more than 1500 CoC accredited facilities. Data collected include demographic and clinical patient characteristics, cancer staging and tumor histology, and type of treatment administered.11,12 NCDB data are de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA). Institutional review board approval was not required for this study as no patient, physician, or hospital identifiers were evaluated.
From 2004 to 2016, patients with nonmucinous adenocarcinoma histology [Surveillance, Epidemiology, and End Results (SEER) ICD-0-3 histology codes 8140–8148, 8210–8213, 8220–8221, 8255, 8260–8263, 8440–8441, 8490, and 8570–8576] of the appendix (SEER primary site code C181) without distant metastases who underwent margin negative (R0) surgical resection via either a hemicolectomy [including right colectomy or subtotal colectomy, with or without contiguous organ resection (Facility Oncology Registry Data Standards code 40–41), defined as RHC for this study] or appendectomy [including partial colectomy, with or without contiguous organ resection (Facility Oncology Registry Data Standards code 30–32), defined as A/PC for this study] were identified.6,13,14,15 Patients with and without LN metastases were compared to identify factors associated with nodal spread. Patients were then stratified into two groups based on the presence or absence of factors associated with LN metastasis (low-risk group, which included patients with factors associated with a low likelihood of LN metastasis based on this analysis and on data for other adenocarcinomas7,8,9 and non-low-risk group, which included patients who did not have all low-risk factors for LN metastasis), and the rates of LN positivity for these two groups were calculated. Finally, survival outcomes were compared between patients who underwent A/PC and RHC.
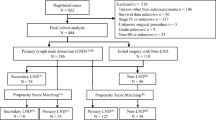
Patients with histology codes other than those previously stated, including goblet cell histology, with nonappendiceal located tumors, who did not undergo either RHC or A/PC, who had distant metastatic disease, who had unknown/inconsistent staging information, or who did not have an R0 resection were excluded. Patients with prior cancer diagnoses were also excluded to avoid biasing survival results due to prior diagnoses or treatments. The resulting final cohort consisted of 2487 patients (Fig. 1).
Variables and Outcomes
Clinical variables evaluated included age, sex, race, Charlson–Deyo comorbidity score, insurance status, treatment facility type (academic or nonacademic), type of surgical procedure performed (A/PC or RHC), and treatment with adjuvant systemic therapy.16 Evaluated tumor variables included size, preoperative carcinoembryonic antigen (CEA) level, histologic subtype (signet ring or non-signet ring), T stage, LN status (positive or negative), pathologic stage, histologic tumor grade, and presence or absence lymphovascular invasion (LVI). Age (≥ 60 or < 60 years) and tumor size (≥ 2 or < 2 cm) were further dichotomized using the Liu method.17 Variables with missing data were recorded as unknown. The primary study outcome was the rate of LN positivity. Secondary study outcomes included identification of factors associated with LN positivity, and comparison of overall survival (OS) between patients undergoing A/PC and RHC.
Statistical Methods
Univariable analysis was performed using Pearson’s chi-squared or Fisher’s exact test, as appropriate, for categorical variables, and the Wilcoxon rank-sum test for continuous variables. Multivariable logistic regression analysis was performed to evaluate factors associated with LN positivity. Factors with a p-value ≤ 0.10 on univariable analysis were included in this multivariable analysis. Proportions of patients with positive LNs were calculated, and 95% confidence intervals (CI) for proportions were estimated using the Wilson method.18 Survival analyses were estimated using the Kaplan–Meier method and compared using the log-rank test. Associations between variables and survival outcomes were determined using the Cox proportional hazards model, and the proportionality of the model was ensured using the Schoenfeld residuals test. The start time for follow-up and survival analyses was the day of diagnosis.19 All tests were two sided. p-Values < 0.05 were considered statistically significant. All statistical analyses were performed using Stata Version 17.20
Results
Patient Cohort and Factors Associated with LN Metastasis
Of the 2487 patients included, 652 [26.2%; 95% confidence interval (CI) 24.5–28.0%] had LN metastases. The median age of the study cohort was 61 [interquartile range (IQR) 52–71] years, and 1365 patients (54.9%) were male. Baseline descriptive statistics for the study cohort and univariable analysis comparisons between patients with and without LN metastases are presented in Table 1.
Among the 2236 patients with ≥ 1 LN evaluated (Supplementary Table 1), on multivariable analysis, T4 T stage [odds ratio (OR) 4.2, 95% CI 1.1–15.3, p = 0.032], poorly/undifferentiated histologic grade (OR 2.2, 95% CI 1.3–3.7, p = 0.004), and presence of LVI (OR 4.4, 95% CI 2.8–7.1, p < 0.001) were associated with LN metastasis (Table 2).
Comparison of Cohorts at Low Risk and Non-low Risk for LN Metastasis
Based on the results of the multivariable analysis evaluating factors associated with LN metastasis for patients with ≥ 1 LN evaluated, 113 patients (4.5% of the entire study cohort) were identified as having tumors at low risk for LN metastasis (T1 T stage tumors with well- or moderately differentiated histology and without LVI). In this low-risk group, two patients were found to have LN metastases, yielding a LN metastasis rate of 1.8% (95% CI 0.5–6.2%) for this group. Conversely, among the 2374 non-low-risk patients (those with ≥ T1 T stage, poorly/undifferentiated histologic grade, or presence of LVI), 650 patients were found to have LN metastases, yielding a LN metastasis rate of 27.4% (95% CI 25.6–29.2%) for this group.
No significant differences in sociodemographic features were seen between low-risk and non-low-risk patients. Compared with non-low-risk patients, low-risk patients were significantly more likely to have tumors < 2 cm [n = 53 (46.9%) low risk versus n = 447 (18.8%) non-low risk, p < 0.01] and to have non-signet ring histology [n = 112 (99.1%) low risk versus n = 2035 (85.7%) non-low risk, p < 0.01], and significantly less likely to receive adjuvant systemic therapy [n = 6 (5.3%) low risk versus n = 884 (37.2%) non-low risk, p < 0.01].
On subgroup analysis of patients with specifically low-grade tumors without LVI (n = 397), for patients with T1, T2, T3, and T4 tumors, 1 of 72 patients (1.4%, 95% CI 0.2–7.5%), 7 of 98 patients (7.1%, 95% CI 0.4–14.0%), 16 of 149 patients (10.7%, 95% CI 6.7–16.7%), and 11 of 78 (14.1%, 95% CI 8.1–23.5%), respectively, had LN metastases. For these patients with low-grade tumors, as compared with those with T1 T stage tumors, the OR for LN metastasis associated with T2, T3, and T4 tumors was 0.6 (95% CI 0.1–17.3, p = 0.763), 2.6 (95% CI 0.2–30.9, p = 0.445), and 3.2 (95% CI 0.2–45.8, p = 0.238), respectively.
Comparison of Patients Who Underwent A/PC Versus RHC
Of the entire study cohort, 672 patients (27.0%) underwent A/PC, 37 (1.5%) of whom were in the low-risk cohort, and 635 (25.5%) of whom were in the non-low-risk cohort. On comparison of patients who underwent A/PC versus RHC among the entire cohort, the median number of LNs evaluated was significantly less for those who underwent A/PC [11 (IQR 0–18)] versus RHC [17 )IQR 13–23), p < 0.01], and patients who underwent A/PC were significantly less likely to have LN metastases [n = 130 (19.4%) A/PC versus n = 522 (28.8%) RHC, p < 0.01] and to receive adjuvant systemic therapy [n = 187 (27.8%) A/PC versus 703 (38.7%) RHC, p < 0.01].
Among the low-risk group, the median number of LNs evaluated remained significantly less for those who underwent A/PC [1 (IQR 0–12) versus RHC 17 (IQR 13–23), p < 0.01], but no association was seen between type of resection and presence of LN metastases [n = 0 (0.0%) A/PC versus n = 2 (2.6%) RHC, p = 0.32]. One patient (2.7%) who underwent A/PC and five patients (6.6%) who underwent RHC received adjuvant systemic therapy (p = 0.39).
Follow-Up and Survival Analysis
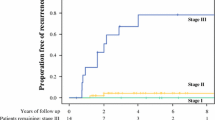
Median follow-up time for patients alive at last follow-up was 55.0 (IQR 31.0–83.7) months and did not differ between patients who underwent A/PC versus RHC: 51.9 (IQR 29.9–83.7) months for the A/PC cohort versus 56.3 (IQR 31.8–83.5) months for the RHC cohort (log-rank p = 0.243). On multivariable survival analysis of the entire cohort, performance of A/PC was associated with an OS disadvantage as compared with performance of RHC [hazard ratio (HR) 1.5, 95% CI 1.0–2.4, p = 0.049] (Table 3), and with significantly with worse 5-year OS (64.3% A/PC versus 70.0% RHC, log-rank p = 0.029) (Fig. 2). However, on subgroup analysis of patients in the low-risk LN metastasis group [n = 113 (37 of whom underwent A/PC)], 5-year OS did not significantly differ between those underwent A/PC versus RHC (88.3% A/PC versus 92.7% RHC, log-rank p = 0.305).
Multivariable survival analysis of the subgroups of patients with stage I/II and III disease, controlling for the same factors as were accounted for on analysis of the entire study cohort, demonstrated no significant difference in survival based on type of surgical procedure performed within each subgroup: stage I/II (HR 1.8, 95% CI 0.95–3.45, p = 0.069), stage III (HR 1.2, 95% CI 0.60–2.32, p = 0.639).
Additional subgroup analyses were performed, comparing patients who underwent A/PC with ≤ 2 LNs evaluated (n = 232) with patients who underwent RHC. Among this subgroup, performance of A/PC remained associated with an OS disadvantage (HR 2.4, 95% CI 1.1–5.3, p = 0.036), and with significantly worse 5-year OS (63.1% A/PC versus 70.0% RHC, log-rank p = 0.012). However, among the low-risk cohort [n = 99 (23 of whom underwent A/PC)] of this subgroup, 5-year OS did not significantly differ based on type of surgical procedure performed (80.2% appendectomy/partial colectomy versus 92.7% right hemicolectomy, p = 0.079).
Discussion
NMACA is a rare disease without dedicated evidence-based management guidelines. Many treatment recommendations are extrapolated from those for colonic adenocarcinoma, including the general agreement that these patients should undergo full oncologic resection with RHC for complete nodal staging. However, both NCCN and Japanese consensus guidelines indicate that, for endoscopically completely resected malignant colon polyps (defined as a pT1 cancer) without adverse features, observation without additional surgical resection is an acceptable treatment option given the low rates of LN metastases and recurrence for these favorable tumors.4,21 While some reports support treating early appendiceal NMACA similar to malignant colon polyps, there is limited data directly evaluating the rate of LN metastasis for patients with early, favorable NMACA, and thus determining whether these patients may be able to undergo less extensive resections with deferral of LN staging. The current study found that overall, the LN metastasis rate for patients with NMACA was equivalent to that reported in other studies,3 but that patients with early NMACA with low-risk features for LN metastasis (specifically T1, well- or moderately differentiated tumors without LVI) had very low rates of nodal metastases upon resection.
Because the primary rationale for performing a full oncologic resection with RHC in these patients is for adequate nodal staging, the findings of the current study suggest that among patients with these low-risk disease features, full oncologic resection with RHC may not be necessary given the very low rate of LN metastasis in these patients. As such, these patients may be able to undergo less extensive resections for primary tumor removal, such as with an appendectomy alone without full nodal staging, without concern of missing occult nodal metastases. NCCN guidelines support less extensive resection options for early adenocarcinomas with similar low-risk features in other anatomic locations, such as esophageal, gastric, or rectal adenocarcinoma, with the rationale in each of these cancers being that a full oncologic resection to obtain appropriate LN staging is not needed due to the low rate of LN metastases for these early cancers.7,8,9 It is likely that early NMACA behaves similarly to these other early adenocarcinomas regarding LN metastases, and thus, less extensive resection may also be an appropriate treatment option for early, pathologically favorable NMACA.
The current study found a survival advantage with the performance of RHC rather than A/PC among all-comers with NMACA, but this survival difference did not persist among the subgroup of patients with low-risk tumors. This is likely secondary to a stage migration effect because RHC is able to provide more LNs for evaluation, and thus has a higher probability of finding nodal metastases.22 In fact, in the present study, patients who underwent RHC had significantly more nodes evaluated than those who underwent A/PC, and when comparing RHC with less extensive resection in similarly staged patients, no appreciable survival difference was identified within each stage group. Additionally, there was no association between LN positivity and resection type for patients with low-risk tumors, likely because the rate of LN positivity in general was so low among this subgroup. Given the low rate of LN metastasis for patients with early, favorable NMACA as identified in this study, performance of a less extensive resection appears to be a tenable option for these patients without compromising oncologic outcomes.
A limitation of the current study is the inability to determine the extent of resection among patients who underwent A/PC, as the NCDB does not delineate this detail. Although these patients underwent a resection less extensive than a formal RHC, it is not clear how many patients in the A/PC cohort underwent a traditional appendectomy versus an appendectomy with partial cecectomy versus a full ileocecectomy. Nonetheless, there are advantages to performing a resection less extensive than a full oncologic RHC when possible, with studies showing lower rates of morbidity following appendectomy rather than right colectomy for other appendiceal pathology, such as carcinoid tumors < 2 cm.23 While complications following RHC are low, a major benefit to performing an appendectomy rather than RHC is the avoidance of an anastomosis and its associated potential complications.24 Additionally, although this unfortunately cannot be discerned from the NCDB either, it is likely that many of the patients in the low-risk group were diagnosed incidentally on final pathologic review of an appendectomy for other etiologies.3 Deferral of RHC for these patients prevents additional risks from a second anesthesia event and/or any surgical or nosocomial complications, which may potentially occur with performance of an additional procedure. Although the number of patients in the current study who met criteria to be in the low-risk group was < 5% of the entire cohort, these still represent an important group of patients to identify as they are a select group of patients who may be able to benefit from the opportunity to have a less extensive operation without compromising oncologic outcomes. For the vast majority of patients with NMACA, right hemicolectomy continues to remain the operation of choice to ensure optimal nodal staging.
In addition to those already discussed, several other limitations should be considered when interpreting this study. This study included patients with any number of lymph nodes evaluated, rather than only those who had complete nodal staging (i.e., ≥ 12 LNs harvested). The decision to include those with < 12 LNs evaluated was to make the results of this study more generalizable, as many patients who undergo an appendectomy for a low-risk NMACA may not have 12 LNs evaluated, but would still be at low risk for LN metastasis given their favorable tumor. Additionally, inclusion of only those with ≥ 12 nodes evaluated would have led to the exclusion of many patients, which may have significantly reduced the study power and introduced additional selection biases. As a retrospective analysis, it is possible that potential confounding variables were not captured and could have impacted the results in undefined ways. For example, the individual decisions regarding whether to perform A/PC versus RHC for each patient cannot be determined. Also, OS, rather than disease-specific survival was evaluated as a secondary outcome due to the limitations of the NCDB, and it is possible some patients in each group died due to noncancer-related causes. There is no strong reason to believe however there should be a significant imbalance between non-disease-related deaths in the comparison groups, and notably Charlson–Deyo comorbidity scores were accounted for in the analyses. Finally, the NCDB includes only patients who have received some element of their care at an accredited CoC facility, and thus the results may not be generalizable to patients treated at other centers.
Conclusions
Although relatively uncommon, patients with early NMACA with favorable pathologic features (T1, well- or moderately differentiated tumors without LVI) have a very low rate of LN metastasis. Furthermore, performance of a less extensive resection among this low-risk cohort does not appear to be associated with worse survival outcomes as compared with performance of a full oncologic resection with RHC. While the vast majority of patients with NMACA should undergo RHC for complete nodal staging, the subset of patients with early NMACA with low-risk features may be able to be spared the morbidity of more extensive surgery without compromising oncologic outcomes.
References
McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer. 2002;94(12):3307–12. https://doi.org/10.1002/cncr.10589.
Marmor S, Portschy PR, Tuttle TM, Virnig BA. The rise in appendiceal cancer incidence: 2000–2009. J Gastrointest Surg. 2015;19(4):743–50. https://doi.org/10.1007/s11605-014-2726-7.
Kelly KJ. Management of appendix cancer. Clin Colon Rectal Surg. 2015;28(4):247–55. https://doi.org/10.1055/s-0035-1564433.
National Comprehensive Cancer Network. Colon Cancer, Version 4.2020, NCCN Clinical Practice Guidelines in Oncology. Accessed November 17, 2020, https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf
Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425–38. https://doi.org/10.1097/DCR.0000000000001530.
Gahagan JV, Whealon MD, Phelan MJ, et al. Lymph node positivity in appendiceal adenocarcinoma: should size matter? J Am Coll Surg. 2017;225(1):69–75. https://doi.org/10.1016/j.jamcollsurg.2017.01.056.
National Comprehensive Cancer Network. Esophageal and esophageal junction cancers, Version 4.2021, NCCN Clinical Practice Guidelines in Oncology. Accessed August 26, 2021. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
National Comprehensive Cancer Network. Gastric Cancer, Version 4.2021, NCCN Clinical Practice Guidelines in Oncology. Accessed August 26, 2021. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
National Comprehensive Cancer Network. Rectal Cancer, Version 1.2021, NCCN Clinical Practice Guidelines in Oncology. Accessed August 26, 2021. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
Hata K, Tanaka N, Nomura Y, Wada I, Nagawa H. Early appendiceal adenocarcinoma. A review of the literature with special reference to optimal surgical procedures. J Gastroenterol. 2002;37(3):210–4. https://doi.org/10.1007/s005350200023.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90. https://doi.org/10.1245/s10434-007-9747-3.
The American College of Surgeons. About the National Cancer Database. National Cancer Database. Accessed October 7, 2020. https://www.facs.org/quality-programs/cancer/ncdb/about
Turaga KK, Pappas S, Gamblin TC. Right hemicolectomy for mucinous adenocarcinoma of the appendix: just right or too much? Ann Surg Oncol. 2013;20(4):1063–7. https://doi.org/10.1245/s10434-012-2783-7.
Nussbaum DP, Speicher PJ, Gulack BC, et al. Management of 1- to 2-cm carcinoid tumors of the appendix: using the national cancer data base to address controversies in general surgery. J Am Coll Surg. 2015;220(5):894–903. https://doi.org/10.1016/j.jamcollsurg.2015.01.005.
Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2016;122(2):213–21. https://doi.org/10.1002/cncr.29744.
American College of Surgeons. National Cancer Data Base Participant User File Dictionary. Accessed December 21, 2020. http://facs.org/-/media/files/quality-programs/cancer/ncdb/puf_data_dictionary.ashx
Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676–86. https://doi.org/10.1002/sim.4509.
Erdoğan S, Gülhan OT. Alternative confidence interval methods used in the diagnostic accuracy studies. Comput Math Methods Med. 2016;2016:7141050. https://doi.org/10.1155/2016/7141050.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. https://doi.org/10.1016/0197-2456(96)00075-x.
StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021.
Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–39. https://doi.org/10.1007/s10147-015-0801-z.
Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604–8. https://doi.org/10.1056/NEJM198506203122504.
Bamboat ZM, Berger DL. Is right hemicolectomy for 2.0-cm appendiceal carcinoids justified? Arch Surg. 2006;141(4):349-52; discussion 352. https://doi.org/10.1001/archsurg.141.4.349
Veyrie N, Ata T, Muscari F, et al. Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg. 2007;205(6):785–93. https://doi.org/10.1016/j.jamcollsurg.2007.06.284.
Funding
No external funding was received for this study.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data: Straker, Grinberg, Sharon, Shannon, Fraker, Shanmugan, Miura, Karakousis. Drafting the article or revising it critically for important intellectual content: Straker, Grinberg, Sharon, Shannon, Fraker, Shanmugan, Miura, Karakousis. Final approval of the version to be published: Straker, Grinberg, Sharon, Shannon, Fraker, Shanmugan, Miura, Karakousis. All authors have reviewed and approved the submitted manuscript and agree to be accountable for all aspects of the work submitted.
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to declare. This study has been submitted for consideration for presentation at the 17th Annual Academic Surgical Congress, scheduled for 1–3 February 2022 in Orlando, FL.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Straker, R.J., Grinberg, S.Z., Sharon, C.E. et al. Pathologic Factors Associated with Low Risk of Lymph Node Metastasis in Nonmucinous Adenocarcinoma of the Appendix. Ann Surg Oncol 29, 2334–2343 (2022). https://doi.org/10.1245/s10434-021-11213-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11213-5